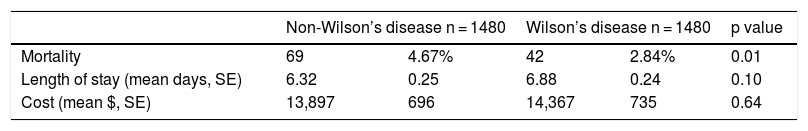Wilson’s disease (WD) is a rare genetic disorder characterized by excessive copper disposition predominantly in the liver and brain. Hospitalization data on patients with WD are scarce. Hence, we sought to examine the inpatient characteristics and outcomes of patients with WD.
Patients and MethodsWe utilized the National Inpatient Database (2006–2011) and analyzed all adult patients with a diagnosis of WD.
ResultsThere were 9046 hospitalizations during the study period. The leading etiologies for admissions were chronic liver disease (8.58%), WD (6.49%) and infections (septicemia 3.10% and pneumonia 2.50%). The overall inpatient mortality rate for WD patients was 2.58%. Independent predictors of mortality in WD patients were acute respiratory failure (OR: 4.53; 95% CI: 2.44–8.42), acute renal failure (OR: 4.09; 95% CI: 2.19–7.65), decompensated liver disease or liver failure (OR: 3.37; 95% CI: 1.72–6.59), and advanced age (every 10 year increase, OR: 1.48; 95% CI: 1.25–1.75). Propensity-score matched analysis revealed better inpatient survival in WD patients compared to matched non-WD patients (2.84% vs. 4.67%, p = 0.01).
ConclusionsOur study demonstrated the clinical characteristics and outcomes of hospitalized patients with WD. These findings add important knowledge to our understanding of the healthcare utilization and outcomes of this rare disease in the United States.
Wilson’s disease (WD) is a rare autosomal recessive disorder of defected copper metabolism in the liver. Excessive copper deposition causes oxidative stress, impaired mitochondrial function, and eventually leads to hepatic, neurologic, renal, and other organ dysfunction [1]. Diagnosis of WD can be challenging due to its rarity, highly variable clinical manifestations, and non-specific diagnostic tests that are currently available [2,3]. Since the first description of WD in 1912, there have been revolutionary advances in the treatment of the disease. Introduction of copper chelating agent in 1950’s finally started to turn the tide in the battle against this fatal disease [4]. Currently effective medical therapy with chelating agents and copper antagonists can be achieved in many patients with satisfactory clinical response [5]. Liver transplantation also becomes an excellent treatment option for WD patients with end-stage liver disease and fulminant hepatic failure. Long-term outcome after transplantation has also improved considerably over the last decade with improvement in healthcare system [6,7]. A recent cohort study from Europe showed an 81% 10-year survival rate among pediatric patients receiving liver transplants for WD [7].
Longitudinal follow-up from WD-specialized centers have shed light on the natural history and prognosis of the disease. There have been a few published patient cohorts on WD worldwide [8–14]. For example, the study from Austria analyzed 229 WD patients from 1961 to 2013 with mean observation period 14.8 years [8]. In this cohort, 7.4% WD patients died, among which 71% of deaths were related to WD. Cirrhosis at diagnosis was the best predictor of death and need for transplant. Despite overall promising outcomes in WD, there is limited knowledge on the outcomes of hospitalized patients with WD in the United States. Hence, we sought to investigate the inpatient outcomes including mortality among adult WD patients using a large national database in the United States.
2Materials and methods2.1Study population and covariatesAll data were extracted from the National Inpatient Sample (NIS) database between 2006 and 2011. The NIS database is the largest publicly available all-payer inpatient healthcare database in the United States [15]. The NIS database is a 20% sample of discharges from all community hospitals participating in Healthcare Cost and Utilization Project (HCUP), representing more than 95% of the U.S. population. The NIS database contains un-weighted data from more than 7 million hospitalizations nationally every year.
We queried the NIS database for hospital data on discharges with ICD-9-CM (International Classification of Disease, Ninth Revision, Clinical Modification) code of WD 275.1 as either principle or secondary diagnosis in order to capture all the hospitalized WD patients. NIS database cannot differentiate between newly diagnosed and established WD. Discharges under the age of 18 were excluded to represent the adult patient population. Discharges with a principal diagnosis or procedure related to pregnancy were further excluded. Patient variables included age, gender, race, median household income for patient’s zip code (quartiles), and insurance status. Race was categorized as White, Black, Hispanic, Asian, and others. Insurance status was categorized as Medicare, Medicaid, private insurance, and others based on the primary payer listed on the discharge record. Comorbidities for risk adjustment were derived from Agency for Healthcare Research and Quality (AHRQ) comorbidity measures based on the methods by Elixhauser et al. Patients were stratified into a score of <3 or ≥3 based on the number of comorbidities. Hospital-related factors were hospital location (urban, rural), hospital bed size (large, medium, and small), and hospital teaching status (teaching, non-teaching). Hospital region was classified as Northeast, Midwest, South, or West. ICD-9 codes of all principle diagnoses were reviewed to determine admission etiologies (Supplementary Table 1).
This research protocol was deemed exempt from the Institutional Review Board of the Ohio State University because the data contained in the NIS database are not identifiable.
2.2Statistical analysisAll statistical calculations were conducted using SAS 9.4 (SAS Institute, Cary, NC). Categorical variables were expressed as weighted frequency (percentage) and differences between groups were analyzed by χ2 tests. Continuous variables were expressed as mean ± standard error (SE) and differences were analyzed with t tests. Temporal trends were assessed using the Cochrane-Armitage trend test. Statistical significance was defined as p value <0.05. A multivariable logistic regression model was used to identify independent predictors for in-hospital mortality where results are presented with odds ratios (OR) and 95% confidence intervals (CI). Terms included in the model were used by stepwise selection to determine the independent predictors of inpatient mortality among patients with WD.
Propensity score matching was further used to compare outcomes between patients with WD and other hospitalized patients. Propensity scores were calculated using a multivariable logistic regression model for WD containing all demographic variables and 29 AHRQ comorbidities. Patients in each group were then matched 1:1 using a greedy matching algorithm with a caliper of 0.2 times the standard deviation of the propensity scores. A total of 1480 pairs were formed (Supplementary Table 2). In our original un-weighted sample there were 1838 discharges with WD. Conditional logistic regression and paired t-test were used to evaluate the outcomes in the matched sample. The study schema is outlined in Fig. 1.
3Results3.1Trend of hospitalization in WD patientsThere were an estimated total of 9046 hospitalizations for adult patients with WD between 2006 and 2011 in the United States. There was an increasing trend of hospitalization for WD annually, from 1145 cases (40.6 per 1,000,000 hospitalizations) in 2006 to 1823 cases (63.4 per 1,000,000 hospitalizations) in 2011, p < 0.001 (Fig. 2).
3.2Baseline characteristics and outcomes for patients with WDThe baseline characteristics and outcomes for hospitalized patients with WD were described in Table 1. The mean age of admission was 50.4 ± 0.6 years old. Among the hospitalized patients, 54.23% were female and 73.35% were White. Majority of WD patients had Medicare and Private insurance. WD patients were more likely admitted to large and teaching hospitals. Additionally, 38.81% of hospitalizations occurred in the South of the United States compared to other regions. Almost half of hospitalized WD patients had an Elixhauser comorbidity index of more than 3. A total of 233 (2.58%) patients with WD died during their hospitalizations between 2006 and 2011 (Table 1). Mean length of stay was approximately 7 ± 0.23 days with a cost of $13,999 ± 730 for each hospitalization.
Baseline characteristics and outcomes for patients with Wilson’s disease in the National Inpatient Sample (2006–2011).
| Wilson’s disease, n = 9046 (%) | ||
|---|---|---|
| Age (mean years, SE) | 50.37 | 0.59 |
| Gender | ||
| Male | 4135 | 45.77% |
| Female | 4900 | 54.23% |
| Race | ||
| White | 5632 | 73.35% |
| Black | 865 | 11.26% |
| Hispanic | 698 | 9.09% |
| Asian | 167 | 2.18% |
| Other | 316 | 4.12% |
| Income (National Quartile) | ||
| Low | 2478 | 28.26% |
| Moderate | 2434 | 27.76% |
| High | 2118 | 24.15% |
| Very High | 1739 | 19.83% |
| Type of insurance | ||
| Medicare | 3599 | 39.87% |
| Medicaid | 1575 | 17.44% |
| Private | 2996 | 33.19% |
| Other | 858 | 9.50% |
| Location of hospital | ||
| Rural | 1141 | 12.83% |
| Urban | 7751 | 87.17% |
| Teaching Status | ||
| Non-Teaching | 4071 | 45.79% |
| Teaching | 4820 | 54.21% |
| Hospital size | ||
| Small | 936 | 10.53% |
| Medium | 1784 | 20.06% |
| Large | 6171 | 69.41% |
| Hospital region | ||
| Northeast | 1518 | 16.78% |
| Midwest | 2351 | 25.98% |
| South | 3510 | 38.81% |
| West | 1667 | 18.43% |
| Elixhauser Comorbidity Index | ||
| 0 | 903 | 9.98% |
| 1 | 1546 | 17.09% |
| 2 | 2173 | 24.02% |
| ≥3 | 4425 | 48.92% |
| Inpatient outcomes | ||
| Mortality | 233 | 2.58% |
| Length of Stay (days, SE) | 6.95 | 0.23 |
| Cost ($, SE) | 13,999 | 730 |
SE: Standard Error.
There were significant differences among comorbidities between patients with WD and without as shown in Supplementary Table 2. Patients with WD had significantly higher prevalence of liver diseases (20.93%), neurological diseases (10.86%), psychosis (7.59%), and depression (15.45%) compared to non-WD patients. Among hospitalized WD patients 8.44% reported alcohol abuse and 5.78% drug abuse. Anemia (25.12%) and coagulopathy (14.82%) were also common in patients with WD. In contrast, hospitalized non-WD patients had significantly higher prevalence for cardiovascular disorders, chronic lung disease, diabetes, hypertension, malignancy, and renal failure.
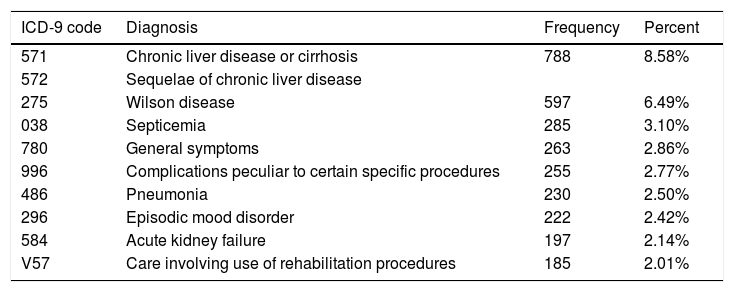
3.3Principal diagnosis for admission in patients with WDThere was a wide spectrum of principle diagnoses for hospitalization in patients with WD (Table 2). The most common diagnosis for admissions in patients with WD was chronic liver disease which accounted for 8.58% of total admissions. Most common chronic liver diseases were cirrhosis (40.00%) and hepatic encephalopathy (38.92%). Other leading diagnoses for admissions were WD (6.49%), infection (5.60%) including septicemia (3.10%) and pneumonia (2.50%). Among the full population of WD, 1.09% of the patients had liver failure as their principle diagnosis.
Top principle diagnoses for patients with Wilson’s disease in National Inpatient Sample (2006–2011).
| ICD-9 code | Diagnosis | Frequency | Percent |
|---|---|---|---|
| 571 | Chronic liver disease or cirrhosis | 788 | 8.58% |
| 572 | Sequelae of chronic liver disease | ||
| 275 | Wilson disease | 597 | 6.49% |
| 038 | Septicemia | 285 | 3.10% |
| 780 | General symptoms | 263 | 2.86% |
| 996 | Complications peculiar to certain specific procedures | 255 | 2.77% |
| 486 | Pneumonia | 230 | 2.50% |
| 296 | Episodic mood disorder | 222 | 2.42% |
| 584 | Acute kidney failure | 197 | 2.14% |
| V57 | Care involving use of rehabilitation procedures | 185 | 2.01% |
A total of 43 patients with WD died during hospitalizations between 2006 and 2011 in the unweighted sample. Most of the patients died due to liver-related cause (30.23%) followed by infections (18.60%). Independent predictors of inpatient mortality among patients with WD are shown in Table 3. For every 10-year increment of age, there was increased risk of death during hospitalization (OR: 1.48; 95% CI: 1.25–1.75). Other predictors of inpatient mortality in WD patients were acute respiratory failure (OR: 4.53; 95% CI: 2.44–8.42), acute renal failure (OR: 4.09; 95% CI: 2.19–7.65) and decompensated liver disease or hepatic failure (OR: 3.37; 95% CI: 1.72–6.59).
Logistic regression model for mortality among patients with Wilson’s disease in National Inpatient Sample (2006–2011).
| Predictive factors | OR (95% CI) | |
|---|---|---|
| Age (10 year increase) | 1.48 | (1.25, 1.75) |
| Acute respiratory failure | 4.53 | (2.44, 8.42) |
| Acute renal failure | 4.09 | (2.19, 7.65) |
| Decompensated liver disease or liver failure | 3.37 | (1.72, 6.59) |
CI, confidential interval.
Covariates in the propensity score analysis were all well balanced between the WD and non-WD groups except age (Supplementary Table 3). Inpatient mortality was lower in WD patients compared to matched non-WD patients (2.84% vs. 4.67%, p = 0.01) (Table 4). There were no significant differences in length of stay, or total cost between the two cohorts in the propensity-matched sample.
Analysis of outcomes of propensity-matched Wilson’s disease and non-Wilson’s disease patients National Inpatient Sample (2006–2011).
| Non-Wilson’s disease n = 1480 | Wilson’s disease n = 1480 | p value | |||
|---|---|---|---|---|---|
| Mortality | 69 | 4.67% | 42 | 2.84% | 0.01 |
| Length of stay (mean days, SE) | 6.32 | 0.25 | 6.88 | 0.24 | 0.10 |
| Cost (mean $, SE) | 13,897 | 696 | 14,367 | 735 | 0.64 |
We reported the outcomes of hospitalization in patients with WD in the United States based on analysis of the largest national inpatient database. Our results showed an increasing annual trend of hospitalization in patients with WD from 2006 to 2011. The leading causes for hospitalizations are chronic liver disease and infection. Compared to general hospitalized patients, WD patients had favorable survival. Chronic organ system comorbidities (respiratory, renal, and liver) and advancing age are associated with increased mortality in patients with WD.
Despite being a rare disease with an estimated prevalence of 1 in 30,000 [16], our study demonstrated an increased hospital utilization for WD. The reason is likely multifactorial and may include the chronicity of disease, increased awareness of the disease, disease related complications, and side effects related to medical therapy.
The clinical manifestations and comorbidity profile of WD largely depend on the organs that are involved, most commonly liver and brain. Patients with WD often present with acute or chronic hepatitis, cirrhosis, liver failure, or neuropsychiatric disorders [16]. Our study also demonstrates significantly higher hepatic and neuropsychiatric comorbidities in hospitalized WD patients compared to general population. In addition, our study showed that liver disease is the leading cause of hospitalization and death among patients with WD.
Notably, the prevalence of drug and alcohol abuse was almost 2 times higher in WD patients compared to hospitalized non-WD patients in the present study. This finding is in line with the observation of increased substance and alcohol abuse in patients with mental disorders [17] and WD [18]. One study from Serbia showed particularly high suicidal deaths in their WD cohort [11]. Half of the subjects who committed suicide had no history of depression and were motivated by other suffering such as substance abuse and alcoholism [11]. WD related neuropsychiatric disorders may also contribute to increased hospital utilization.
Infection, particularly sepsis and pneumonia, is one of the major contributors for admissions among WD patients in the present study. Although immune system requires copper to perform several critical functions, suppressed immune response is observed with excessive copper exposure in mice [19,20] and healthy humans [21]. Some studies also found impaired bactericidal activity and copper-induced inhibitory effect on cell-mediated immunity, which was improved with D-penicillamine treatment in WD [22,23]. It is noteworthy that 2.50% of WD patients in our study are admitted for pneumonia, which echoes Czlonkowska’s finding that pneumonia is a common complication in this population [10]. Therefore, early control of infection and preventive measures such as pneumococcal vaccination in patients with WD might be beneficial in reducing hospitalizations.
There have been a few WD patient cohorts in the literature that reported the causes of death [8,10,11]. Czlonkowska’s group from Poland showed 20 out of 164 WD patients died during 11-year follow-up [10]. Almost half of the deaths were secondary to liver failure and ten of them also had pneumonia or sepsis. Similarly, large WD cohorts from Austria and Serbia also showed hepatic failure remain the leading cause of death [8,11]. Our study is in concordance with current literature. In addition, the present study shows that WD patients who presented with respiratory and renal failure also had increased mortality. Respiratory failure, as a result of severe hypokalemia related to renal tubular dysfunction has been reported in WD [24]. Copper induced renal disturbances have long been described in WD [25]. In addition, therapeutic drugs like penicillamine, and decompensated cirrhosis, may also lead to renal dysfunction [26]. Therefore, early referral and comprehensive management of organ failures in a transplant center may be beneficial.
The mortality of patients with WD ranges from 1.8% to 21.1% worldwide based on cohort studies [2,8–13]. The inpatient mortality of WD was 2.58% in the current study. Long-term survival of patients with WD in comparison to general populations has not been consistently reported. Some studies showed comparable survival of WD patients to general population [2,14], while others reported worse outcomes in WD [8,10]. Our study demonstrates the overall survival of WD is reassuring compared to their counterparts, a cohort of non-WD patients with matched comorbidities instead of healthy population. However, it’s worth pointing out that our patients with WD are slightly (2 years) younger than those without despite propensity match. The discrepancy of long-term survival in WD may include different patient population, small sample size, stage of the disease, treatment response and compliance, and advances in both medical and surgical treatments during long observation period.
Our study has several limitations which are inherent to the use of administrative data. First, the NIS database counts each single admission rather than individual patient. In the study of a chronic disease, the findings may be potentially skewed owing to the rate of re-admissions. Second, the data collection of NIS relies on accuracy of the ICD coding. Third, the NIS does not contain non-coded clinical data such as laboratory or medications. Despite these limitations, the NIS enhances statistical power by providing a large sample size to analyze outcomes of a rare disease.
In conclusion, our study demonstrates the clinical characteristics and outcomes among hospitalized patients with WD. These findings add important knowledge to our understanding of the healthcare utilization and outcomes of this rare disease in the United States.
Conflict of interestThe authors do not declare any conflict of interest.