The Choosing Wisely (CW) initiative aims to improve daily practice supported by evidence concerning unnecessary medical tests, procedures, and treatments. This philosophy is essential in managing viral hepatitis (VH), which primary care physicians increasingly carry out. It is also essential to achieving disease elimination. Thus, the aim of our study was to propose evidence-based CW recommendations in VH.
Materials and MethodsThe Brazilian Society of Hepatology (SBH) formed a panel of experts in VH who selected evidence-based CW recommendations, which were subsequently scrutinized and ranked by all members of SBH using a web-based approach.
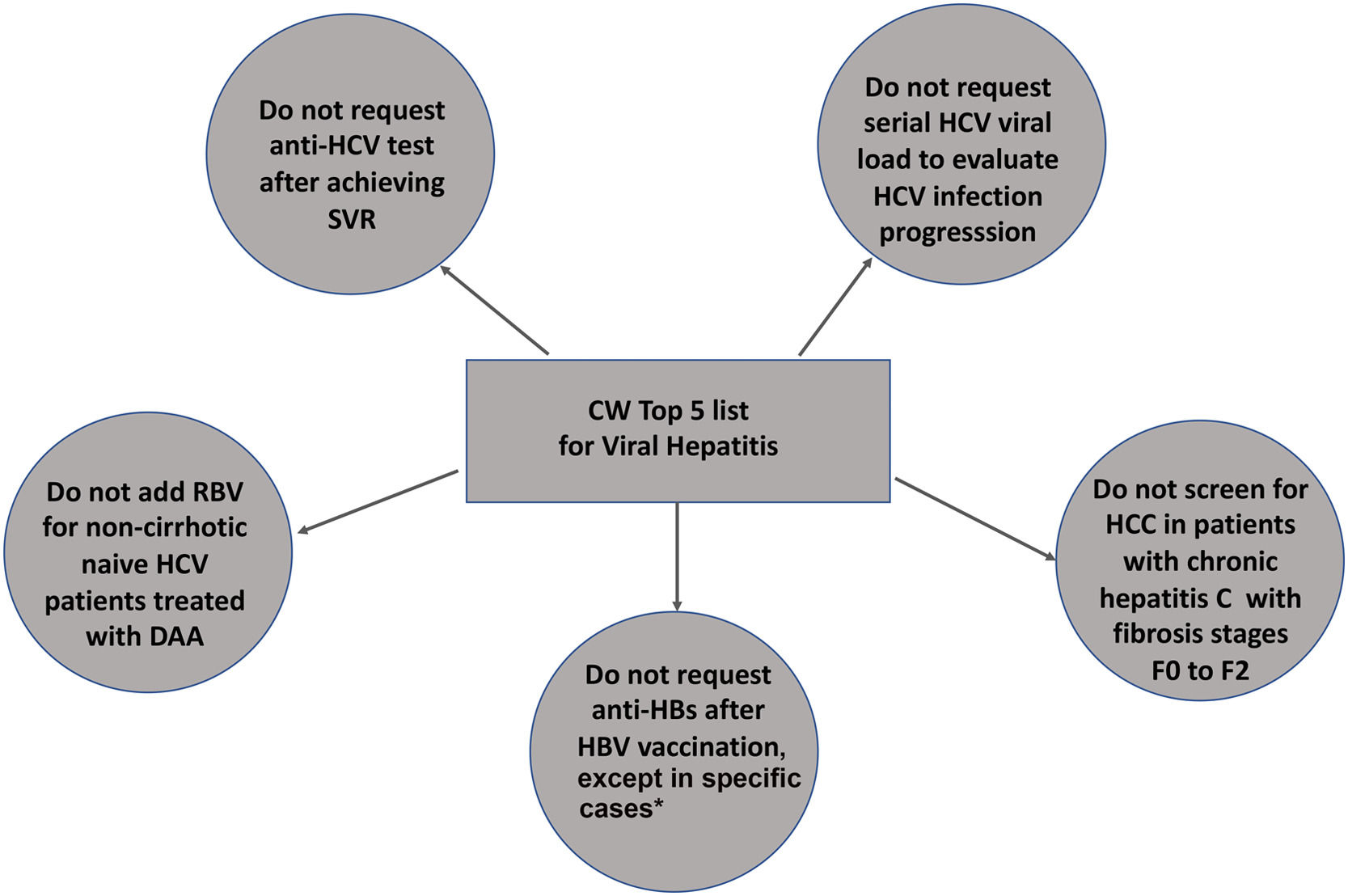
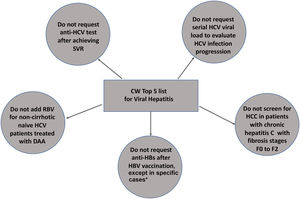
ResultsFive recommendations were chosen in order of importance: 1) do not order anti-HCV testing after achieving sustained virological response; 2) do not request serial HCV viral load to evaluate HCV progression, 3) do not add ribavirin to direct-acting antivirals in non-cirrhotic, naïve HCV patients; 4) do not screen for hepatocellular carcinoma in HCV patients with none to moderate fibrosis (≤ F2); 5) do not request anti-HBs after HBV vaccination, except for children born to HBV-infected mothers, hemodialysis patients, healthcare professionals, people who have had sexual contact with chronic HBV carriers, HIV-positive persons and immunocompromised individuals (hematopoietic stem-cell transplant recipients or persons receiving chemotherapy).
ConclusionsCW recommendations may help general practitioners adopt a more rational and cost-effective approach in managing patients with VH in Brazil and Latin America, leading to lesser waste or harm to patients.
In 2012, the American Board of Internal Medicine Foundation and Consumer Reports launched the Choosing Wisely [1] campaign [2] to counteract the increasing healthcare expenditure associated with low-value medicine [3]. Most low-value related practices were non-evidence-based prescriptions of unnecessary laboratory tests, procedures and treatments with no benefit or potential harm to patients [4]. This initiative has gained widespread support from more than seventy medical societies worldwide [5]. Based on CW philosophy, these societies reported the top five recommendations in their field of knowledge that should be avoided. Recently, CW Canada has reported their list of low-value medical practices in caring for patients with chronic liver diseases (CLD). However, only one recommendation was related to viral hepatitis [1]. Currently, around 240 and 71 million people have been infected with hepatitis B [6] and C (HCV) viruses, with an increased lifetime risk of developing end-stage liver disease (ESLD) and hepatocellular carcinoma (HCC) [7, 8].
Viral hepatitis elimination is possible shortly by implementing different strategies and a cascade of care. For HBV infection, it comprises universal HBV vaccination, HBV screening of at-risk subjects, pregnant women, and nucleot(s)ide analogs (NUCs) to provide long-term HBV suppression [9, 11, 12]. For HCV, the Center for Diseases Control (CDC) and the American Association for the Study of Liver Diseases (AASLD) guidelines recommend a one-time universal screening for all adults (above 18 years old) and ongoing screening based on risk behaviors and/or exposures. In addition, the employment of highly effective direct-acting antivirals (DAAs) for hepatitis C treatment has also been recommended [7].
It is not uncommon to face misconduct regarding the prevention and diagnosis of both HBV and HCV hepatitis. CW recommendations consist of declarative statements that aim to identify practices that should be reduced or eliminated because they lack proven benefit or may cause harm to patients. The Brazilian Ministry of Health has endorsed World Health Organization (WHO) goals to achieve disease elimination by 2030 [8, 10]. It has recently issued a national plan to implement nurse-led screening of viral hepatitis in primary care and HCV and HBV treatment conducted outside viral hepatitis clinics by primary care physicians [13]. CW recommendations related to viral hepatitis may be valuable in this scenario. Thus, to assist in managing HBV and HCV infection without wasting resources, the Brazilian Society of Hepatology (SBH) sought to create a Top 5 list of low-value healthcare practices with the support of CW Brazil in the field of viral hepatitis. This Top-5 list is summarized in the present manuscript.
2MethodsThe Brazilian Society of Hepatology (SBH) appointed one leading expert in viral hepatitis to engage with CW Brazil to be acquainted with a suggested methodology for establishing those top five recommendations on managing viral hepatitis. The governing board also chose a committee of seven additional experts from the SBH focused on interest groups of HBV and HCV infections. They developed and discussed a draft of eight recommendations to be voted on and graded by all society members through a web-based platform using Survey Monkey. Based on the results, the same group of experts issued the Top 5 List of unnecessary tests, procedures and treatments to be avoided by primary healthcare personnel concerning viral hepatitis B and C. Those recommendations were further submitted to the governing board of ALEH for review and endorsement.
2.1Ethical statementThis study was supported by the Brazilian Society of Hepatology and the Latin American Association for the Study of the Liver. There are no ethical concerns related to the study. It does not contain any intervention involving human participants performed by any of the authors.
3Results3.1The initial set of recommendations suggested by the SBH committee- 1)
Do not request anti-HCV testing after achieving sustained virological response (SVR).
- 2)
Do not request serial HCV-viral loads to evaluate HCV progression.
- 3)
Do not add ribavirin (RBV) for non-cirrhotic, naïve HCV patients treated with DAAs.
- 4)
Do not screen HCV patients with no or mild and moderate fibrosis (F ≤ 2) for HCC with ultrasonography (US) and/or alpha-fetoprotein (AFP).
- 5)
Do not request anti-HBs after HBV vaccination, except for children born to HBV-infected mothers, patients on renal replacement therapy, healthcare personnel and sexual contacts of chronic HBV carriers, HIV-positive subjects and those who are immunosuppressed.
- 6)
For suspected acute hepatitis B, do not request a complete HBV serology panel. Otherwise, order only HBsAg and anti-HBc IgM.
- 7)
Do not request complete serology to screen for HBV infection, as HBsAg and anti-HBc IgG may adequately identify carriers or previous HBV contacts.
- 8)
Think carefully before treating chronic hepatitis B based on a single ALT level and a single viral load result, except for cirrhotic patients.
Anti-HCV is lifelong positive after SVR or spontaneous HCV elimination and in individuals with chronic HCV infection. This way, it should not be requested to confirm viral clearance of HCV reinfection. If suspecting reinfection or evaluating SVR, the correct test to perform is the HCV-RNA. It is not uncommon that patients with SVR are submitted to unnecessary anti-HCV tests many times after HCV cure, leading to unnecessary costs like asking for a new HCV-RNA and anxiety for the patients. It is, therefore, essential to highlight this recommendation to patients and healthcare providers [18–20].
Top 5 list of low-value medical practices for viral hepatitis and its rationale according to CW philosophy
| Number | Recommendation | Rational |
|---|---|---|
| 1 [18–20] | Do not request anti-HCV testing after achieving SVR. | Anti-HCV remains positive lifelong after SVR or spontaneous HCV elimination. Anti-HCV should not be requested to confirm viral clearance of HCV reinfection. |
| 2 [18–20] | Do not request serial HCV viral loads to evaluate HCV progression. | HCV-RNA is an invaluable tool for the diagnosis of HCV viremia and usually indicates HCV treatment. Reassessment of HCV-RNA after treatment is suitable only for assessing SVR 12 to 24 weeks after treatment or for reinfection assessment. |
| 3 [14] | Do not add RBV for non-cirrhotic, naïve HCV patients treated with DAAs. | The addition of RBV has no impact on HCV SVR in treatment-naïve patients without cirrhosis regardless of the DAA regimen chosen [18–20]. |
| 4 [19] | Do not screen HCC in patients with chronic hepatitis C with fibrosis stages F0 to F2. | HCC remains a threat for HCV patients with cirrhosis or advanced fibrosis (F3) even after SVR. So far, only this group of individuals should undergo HCC screening before or after SVR due to the negligible risk for HCC in F0-F2 patients. |
| 5 [12] | Do not request anti-HBs after HBV vaccination, except for children born to HBV-infected mothers, patients on renal replacement therapy, healthcare personnel and sexual contacts of chronic HBV carriers, HIV-positive subjects and individuals at risk of HBV reactivation, like those under immunosuppressive therapy. | Hepatitis B vaccination response with the complete 3-dose series results in persistent seroprotection (anti-HBs ≥10 mIU/mL) in healthy adults under 40 years and children. Serological testing after vaccination is recommended only for people whose clinical management depends on knowledge of their immune status. |
SVR, sustained virological response; HCV-RNA, RNA for hepatitis C virus; HCV, hepatitis C virus; DAAs, Direct-acting antivirals; RBV, ribavirin; HCC, hepatocellular carcinoma; the US, ultrasonography; HBV, hepatitis B virus.
Top 5 list for viral hepatitis in Brazil and Latin America regarding Choosing Wisely criteria.
*Exceptions: children born to HBV-infected mothers, patients on renal replacement therapy, healthcare personnel in contact with chronic HBV carriers, HIV-positive subjects and individuals at risk of HBV reactivation such as those under immunosuppressive therapy.
CW, Choosing Wisely; SVR, sustained virological response; anti-HCV, antibody against hepatitis C virus; RBV, ribavirin; DAAs, direct-acting antivirals; HCV, hepatitis C virus; HBV, hepatitis B virus; anti-HBs, antibody against hepatitis B virus surface antigen; HCC, hepatocellular carcinoma; F, fibrosis.
HCV-RNA is invaluable for diagnosing hepatitis C viremia. When detected, it indicates the need for HCV treatment. It is crucial to reinforce that HCV viral load neither impacts disease progression nor correlates with fibrosis development or progression in chronic HCV-infected immunocompetent patients [18–20]. With the current pan-genotypic and highly efficacious DAAs, it is also not required to tailor therapy with viral load to preview the chance of SVR [18–20]. Hence, reassessment of HCV-RNA after treatment is only suitable for assessing SVR 12 to 24 weeks after treatment to define a virological cure or when reinfection is suspected [18–20].
3.2.3Recommendation 3: do not add RBV for non-cirrhotic, naïve HCV patients treated with DAAsThe addition of RBV has no impact on HCV SVR in treatment-naïve patients without cirrhosis, regardless of the chosen DAA regimen. Likewise, regardless of genotype, those patients' response rates were very similar with or without ribavirin [14]. Therefore, ribavirin is not indicated for treating mild to moderate fibrosis patients with DAAs [18–20]. Due to the high prevalence of adverse events that sometimes impact the maintenance of the treatment, the option of extension of treatment from 12 to 24 weeks in decompensated cirrhotics could be considered at the discretion of the prescriber [18–20].
3.2.4Recommendation 4: do not screen HCC in patients with chronic hepatitis C with fibrosis stages F0 to F2HCC remains a threat for HCV patients with advanced fibrosis and cirrhosis even after SVR. Whether all of those subjects should be submitted to HCC screening lifelong with the US with or without AFP measurements is currently under debate. However, there is no need to perform HCC screening in patients with milder degrees of fibrosis before or after SVR due to their negligible risk for HCC development.
3.2.5Recommendation 5: do not request anti-HBs after HBV vaccination, except for children born to HBV-infected mothers, patients on renal replacement therapy, healthcare personnel and sexual contacts of chronic HBV carriers, HIV-positive subjects and individuals at risk of HBV reactivation, like those under immunosuppressive therapyHepatitis B vaccination with the complete 3-dose series results in seroprotection (anti-HBs ≥10 mIU/mL) of greater than 90% in healthy adults younger than 40. The immune response declines with age, 75% in adults aged > 60 years. However, the vaccine response is persistent in healthy adults and children. Serological testing after vaccination is recommended only for people whose clinical management depends on knowledge of their immune status.
4DiscussionThis study describes the Top 5 List of unnecessary laboratory tests, including AFP, anti-HCV, HCV viral load, serology markers of HBV infection or vaccination; procedures such as US and treatments such as RBV to avoid in specific common clinical scenarios in the management of viral hepatitis using the CW methodology endorsed by SBH and ALEH. To our knowledge, this is the first set of recommendations devoted solely to hepatitis B and C based on CW methodology. Brahmania et al. [1] have previously reported on the Canadian Top 5 List of low-value medical interventions in general Hepatology. Only one recommendation dealt with viral hepatitis concerning serial HCV viral load measurements in subjects with chronic hepatitis C. Interestingly, it was also included as one of those Top 5 recommendations chosen to be avoided in Brazil and Latin America. All of them would be very useful in accomplishing WHO goals for viral hepatitis elimination in low to middle-income Latin American countries where resource allocation to healthcare is even lower compared to high-income nations [8]. In order to reduce waste in the viral hepatitis continuum of care, it is crucial to publicize those recommendations. They would be suitable not only for hepatologists and infectious disease physicians but also for general practitioners in primary care to increase their awareness of the futility and potential harm of unnecessary healthcare services. It is worth mentioning that patient-driven anti-HCV testing after HCV SVR is common in Brazil during screening campaigns. Approximately 10% of anti-HCV-positive subjects are HCV-RNA negative subjects previously treated with SVR (not published). Hence, it is important to reinforce that the request for the anti-HCV test for individuals who have cured a previous HCV infection will be futile independent of any new HCV-screening policy. In current practice, post-vaccination anti-HBs testing and serial HCV viral load assessment in non-responders to interferon-based therapies were also patient-driven due to low awareness.
On the other hand, RBV add-on therapy to DAA regimens was commonly prescribed for non-cirrhotic treatment naïve HCV patients in Brazil until 2019, when the Brazilian Ministry of Health stated this was an unnecessary practice [14]. Hepatitis B is considered a low prevalence disease in Brazil, despite its high prevalence in remote parts of the Amazon basin and some inner counties in Southeastern and Southern Brazil [15]. In those parts of Brazil, management of HBV infection will be conducted in primary care. Therefore, all those recommendations regarding low-value care related to HBV infection, including post-vaccination anti-HBs testing and the appropriate use of the diagnostic panel for hepatitis B serology in different clinical settings, will be necessary for their decision-making. It is also important to note that low-care medical practices impact direct and indirect medical costs funding by the Brazilian Health System (SUS) and direct non-medical costs related to transportation and lost labor days [16]. They may also be challenging for underprivileged patients, particularly from low-income areas of Latin America.
It is worth mentioning that CW philosophy must always be based on evidence. Still, nevertheless, it might also lead to underuse and misconduct, avoiding specific steps in care essential to achieving and granting good clinical practice. Thus, each recommendation must be clear, preventing other doubts from those who will use them in daily clinics.
This study has some limitations. Brazilian experts elaborated all recommendations for subsequent endorsement by SBH members and the ALEH direction board. Therefore, it may be more adjusted to circumstances related to managing viral hepatitis in the context of SUS rather than in all other countries in Latin America, each with its proper strategies for viral hepatitis elimination. In Brazil, the continuum of care for viral hepatitis will be centered on primary care, which may not be the case in other Latin American Countries. It is also essential to emphasize that viral hepatitis guidelines are dynamic and may change periodically according to current scientific evidence, knowledge level, and healthcare resources. It is, therefore, possible that some of these recommendations need to be updated shortly.
5ConclusionsFor the first time, this study disclosed the Top 5 recommendations regarding managing viral hepatitis according to Choosing Wisely methodology. These recommendations may demonstrate how sometimes, overusing resources makes it expensive, confusing, slows down and leads to wrong decisions. Sometimes less is more (or better). The CW's guidance is based on this premise.[17] They may help achieve lower viral hepatitis daily care costs and elimination worldwide, particularly in Brazil and Latin America. Additionally, they may aid not only hepatologists and infectious disease physicians but particularly general practitioners in primary care to adopt a more rational and cost-effective approach to handling their patients with viral hepatitis.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsAll authors contributed to the study design, data interpretation, and intellectual content. The draft of the manuscript was written by CAVN and PLB. All authors read and approved the final manuscript.
Declaration of interestNone
The authors acknowledge the contribution of Choosing Wisely Brazil and the support of the Brazilian Society of Hepatology (SBH) and the Latin American Association for the Study of the Liver (ALEH).