One of the loop diuretics, furosemide, was found useful in bronchial asthma. It enhanced anti-asthmatic effects of albuterol. The underlying mechanism is still unclear.
ObjectiveThis study was planned to investigate whether the enhancing effect of furosemide for albuterol in ovalbumin-induced asthmatic BALB/c mice is diuretic-related or not.
MethodsTwo sets of experiments were performed. In the first, effects of inhaled subdiuretic doses of furosemide and bumetanide (another loop diuretic) were compared. Treatments (mg/mL) were given as 15 minute-inhalation before final ovalbumin provocation as follows: albuterol (2.5), furosemide (0.08), bumetanide (0.005), (albuterol+furosemide, 2.5+0.08), and (albuterol+bumetanide, 2.5+0.005). Airway hyperreactivity (AHR) to inhaled methacholine, levels of IL-6, TNF-α, and differential white blood cells in bronchoalveolar lavage fluid (BALF), and lung histopathology were evaluated. In the second set, effects of oral diuretic doses (mg/kg) of furosemide (10) and bumetanide (0.25) were given before final ovalbumin provocation. Urine volume and asthma parameters were measured.
ResultsOvalbumin-asthmatic mice showed significant increases in AHR, levels of IL-6, TNF-α, and inflammatory cells in BALF, and lung inflammatory cell infiltration. Inhaled furosemide significantly decreased these changes while inhaled bumetanide failed. Albuterol and albuterol+bumetanide significantly decreased these changes more than furosemide while albuterol+furosemide produced the most significant decreases. Both oral furosemide and bumetanide exerted equivalent diuretic effects but failed to improve asthma.
ConclusionsInhaled subdiuretic dose of furosemide enhanced effects of albuterol more in ovalbumin-asthmatic mice rather than bumetanide, while oral diuretic doses of both drugs failed to improve asthma, indicating that this enhancing effect is not diuretic-related.
Bronchial asthma affects 100–150 million people from different ethnicities and age groups with annual asthma-related deaths more than 180,000.1 Treatments of asthma include both short-term relievers (short-acting β2 agonists (SABAs), antimuscarinics, and theophyllines) and long-term controllers (corticosteroids, long-acting β2 agonists (LABAs), leukotrienes modifiers, and mast cell stabilizers).2 Being tolerable, efficient, cheap, and rapidly-acting, SABAs are widely accepted relievers especially albuterol. But due to their short duration of action, SABAs are not suitable for controlling nocturnal symptoms.3 Methylxanthines inhibit production of inflammatory mediators but they are cardiotoxic, neurotoxic, and have unfavorable saturable kinetics.4 Corticosteroids are the most effective controller therapy in asthma but some flare ups were reported in children and their systemic use is associated with many adverse effects.5 Long-acting β2 agonists (LABAs) do not have any clinically important anti-inflammatory effects and they are always combined with inhalational corticosteroids (ICS) because LABAs monotherapy causes more asthma deterioration.6 Leukotrienes modifiers are used alone or with steroids in severe cases but they are expensive and their side effects are common especially in children.7 Mast cell stabilizers such as cromolyn and nedocromil are used prophylactically and they are ineffective during an acute attack.8
The search for new anti-asthmatic treatments with more efficacy and fewer adverse effects is necessary. Relief of airway edema is a useful effect of many anti-asthmatic medicines; hence inhaled diuretics were tested for potential benefits in treatment of bronchial asthma. One of the loop diuretics, furosemide, acted through non-specific inhibition of the Na+/K+/Cl− cotransporter (NKCC) and exerted a bronchoprotective effect, and decreased airway hyperresponsiveness (AHR) in asthmatic patients.9 Also nebulized furosemide was found beneficial for treatment of dyspnea related to different diseases and experimentally-induced dyspnea.10 The NKCC1 has been identified in airways and T lymphocytes.11 Bumetanide; another loop diuretic which specifically inhibits NKCC1; was found less protective in asthma than furosemide.12 As a diuretic, bumetanide is 40 times more potent than furosemide but their equipotent doses are equally effective.13 It is still unclear whether the anti-asthmatic effect of furosemide is molecule-related or class-related. Thus, the present study was designed to test the effects of furosemide and bumetanide separately and combined with albuterol against airway hyperresponsiveness and inflammatory response in ovalbumin-induced asthma in mice.
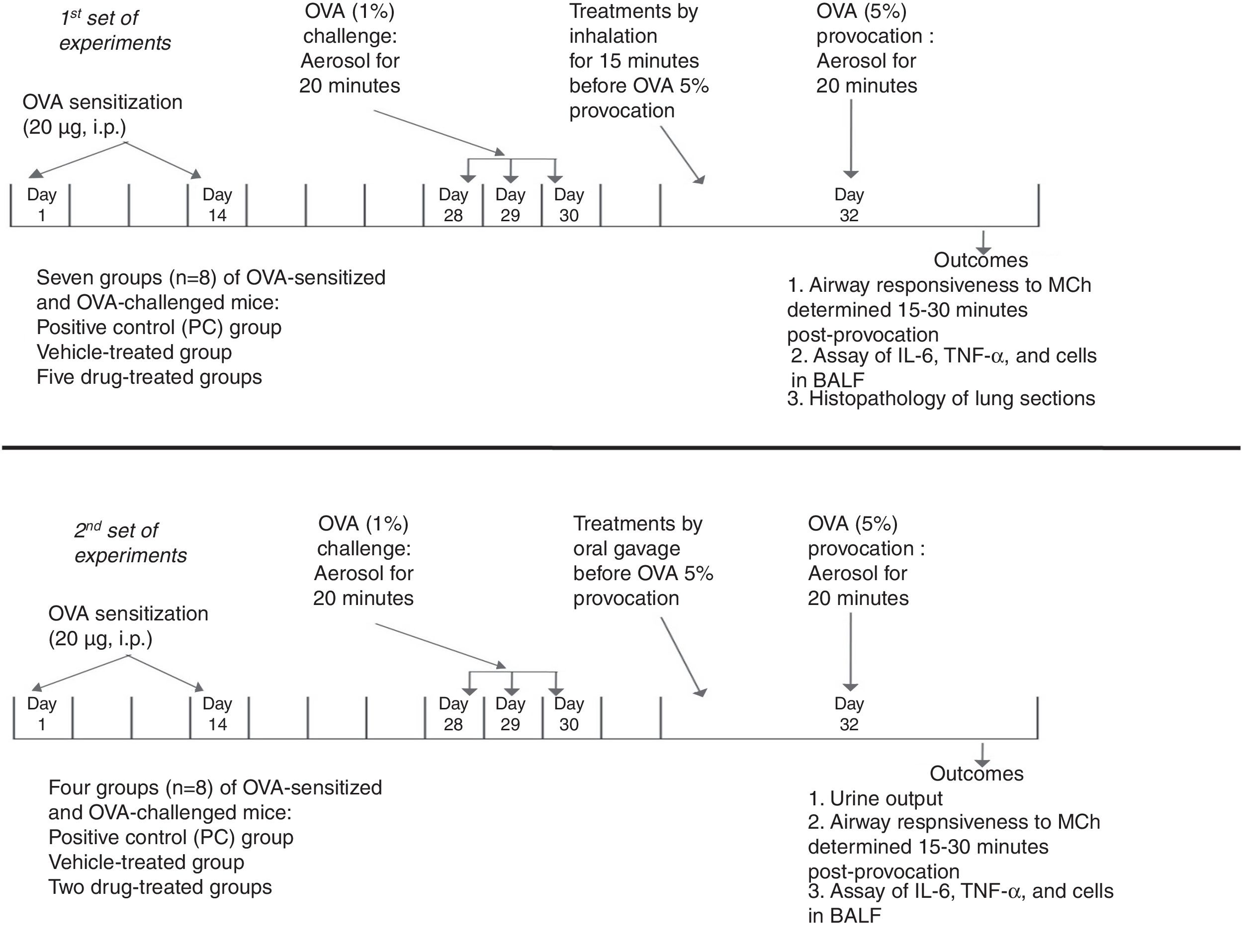
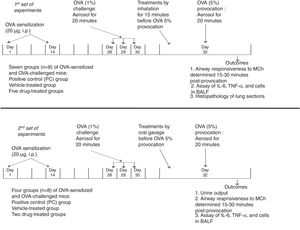
MethodsExperimental designThe study design is summarized in Fig. 1. The protocol of the study was approved by the King Abdulaziz University Research Ethics Committee and adhered to the National Institutes of Health guide for the care and use of laboratory animals. All drugs and chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless mentioned otherwise. Female BALB/c mice (8–10 weeks old and about 30g weight) were housed in cages at 24°C with constant humidity (50–60%), in a 12-h light–dark cycle. All mice were acclimatized for at least one week before experimentation. Standard pellet diet and water were available ad libitum.
Summary of the experimental design in BALB/c mice showing the time points for ovalbumin-sensitization, ovalbumin-challenge, and ovalbumin-provocation, time of administration of treatments, and time for measurement of the outcomes. In the 1st set of experiments, the treatments were given by inhalation. They included albuterol (A, 2.5mg/mL solution), furosemide (F, 0.08mg/mL), bumetanide (B, 0.005mg/mL), albuterol+furosemide (A+F), and albuterol+bumetanide (A+B). In the 2nd set of experiments, the treatments were given by oral gavage. They included furosemide (10mg/kg) and bumetanide (0.25mg/kg).
Mice were sensitized by intraperitoneal (i.p.) injection of 20μg ovalbumin (OVA) emulsified in 2.25mg Alum (aluminum hydroxide) in a total volume of 0.1ml on days 1 and 14. Mice were challenged via the airways with OVA 1% to establish lung inflammation or phosphate buffered saline (PBS) in the control group for 20min on days 28, 29, and 30 by ultrasonic nebulization. On day 32, mice were provoked with OVA 5%. Airway responsiveness was determined 15–30min post-provocation, using double-chamber plethysmography.14,15
Administration of drugs and treatment groupsIn the first set, in addition to the negative control (NC) group (sensitized and challenged with PBS), the OVA-sensitized and OVA-challenged were randomly divided into seven groups (n=8): positive control (PC) group (saline-treated), vehicle (PBS)-treated group, and five drug-treated groups. Treatments were given by inhalation for 15min once before the OVA 5% provocation. They included albuterol (A, 2.5mg/mL solution),15,16 furosemide (F, 0.08mg/mL), bumetanide (B, 0.005mg/mL), albuterol+furosemide (A+F), and albuterol+bumetanide (A+B).15,17 Airway responsiveness to methacholine, levels of IL-6, TNF-α, and cells in the bronchoalveolar lavage fluid, and lung histopathology were evaluated.
In the second set, experiments were done to detect the effect of oral diuretic doses of furosemide and bumetanide on the asthma parameters. Treatments were given by oral gavage before the OVA 5% provocation. The treatments given included furosemide (10mg/kg) and bumetanide (0.25mg/kg).18 Urine output was collected for 6h and its volume (ml) was measured.19 Airway responsiveness to methacholine and levels of IL-6, TNF-α, and cells in the bronchoalveolar lavage fluid were evaluated.
Determination of airway responsiveness to methacholine (MCh)Mice were accommodated to the double-chamber plethysmograph (Emka Tech., France). The specific airway resistance (sRaw, cmH2O/second) was calculated in restrained conscious mice. On day 32, mice were placed in the plethysmograph and baseline values were recorded. Then, mice were exposed to nebulized saline for 3min, given the specified treatments by nebulization for 15min, and provoked with OVA 5%. Airway responsiveness to inhaled MCh (dissolved in 0.9% NaCl) was measured at 15–30min post-provocation where mice were nebulized with doubling doses of MCh (6.25–50mg/mL) for three minutes each, followed by recording for three minutes after each nebulization to determine the airway resistance values. MCh dose-response curves were generated and “the concentration of MCh that produced a 200% increase (PC200) above baseline resistance” was recorded.14,20
Assay of IL-6, TNF-α, and cells in the bronchoalveolar lavage fluidAfter determination of AHR, the mice were anesthetized with pentobarbital (50mg/kg, i.p.), and bronchoalveolar lavage was done with PBS using a tracheal tube. The recovered bronchoalveolar lavage fluid (BALF) was centrifuged and the supernatant was used for measurements of the levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) using ELISA kits according to the manufacturer's instructions (Biolegend San Diego, CA, USA). The cells in the BALF pellet were washed in saline, and suspended in a lysine buffer to destroy the remaining erythrocytes. Aliquots of the cell pellets were placed on slides and then stained with Field's stain. After drying, 200 cells per slide were counted using a microscope (Optima X5Z-H) and cells were classified as eosinophils, neutrophils, lymphocytes, or macrophages.21
Histopathological examinationThe lung sections were dissected and fixed in 10% buffered formalin. Paraffin sections (3mm) were stained with hematoxylin and eosin (H&E). The sections were scored based on severity of the inflammation around the airways and blood vessels and in the alveoli and on the thickness of airway epithelial cell layer with a scale from 0 (no inflammation) to 4 (severe inflammation).21
Statistical analysisData were expressed as means±SEM and analyzed using SPSS version 18. One-way ANOVA followed by Tukey's post hoc test was used to evaluate differences among groups. p<0.05 was considered to be statistically significant.
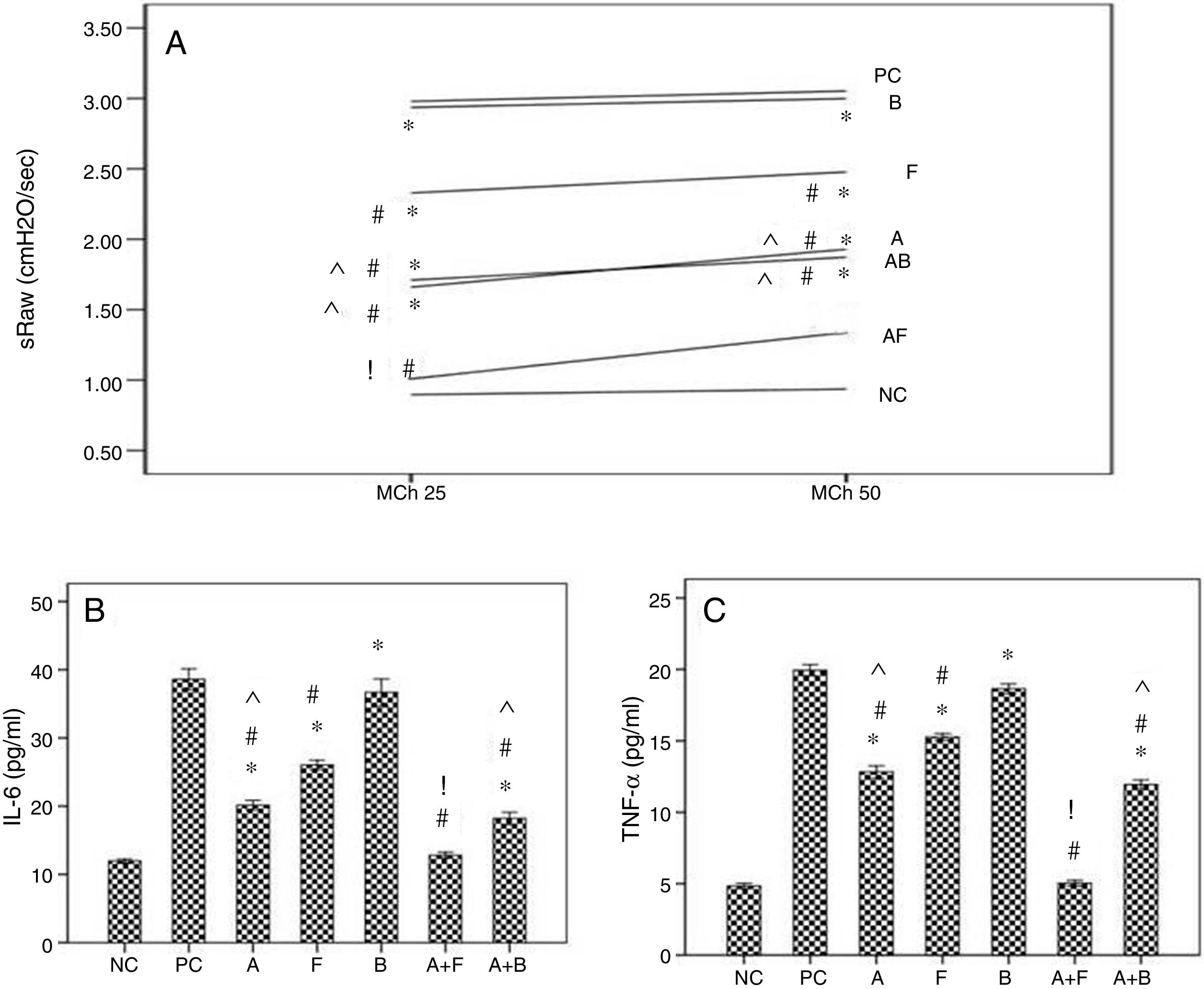
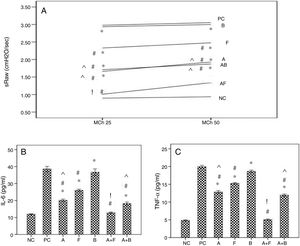
ResultsThe first setAirway hyperresponsiveness to methacholineThe baseline airway resistance did not show significant variations among the different groups. Inhalation of MCh caused concentration-related increases in airway resistance (RxV) compared to baseline level. The asthmatic mice showed significant increases in AHR compared to NC group. Inhaled furosemide significantly decreased AHR while inhaled bumetanide failed. Both albuterol and albuterol+bumetanide treatments significantly decreased AHR more than furosemide. Albuterol+furosemide group showed the most significant decrease of AHR with a non-significant difference from NC group (Fig. 2A).
Effects of inhaled albuterol, furosemide, bumetanide separately and in combinations on: A) Airway responsiveness (AHR), B) IL-6, and C) TNF-α in ovalbumin -sensitized and ovalbumin-challenged mice. AHR to doubling concentrations of methacholine (25 and 50mg/mL) at 15min post-provocation was expressed as specific airway resistance (sRaw). Treatments (mg/mL) included A (albuterol, 2.5), F (furosemide, 0.08), B (bumetanide, 0.005), A+F (albuterol+furosemide, 2.5+0.08), and A+B (albuterol+bumetanide, 2.5+0.005). Data were expressed as mean±SEM (n=8). Comparisons were made using ANOVA with Tukey's post hoc test. *p<0.05: all groups except A+F vs. NC (normal control), #p<0.05: all groups vs. PC and B, ^p<0.05: A and A+B vs. F, !p<0.05: A+F vs. all groups except NC.
The asthmatic mice showed significant increases of levels of IL-6 and TNF-α in BALF compared to NC group. Furosemide significantly decreased them, but bumetanide failed. Albuterol and albuterol+bumetanide treatments significantly decreased them more than furosemide. Albuterol+furosemide group showed the most significant decrease with a non-significant difference from NC group (Fig. 2B and C).
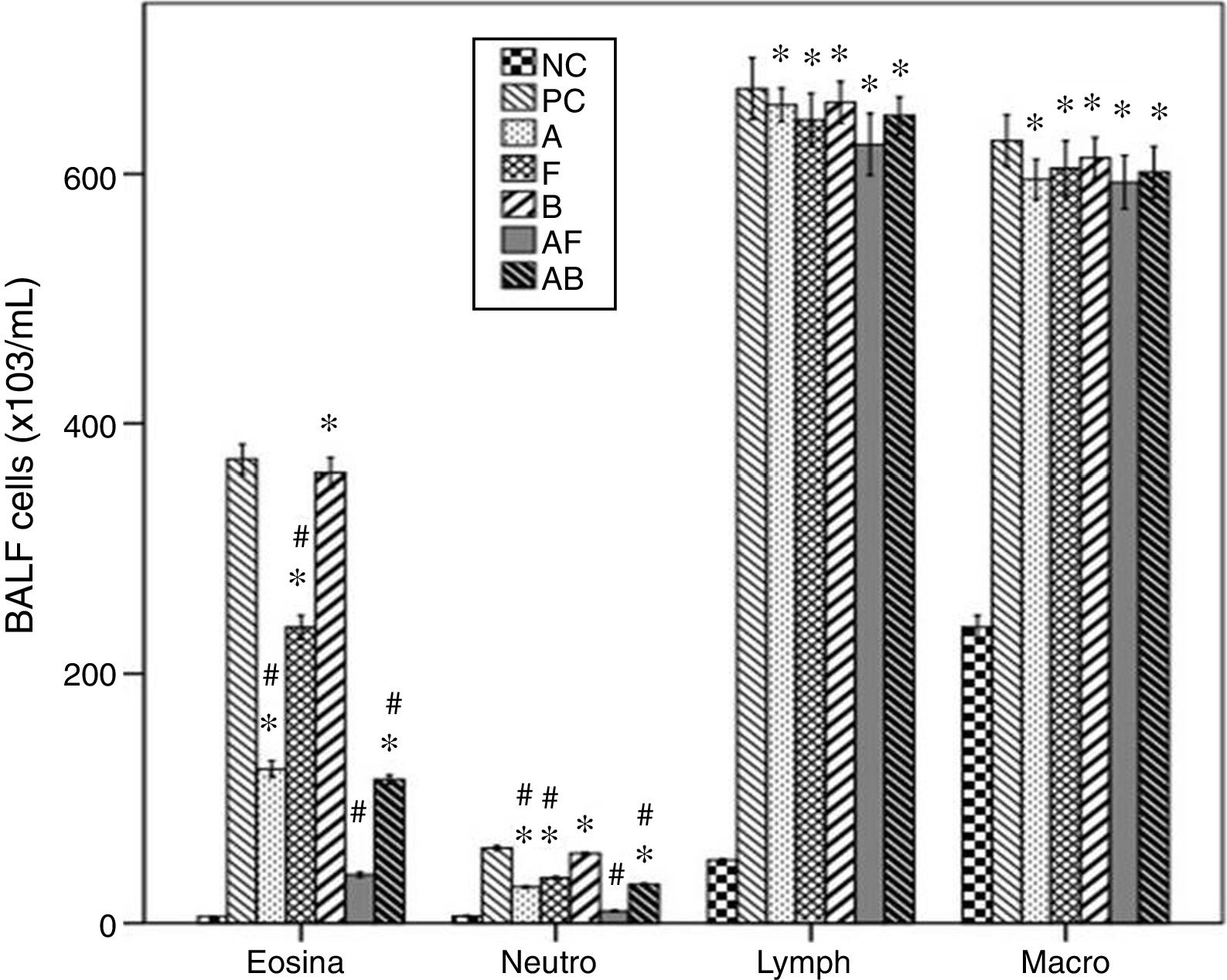
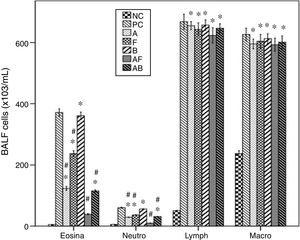
Cellular levels in BALFThe asthmatic mice showed significant increases of levels of inflammatory cells in BALF compared to NC group. Furosemide significantly decreased eosinophils and neutrophils, but bumetanide failed. Albuterol and Albuterol+bumetanide significantly decreased them more than furosemide. Albuterol+furosemide group showed the most significant decrease with a non-significant difference from NC group. All treatments failed to affect levels of lymphocytes and macrophages (Fig. 3).
Effects of inhaled albuterol, furosemide, bumetanide separately and in combinations on bronchoalveolar lavage (BALF) cells in ovalbumin-sensitized and ovalbumin-challenged mice. Treatments (mg/mL) included A (albuterol, 2.5), F (furosemide, 0.08), B (bumetanide, 0.005), A+F (albuterol+furosemide, 2.5+0.08), and A+B (albuterol+bumetanide, 2.5+0.005). Data were expressed as mean±SEM (n=8). Comparisons were made using ANOVA with Tukey's post hoc test. *p<0.05: all groups except A+F vs. NC (normal control), #p<0.05: all groups vs. PC and B, ^p<0.05: A and A+B vs. F, !p<0.05: A+F vs. all groups except NC.
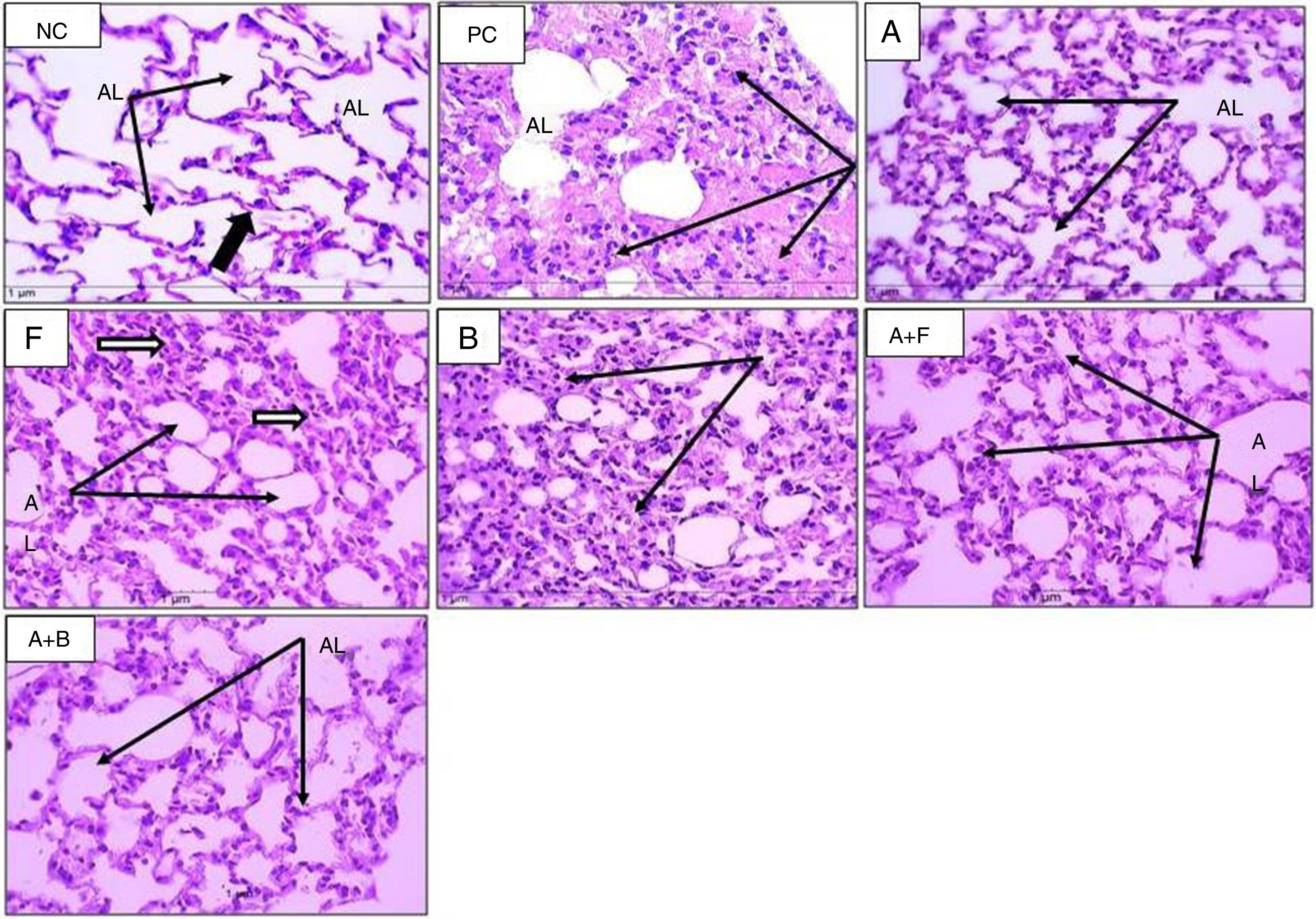
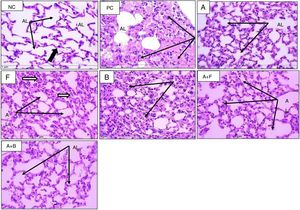
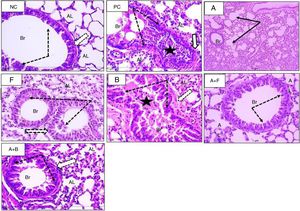
In HE-stained sections (Figs. 4 and 5), the PC group showed abnormal respiratory epithelium, collapsed bronchiole, and alveoli full of mucous secretion and inflammatory cells. The walls were thickened (score 4) compared with NC group (score 0). The furosemide group showed a score 3 appearance, but bumetanide showed a picture nearly similar to positive control. Albuterol and albuterol+bumetanide groups showed score 2 appearances. Albuterol+furosemide group nearly reversed and normalized these OVA-induced changes showing a score 1 picture.
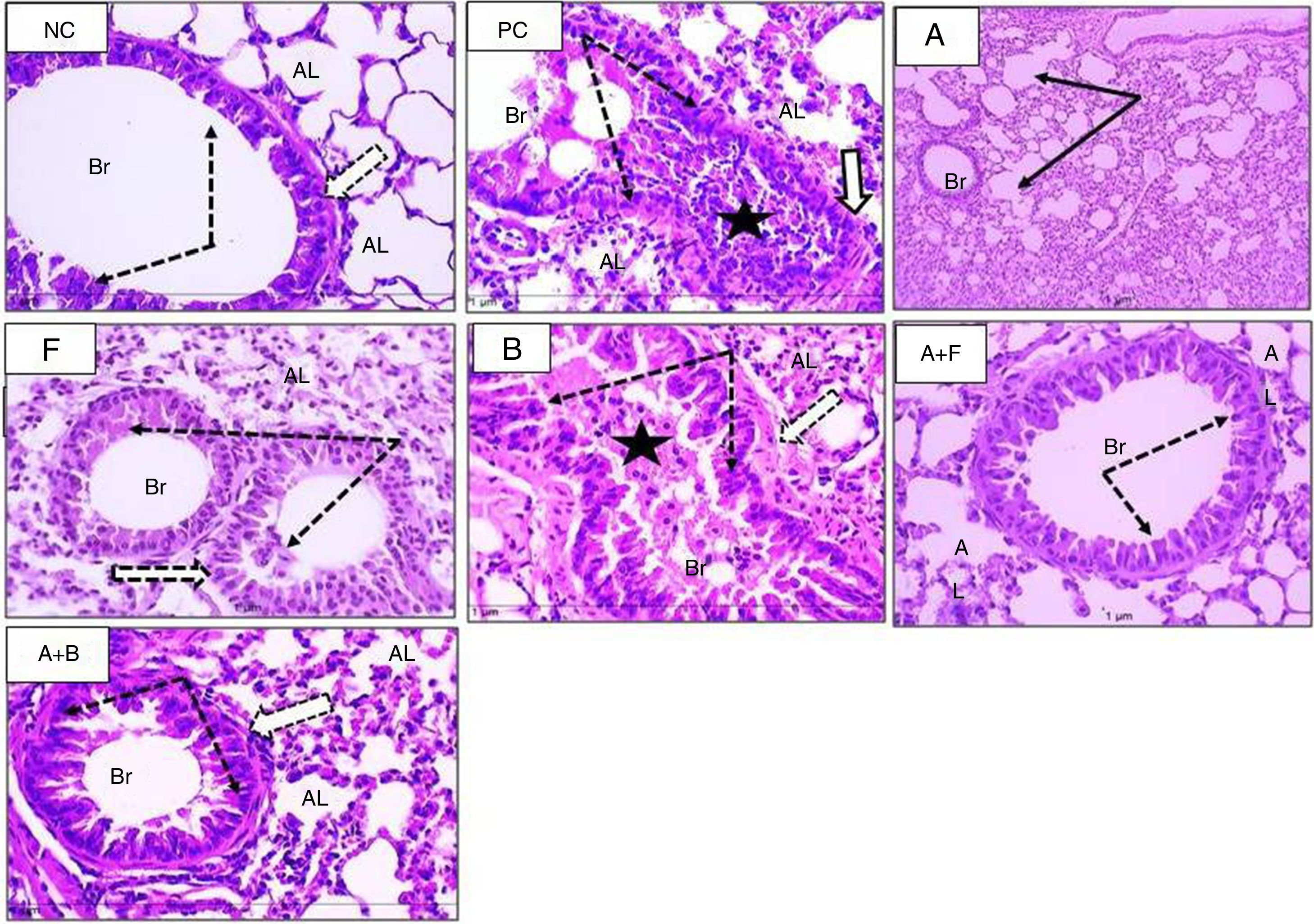
Sections from mice lung (H&E stain) (×400) showing alveoli of normal control (NC), positive control (PC), and inhaled albuterol (A), furosemide (F), bumetanide (B), albuterol+furosemide (A+F), and albuterol+bumetanide (A+B). Normal control (NC) shows patent lumina, thin wall (thin black arrows) and few inflammatory cells (black thick arrow). Positive control (PC) shows consolidated alveoli and thickened walls (arrows). Lumina are filled with pinkish stained mucous and inflammatory cells. Albuterol (A) shows normal patent alveoli (arrows) with thin wall lining epithelium. Furosemide (F) shows patent alveoli with slight thickening of alveolar wall and some contains inflammatory cells (arrows). Bumetanide (B) shows that most alveoli are consolidated with inflammatory cell infiltrate (arrows) and few alveoli are patent. Albuterol+furosemide (A+F) shows healthy patent alveoli (AL) within thin epithelial lining (arrows). Albuterol+bumetanide (A+B) shows that most alveoli (AL) are patents (arrows) and few contain inflammatory cells.
Sections from mice lung (H&E stain) (×400) showing bronchioles of normal control (NC), positive control (PC), and inhaled albuterol (A), furosemide (F), bumetanide (B), albuterol+furosemide (A+F), and albuterol+bumetanide (A+B). Normal control (NC) shows patent bronchioles (Br) with normal lining epithelium (dotted arrows) and muscle layer (white arrow). Nearby alveoli (AL) have thin wall and patent lumina. Positive control (PC) shows collapsed bronchioles (Br) full of mucous secretion and mononuclear inflammatory cells (black star). The wall is slightly thickened (white arrow). Nearby alveoli (AL) are collapsed and full of inflammatory cells. Albuterol (A) shows normal patent bronchioles (Br) and slight thickened alveolar wall. Furosemide (F) shows patent bronchioles (Br) with residual thick epithelium and inflammatory cells (dotted arrows). Bumetanide (B) shows marked obstruction of bronchioles (Br) with mucous and inflammatory cells (star). The lining epithelium (dotted arrows) and bronchial wall (white arrow) showed hypertrophy and the alveolar wall is thick (AL). Albuterol+furosemide (A+F) shows patent bronchioles (Br) with normal epithelium (dotted arrows). The alveoli (AL) showed normal epithelial lining and free of cell infiltrate. Albuterol+bumetanide (A+B) shows patent bronchioles (Br) with thick epithelium. The alveoli (AL) are patent with slight thickened wall.
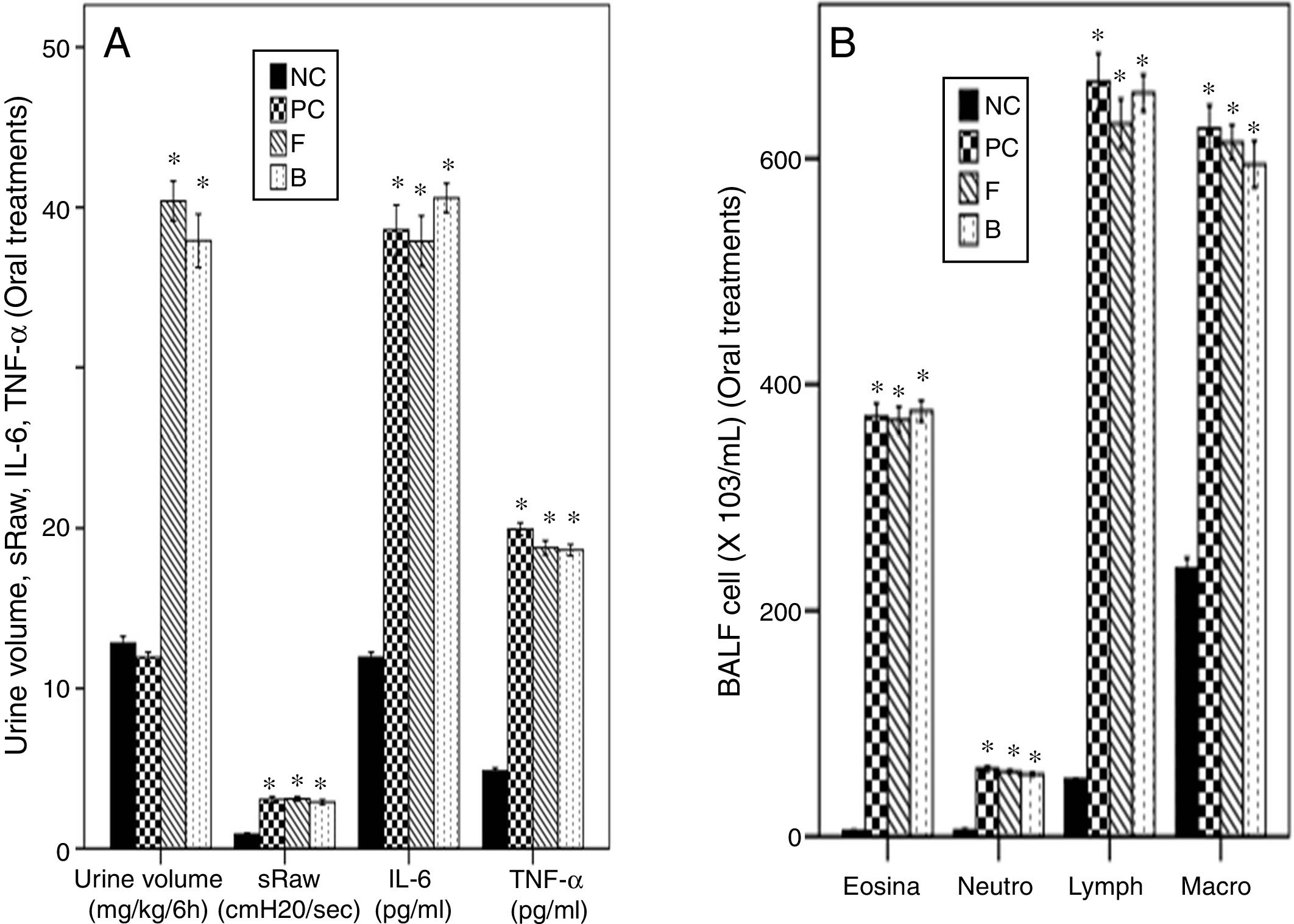
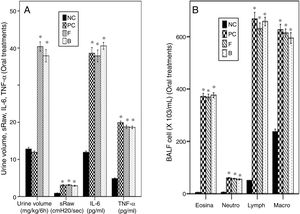
The asthmatic mice showed a non-significant change of urine volume compared to NC group. Both oral furosemide and bumetanide significantly increased urine volume to a similar degree indicating a diuretic effect with a non-significant difference between them (Fig. 6A).
Effects of oral furosemide (10mg/kg) and bumetanide (0.25mg/kg) on: (A) Urine volume, Airway responsiveness (AHR), IL-6, and TNF-α, and (B) Bronchoalveolar lavage (BALF) cells in ovalbumin-sensitized and ovalbumin-challenged mice. Data were expressed as mean±SEM (n=8). Comparisons were made using ANOVA with Tukey's post hoc test. *p<0.05: significant vs. NC (normal control).
Both oral furosemide and bumetanide failed to decrease AHR and levels of IL-6 and TNF-α in BALF (Fig. 6A). They also failed to decrease levels of the inflammatory cells in BALF (Fig. 6B) indicating their failure to improve asthma.
DiscussionThe ovalbumin provocation in OVA-sensitized mice increased eosinophils in lung tissue and BALF as rapidly as 15min post-provocation. Albuterol reduced airway neutrophils, eosinophils, and specific resistance in early phase (10min post-challenge) but did not affect late phase (24h post-challenge).22 In OVA- sensitized and OVA-challenged guinea pigs, acute therapy by an inhaled single dose of salbutamol significantly decreased specific airway resistance values at one hour post-inhalation, rather than at five hours, confirming its bronchodilatory effect.16 In asthmatic patients, inhaled furosemide reversed acute asthma exacerbations and improved pulmonary functions indicating a significant bronchodilatation but less than that of salbutamol.23 In a clinical trial in reactive airway disease patients, combined nebulization of furosemide and salbutamol improved the peak expiratory flow rate more than each agent separately.24 Inhaled furosemide, as an adjunct, effectively attenuated acute asthma attacks in both prophylactic and therapeutic regimens with no evident adverse events.9 In OVA-sensitized mice, NKCC expression has been increased in airway epithelial cells principally on goblet cells.25 Nebulized furosemide improved pulmonary function in asthmatic patients due to its local effect on the lung, rather than its diuretic effect. It inhibited NKCC in airway epithelium, decreasing the intracellular sodium and as a result the intracellular calcium and hence causing airway relaxation. It increased airway epithelium-derived PGE2. Also it reduced production and release of mediators such as LTC4, histamine, neutrophil chemotactics, IL-6, IL-8, and TNF-α. 26–28 Moreover, it decreased the intra-airway thermal gradient due to airway vasodilation.29 It protected against bronchoconstriction induced by exercise, cold air, different allergens, aspirin, methacholine, and others. It showed a pattern of protection similar to that of sodium cromoglycate and nedocromil sodium indicating that it inhibits mediator production and release and improves the sensitivity of inflammatory cells to endogenous glucocorticosteroids.30 Furosemide inhibited leukotriene B4-induced airway eosinophilia through inhibition of anion transport.31 On the other hand, intraperitoneal injection of furosemide decreased basal and allergen-induced airway responsiveness, but unexpectedly increased NKCC1-expressing T lymphocytes lung infiltration and did not affect goblet cell hyperplasia.25,32 Nebulization of furosemide and salbutamol in children with acute asthmatic attack did not improve the clinical or spirometric parameters compared with nebulized salbutamol alone.33 Co-administration of furosemide and albuterol significantly increased the peak flow rate but did not significantly affect the spirometric or clinical scores as compared to albuterol alone.34
In asthmatic patients, nebulization of equidiuretic doses of furosemide and bumetanide attenuated airway reactivity to adenosine 5′-monophosphate (AMP) with a peak effect at 10min. Furosemide was more potent than bumetanide and this could be explained by the restrictive barrier properties of the airway epithelium.35 In vitro, furosemide relaxed human fetal airway constricted by acetylcholine or leukotriene D4. In isolated newborn mouse airways, no differences in relaxation were observed after application of equimolar doses of furosemide (3–300μM) to both epithelial and adventitial surfaces (to mimic aerosolized and systemic administration respectively). Thus, furosemide has a direct non-epithelial-dependent effect on airway smooth muscle tone. Similar results were obtained with bumetanide (0.3–30μM) indicating a 10-fold difference in potency between the two drugs.36 This 10-fold differential potency in airway relaxation between furosemide and bumetanide suggests that airway relaxation may be mediated partly by inhibition of the NKCC.11 Unfortunately, bumetanide blocks the basolateral chloride uptake and depletes the airway surface liquid volume causing mucus stasis.37 Inhaled furosemide effectively improved bronchoconstriction induced by exercise, allergen, distilled water, and AMP indicating suppression of release of inflammatory mediators from mast cells,29 while bumetanide failed.38 The differences between the effects of furosemide and bumetanide in asthma could be attributed to pharmacokinetic differences that lead to inconsistent concentration at the basolateral membrane of airway epithelial cells.39 In this study, the similar diuretic effect of oral equipotent doses of furosemide and bumetanide agrees with previous studies.19 Also, failure of oral diuretic doses of both drugs to improve asthma parameters was reported previously.29 The efficacy of inhaled furosemide; rather than oral; in asthma was partly explained by its differential effects on parameters on the human peripheral blood polymorphonuclear leukocytes (PMNL). Incubation of these cells with furosemide at 10−5, 10−4, and 10−3 M concentrations (equal to levels of inhaled furosemide that can be reached in the bronchial lining fluid) resulted in concentration-dependent decreases of both Ca++ influx and luminal-dependent chemiluminescence signal (an indirect measure of NADPH-oxidase activation). In contrast, furosemide had no effect on phagocytosis and intracellular killing of St. aureus. Moreover, while the 10−5 and 10−4M concentrations decreased chemotaxis of PMNL to n-formyl-methionyl-leucyl-phenylalanine, the 10−3M concentration exerted a weak chemoattractant effect.40
In conclusion, inhaled subdiuretic dose of furosemide enhanced effects of albuterol rather more than bumetanide in ovalbumin-asthmatic mice. Also, oral diuretic doses of furosemide and bumetanide exerted similar diuretic effects but failed to improve asthma. This indicates that the enhancing effect of inhaled furosemide for albuterol in asthmatic mice is not diuretic-related. Further studies are needed to elucidate the exact mechanism.
Conflict of interestThe authors have no conflict of interest to declare.
This project was funded by the Deanship of Scientific Research, (DSR), King Abdulaziz University (KAU), Jeddah under grant number (G 43/828/1438). The authors, therefore, acknowledge with thanks DSR technical and financial support. The participation of the medical students Omar Momina, Bassam Ramzi, Abdullah Abdulkhaliq, Mohammed Alhebshi, and Mohammed Alghamdi is gratefully acknowledged.