Obesity is rapidly increasing and has reached epidemic features worldwide. Obesity is linked to insulin resistance, systemic low-grade inflammation and common pathogenic pathways with a number of comorbidities (including cancer), leading to high mortality rates. Besides change of lifestyles (diet and physical exercise) and pharmacological therapy, bariatric surgery is able to rapidly improve several metabolic and morphologic features associated with excessive fat storage, and currently represents an in vivo model to study the pathogenic mechanisms underlying obesity and obesity-related complications. Studies on obese subjects undergoing bariatric surgery find that the effects of surgery are not simply secondary to gastric mechanical restriction and malabsorption which induce body weight loss. In fact, some surgical procedures positively modify key pathways involving the intestine, bile acids, receptor signaling, gut microbiota, hormones and thermogenesis, leading to systemic metabolic changes. Furthermore, bariatric surgery represents a suitable model to evaluate the gene-environment interaction and some epigenetic mechanisms linking obesity and insulin resistance to metabolic diseases.
Obesity is defined as a chronic state of pathologically increased mass of white adipose cells,1 which represents a global epidemic of the 21st Century.2,3 The prevalence of obesity has markedly increased since the year 20004 and a progressive shift toward obesity has been found at any age since 1980, with a greater surge of obesity prevalence in low- and middle-income developing countries rather than in high-income countries.5,6 Body mass index (BMI) is a clinical surrogate marker of obesity based on weight and height and it is applied to adult men and women, calculated as kg/m2. Adults with a BMI between 25 and 29.9 kg/m2 are defined as overweight, while those with a BMI ≥ 30 kg/m2 are obese.7,8 In the USA, over 60% of adults are overweight and 30% are obese, while 4.7% are extremely obese, i.e. with a BMI ≥ 40 kg/m2.9,10
The pathological expansion of the adipose mass is associated with insulin resistance and a chronic systemic low-grade inflammation, two conditions that pave the way to a number of comorbidities (Table 1). Obesity is associated with the risk of type 2 diabetes mellitus (T2DM) (obesity represents 90-95% of patients with diabetes11), hypertension, hyperlipidemia, pancreatic diseases, nonalcoholic fatty liver disease (NAFLD), and several specific can-cers.12 A high mortality rate is reported not only in patients with morbid obesity, but also in overweight subjects.13 Obesity is also associated with increased risk of cardiovascular disease and contributes to all-cause mortal-ity.11,14 Moreover, T2DM and central obesity are both present in the metabolic syndrome.15-17 The proportion of American adults with obesity reached 50% in 2013,18 and current healthcare costs for obesity-associated conditions were USD 147 billion in the USA.19 The economic costs associated with the obese populations will soon become unbearable.20 Thus, there is an urgent need for a deeper understanding of the fine pathogenic mechanisms (including the gene-environment interactions) linking obesity to associated conditions.
Comorbidities associated with obesity.
|
About 20-30% of the adult obese population might be considered relatively healthy despite their increased weight. These subjects have no metabolic defects and are defined as metabolically healthy obese (MHO). By contrast, there are normal weight or slightly overweight subjects who show the same metabolic abnormalities as obese subjects do, which might be defined as metabolically obese normal weight (MONW). However, they often show an increased waist circumference and an increased fat mass.21 Indeed, recent studies indicate that BMI is not accurate enough in defining metabolically obese subjects and can cause many patients to miss diagnosis. In a group of Spanish adults it has been demonstrated that more than a quarter (29%) of the subjects were considered lean and over two-thirds (80%) were considered overweight on the basis of their BMI that shows a percentage of body fat within the obesity range (cut-off values for obesity were 29 kg/m2 in males and 30 kg/m2 in females).21
Current obesity therapy involves lifestyles (i.e., balanced diet and physical activity), pharmacological interventions, and surgery.22 Bariatric (metabolic) surgery reduces body weight, improves several comorbidities, and is associated with short- and long-term weight-independent beneficial effect which suggest that distinct hormonal and non-hormonal protective factors are involved. Bile acids (BAs) have recently been identified as systemic metabolic regulators and likely trigger several mechanisms leading to improved glucose homeostasis, insulin sensitivity, and lipid metabo-lism.23-28 Indeed, BA pool is altered in obese patients and in T2DM patients, while plasma BA levels change after bariat-ric surgery.26,29,30 BA changes are associated with positive effects on hormone release,26,30,31 post-receptor signaling pathways,32 and gut microbiota.33-37
In this chapter, we summarize the effects of BAs acting as metabolic signaling molecules and thermogenic agents following bariatric surgery. Furthermore, the current physiological interpretations of potential therapeutic value are discussed.
Obesity and InflammationThe main cellular component in obesity, i.e. adi-pocytes, is no longer considered as pure energy-storing cells. This view is supported by studies that investigated the mechanisms regulating the differentiation process of adipocytes and their active involvement in systemic events.38 Thus, the effect of treating obesity and its influence on metabolic and inflammatory biomarkers have gained increasing interest.12,38 The development of excess white fatty tissues in specific regions of the body is meta-bolically more relevant factor for obesity than the increased BMI per se. Excess fat in the upper part of the body (i.e., central obesity) is indeed associated with increased risk of metabolic and cardiovascular diseases.11 Waist circumference becomes the anthropometric marker of central obesity, as a reliable indicator of visceral fat content.39
Obesity has been considered as the consequence of an impaired balance between energy intake and energy expenditure, but current views are far more complicated. The concept of impaired balance only partly explains the mechanisms responsible for fat accumulation. Growing evidence suggests that early exposition to environmental factors (i.e., foetal malnutrition during pregnancy,40,41 endocrine disrupting compounds,42-51 and prenatal exposure to air pollution52-54) contributes also to visceral fat ac-cumulation,55 obesity,52-54,56 and insulin resistance.57-59 Mechanisms are complex and involve gene-environment interactions40,60,61 and epigenetic mechanisms such as DNA methylation,62 histone acetylation/deacetylation and non-coding mRNA.63,64 The early and persistent environmental exposure, in association with lifestyle factors and an “obesogenic” environment,18,64 activates pathogenic pathways involving inflammation and immune system dysfunction,65,66 thereby leading to chronic low-grade inflammation and insulin resistance.67
The adipocyte is the main cell type of adipose tissue, which is devoted to store energy (e.g. triglycerides), but is also active in synthesizing and secreting specific molecules (i.e., adipokines) with endocrine, paracrine and auto-crine functions. The same set of molecules display either pro- and anti-inflammatory properties, e.g., cytokines.68,69 Central obesity is therefore deemed as a chronic low-grade inflammatory state responsible for a deregulated release of adipokines and cytokines into the circulation.68,69 Large adipocytes, moreover, are robustly represented in the fat mass of severe obese subjects and are surrounded by macrophages to form crown-like structures.70-73 Here, a set of chemokines become major players of chronic inflammation by inducing chemotaxis in nearby responsive cells.68,69 Chemotaxis is the mechanism by which the movement of somatic cells and single cells or even multi-cellular organisms is guided by chemicals in their environment. Chemokines are pro-inflammatory factors and recruit cells of the immune system when infection and inflammation occur.
Inflammation is the common feature of central obesity and metabolically obese normal weight subjects and is a major trigger for T2DM. By contrast, subjects with gluteo femoral obesity do not show a peculiar inflammatory profile and have indeed a low risk of developing T2DM.
Bariatric Surgery and Metabolic EffectsSeveral therapeutic strategies have been proposed for treating obesity. Intensive lifestyles interventions achieve only 5-6% body weight loss,74 which is not able to be maintained for a long time,75 despite decreased cardiovascular risk.76 Many studies have shown that bariatric surgery is a very effective approach in reducing body weight.77-82 So far, bariatric surgery remains the most successful option in patients with a BMI ≥ 40 kg/m2 and with or without T2DM, as well as in patients with T2DM and a BMI ≥ 35 kg/m2 and even in patients with difficultly controlled T2DM and a BMI < 35 kg/m2. The term “bari-atric” likely originates from the Hebrew word “bari” which means fat. Also in the Genesis, fat cows were indeed called “bari” cows.83 Other sources indicate that “bar-iatric” comes from two words of the modern Greek baros and iatrikos, meaning “load” and “medicine”, respectively.83
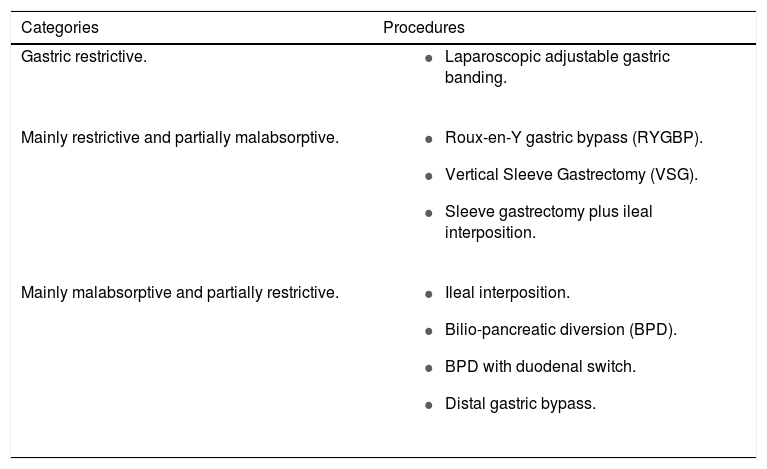
The National Institute of Health (NIH) issued the initial indications for bariatric surgery in 1991, and more recently, the European Association for Endoscopic Surgery (EAES), and the American Society for Metabolic and Bariat-ric Surgery (ASBS) added novel indications and recommended bariatric surgery also for subjects displaying mild obesity (Class 1) associated with severe comorbidities including T2DM.84,85 Category of patients and procedures should be chosen depending on local availability, selection criteria, and surgeon’s experience (Table 2). The mechanisms by which bariatric surgery induces body weight loss are of two types: the reduction of nutrient ingestion by gastric restriction (i.e., restrictive bariatric surgery) or the reduction of nutrient absorption (by reducing the length of intestinal mucosa in contact with nutrients or the cross of the gastrointestinal tract, which is surgically resected or excluded) i.e., malabsorptive body weight loss surgery. Some procedures are purely gastric restrictive (i.e. laparoscopic adjustable gastric banding), other procedures are mainly restrictive and partially malabsorptive (i.e., Roux-en-Y gastric bypass, RYGBP, and vertical sleeve gastrectomy, VSG), and others are mainly malabsorptive and partially restrictive (i.e., Bilio-pancreatic diversion, BPD).
Procedures of bariatric surgery.
| Categories | Procedures |
|---|---|
| Gastric restrictive. |
|
| Mainly restrictive and partially malabsorptive. |
|
| Mainly malabsorptive and partially restrictive. |
|
Bariatric surgery was originally designed for morbid obese subjects. However, the indications to bariatric surgery have changed substantially in the last 10 years, and in the last five years, it has been claimed as an option not only for morbid obesity, but also as ultimate treatment of T2DM in patients with less severe degree of obesity.31,77-82,86 Long-term studies, however, have shown reappearance of diabetes in spite of bariatric surgery.87
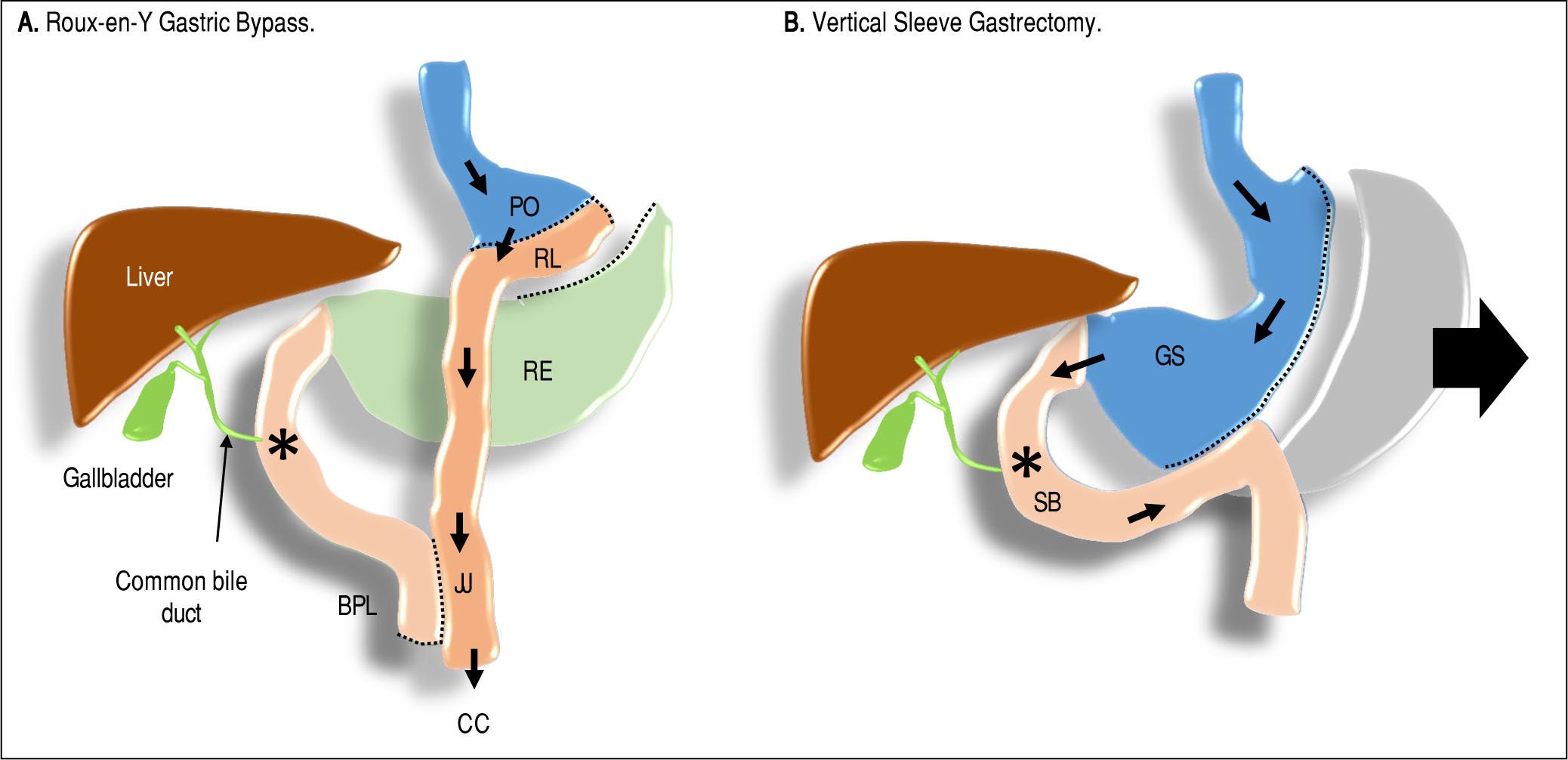
The main indications and contraindications to bariatric surgical procedures are listed in table 3, while a commented comparison of different guidelines is shown in table 4. Surgeons proposed to introduce new classes of obesity (super obese and super morbid obese subjects, Class 4 and 5, respectively) for which malabsorptive surgery is mandatory. The technical features of RYGBP and VSG are illustrated in Figure 1. RYGBP reduces food intake by creating a small stomach pouch: an Y-shaped section of the ileum is connected directly to this small pouch, and food will proceed straight to the ileum without contact with both duodenum and jejunum. Supplementation of calcium and iron is essential following RYGBP because duodenum and jejunum are involved in the absorption of both components. Moreover, RYGBP interferes with the secretion of gut hormones, namely ghrelin, glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and gastric inhibitory polypeptide (GIP), which act as signals for the brain centers and contribute to the control of satiety and hunger. Therefore, the anatomical modifications occurring after RYGBP exclude some intestinal segments from contact with nutrients and modify the eating behavior. Saeidi, et al.88 examined the RYGBP-induced metabolic profile in rats with diet-induced obesity, and with streptozotocin-induced diabetes, as well as in Goto-Kakizaki rats, e.g., non-obese Wistar rats that spontaneously develop T2DM in the early stage of life. The Y-shaped loop is responsible for an intense glucose absorption and utilization, as confirmed by 18-fluoro-2-deoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT). The RYGBP-induced remodeling of duodenum and jejunum greatly influences insulin resistance and glucose tolerance to meet increased bioenergetic demands. Whereas in BPD, the bypass of the duodenum, the entire jejunum and the first portion of the ileum account for the normalization of peripheral insulin sensitivity, and in RYGBP the bypass of a shorter intestinal tract mainly affects the hepatic insulin sensitivity.89
Indications, novel indications and contraindications to bariatric surgery.
| • Indications BMI ≥ 40 kg/m2. BMI 35-39.9 kg/m2 with comorbidities. Insufficient weight loss with medical therapy. |
| • Novel indication (EAES/ASBS) BMI 30-35 kg/m2 with comorbidities. |
| • Contraindications Low compliance to medical therapy. Psychiatric diseases: personality disorders, major depression. |
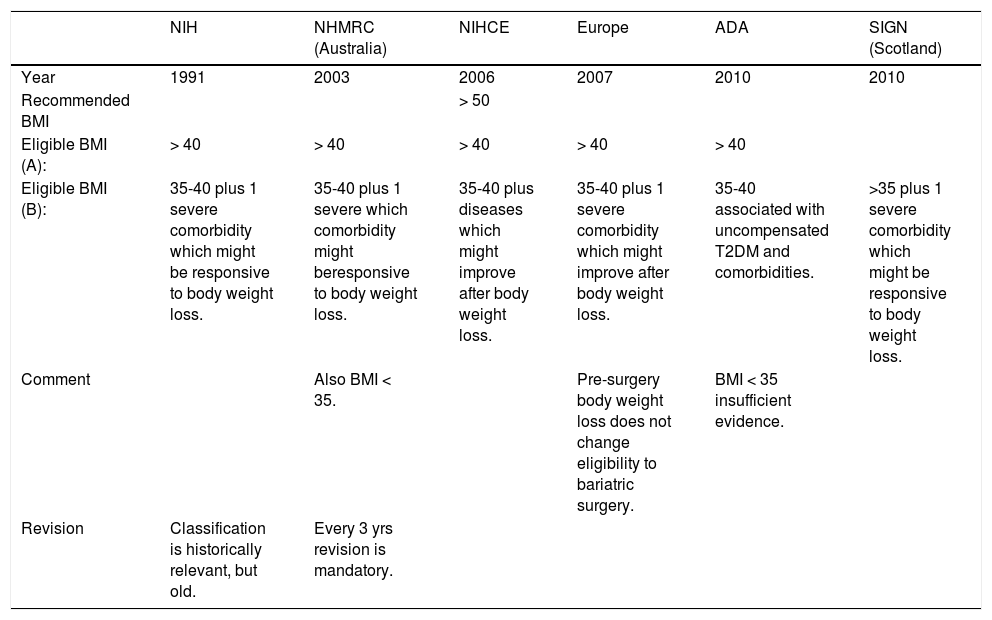
Indications to bariatric surgery according to different Scientific Societies and guidelines.
| NIH | NHMRC (Australia) | NIHCE | Europe | ADA | SIGN (Scotland) | |
|---|---|---|---|---|---|---|
| Year | 1991 | 2003 | 2006 | 2007 | 2010 | 2010 |
| Recommended BMI | > 50 | |||||
| Eligible BMI (A): | > 40 | > 40 | > 40 | > 40 | > 40 | |
| Eligible BMI (B): | 35-40 plus 1 severe comorbidity which might be responsive to body weight loss. | 35-40 plus 1 severe which comorbidity might beresponsive to body weight loss. | 35-40 plus diseases which might improve after body weight loss. | 35-40 plus 1 severe comorbidity which might improve after body weight loss. | 35-40 associated with uncompensated T2DM and comorbidities. | >35 plus 1 severe comorbidity which might be responsive to body weight loss. |
| Comment | Also BMI < 35. | Pre-surgery body weight loss does not change eligibility to bariatric surgery. | BMI < 35 insufficient evidence. | |||
| Revision | Classification is historically relevant, but old. | Every 3 yrs revision is mandatory. |
In the Roux-en-Y Gastric Bypass, the proximal stomach is divided to assemble a stomach pouch (PO) of about 30mL through which nutrient flow. The gastric remnant (RE) is not in contact with enteral nutrients any more, it is sealed proximally and in continuity with the duodenum distally. A segment of jejunum is connected to the stomach pouch forming to form the Roux limb (RL) of variable length (due to factors depending on surgeon and/or patient). The intestinal continuity must be guaranteed by connecting the biliopancreatic limb (BPL) to the Roux limb distally creating a jejunojejunostomy (JJ) which continues distally through the remaining bowel, i.e., the common channel (CC). In the Vertical Sleeve Gastrectomy, about 70% of the stoma ch is removed (arrow); a tube-like gastric sleeve (GS) is left, which is in continuity with an intact small bowel (SB). The result is a significantly decreased stomach volume. Symbols: arrows (→) indicate the nutrient flow. Asterisk (*) indicates the point of contact between bile, BAs, and nutrients.
VSG consists of the removal of about two-thirds of the stomach and the creation of a tube-like gastric sleeve in continuity with an intact small bowel. VSG improves glucose tolerance, and GLP-1 is considered the first suitable candidate. However, experiments examining the effects of VSG in GLP-1 receptor wild-type vs. knockout mice, show a similar GLP-1 response to a mixed meal, suggesting that GLP-1 secretion is not impaired in mice with disrupted GLP-1 receptor signaling. Also, both mouse strains show comparable blood glucose levels that are much lower than those in sham-operated mice. These finding suggest that diabetes remission, after VSG, is not mediated by GLP-1.90 Chambers, et al.91 showed that VSG improves glucose tolerance in ghrelin knockout mice exposed to a high-fat diet for 10 weeks before surgery. Thus, glycemic control is achieved after surgery even in the absence of ghrelin.
Novel and more conservative endoluminal approaches in bariatric surgery have been developed in the last decade. The endoscopic duodenal-jejunal bypass sleeve reproduces the effects of the RYGBP and has been successfully used for treating porcine model of obesity.92 Technically, a tubular device is implanted endoscopically from the proximal duodenum to the jejunum, and this setting avoids the nutrients contact with the mucosa. This device does not produce histological changes as demonstrated in animals. Unfortunately, the duodenal-jejunal bypass surgery without VSG does not normalize glucose tolerance. β-cell function in T2DM remain slower (70%) compared to that in subjects with normal glucose tolerance93 and undergoing duodenal-jejunal bypass surgery, despite normal HbA1c levels.
Current views strongly suggest that the beneficial effects of bariatric surgery are related to the combination of short-term effects of caloric restriction,94 and long-term effect on body weight loss,95 with a number of hormones acting metabolically on distinctive pathways.14 There are two mechanisms that may provide the physiological interpretation of the beneficial effects of bariatric surgery:
- •
According to the “foregut hypothesis”, some unspecified factors acting on the diabetic profile may not be released when nutrients are not in contact anymore with the duodenum;96,97 and
- •
The “hindgut hypothesis”, by contrast, proposes that the rapid transit of nutrients towards the distal intestine, may increase the L-enterocyte mediated secretion of GLP-1 with anti-diabetic effects even for a long time.98,99
Notably, the GLP-1 increase might be mediated by the increased enterohepatic circulation and serum levels of total BAs, which now appear as important mediators of some beneficial effects of bariatric surgery.100
The amount of pancreatic fat is significantly higher in T2DM and in obese subjects than in control subjects, and the reduction following bariatric surgery is associated with improved insulin resistance indices and β-cell func-tion.93 Furthermore, bariatric surgery reverses the increment of the natural killer lymphocytes seen in obese patients with insulin resistance. This finding indicates that bariatric surgery has a positive effect on the chronic inflammatory metabolic status.101
To understand the effect of bariatric surgery on body weight loss, it should be considered that bile and pancreatic fluids released into the duodenum break down carbohydrate, fat and proteins. As a consequence, bilio-pancreatic diversion (BPD), one of the most successful procedures in decreasing body weight, acts by diverting bile and pancreatic fluids away from ingested food.77 In BPD, malabsorption occurs because the stomach is reduced to a small pouch because almost 70% of the stomach is removed and is reconnected directly to the last part of the ileum. In such a way, bile and pancreatic enzymes keep in contact with nutrients shortly only before entering the colon where nutrient absorption is not possible. Therefore, when bile is diverted away, undigested nutrients reach the ileum, leading to malabsorption, production of gas, and flatus.
In humans, VSG has been associated with ileal interposition in order to reduce body weight and improve T2DM and lipid abnormalities. In this regard, ileal interposition would stimulate early secretion of incretins, while gastric resection would reduce ghrelin levels and accelerate gastric emptying.29,102-107 Ghrelin is an orexigenic hormone (appetite stimulant). Its serum levels are elevated before meals and decrease rapidly after food ingestion. In patients undergoing gastric bypass, the circulating ghrelin levels do not rise before meals. Ghrelin has opposite effects compared to GLP-1 since it stimulates the secretion of counter regulatory hyperglycemic hormones (e.g., glucagon, catecho-lamines, cortisol, and growth hormone). Nonetheless, it suppresses the secretion of the insulin-sensitizing hormone adiponectin by the adipocytes, and consequently, blocks insulin signaling in the liver. VSG slightly modify intestinal anatomy, but by removing the fundus, eliminates the ghre-lin-induced hyperglycaemia, if any.
The ileum is the small intestinal tract in which digestive enzymes, especially lipases, keep in contact with ingested food. The ileum is critical in nutrient absorption since fat and fat-soluble vitamins are absorbed here with the help of BAs. Surgical approaches enhancing the delivery of nutrients through the ileum are crucial in stimulating the secretion of incretins. To explain the improved T2DM, incretins secretion and incretin-mediated effects have been evaluated in patients after the most common bariatric surgical procedures, especially following RYG-BP.31 The incretin dependent mechanisms were firstly observed after gastric bypass, and the more extensive is the intestinal bypass the greater are the nutritional and metabolic effects.108
Changes of gut microbiota occurring after bariatric surgery should be also taken into account when interpreting the beneficial metabolic outcomes of the surgery.34 In animals, RYGBP modifies the gut microbiota profile of the alimentary limb and the common channel similarly to those induced by prebiotic treatment or body weight loss by dieting.35 An extensive review on the effects of surgically induced body weight loss on the human gut microbiota suggested the presence of an overabundance of Proteobacte-ria in the distal gut microbiota after RYGBP, which is distinct from changes seen in body weight loss without the surgery.37 Modifications of the gut microbiota can ultimately affect both BA metabolism and likely metabolic pathways.
Changes in Ba Metabolism After Bariatric SurgeryCurrent research has attracted the attention to the additional effects of BAs beyond their physiological digestive function in bile.109 Such additional effects depict BAs as metabolic mediators of glucose homeostasis and insulin sensitivity, which can also explain some effects of bariatric surgery independently from simple body weight loss. Bariatric surgery becomes therefore a pathophysiological-ly relevant model to re-evaluate the function of BAs in health, disease, and following weight-reducing surgical procedures in obesity. Essential aspects related to BA physiology are described in the current issue of Ann Hepa-tology by Di Ciaula, et al.
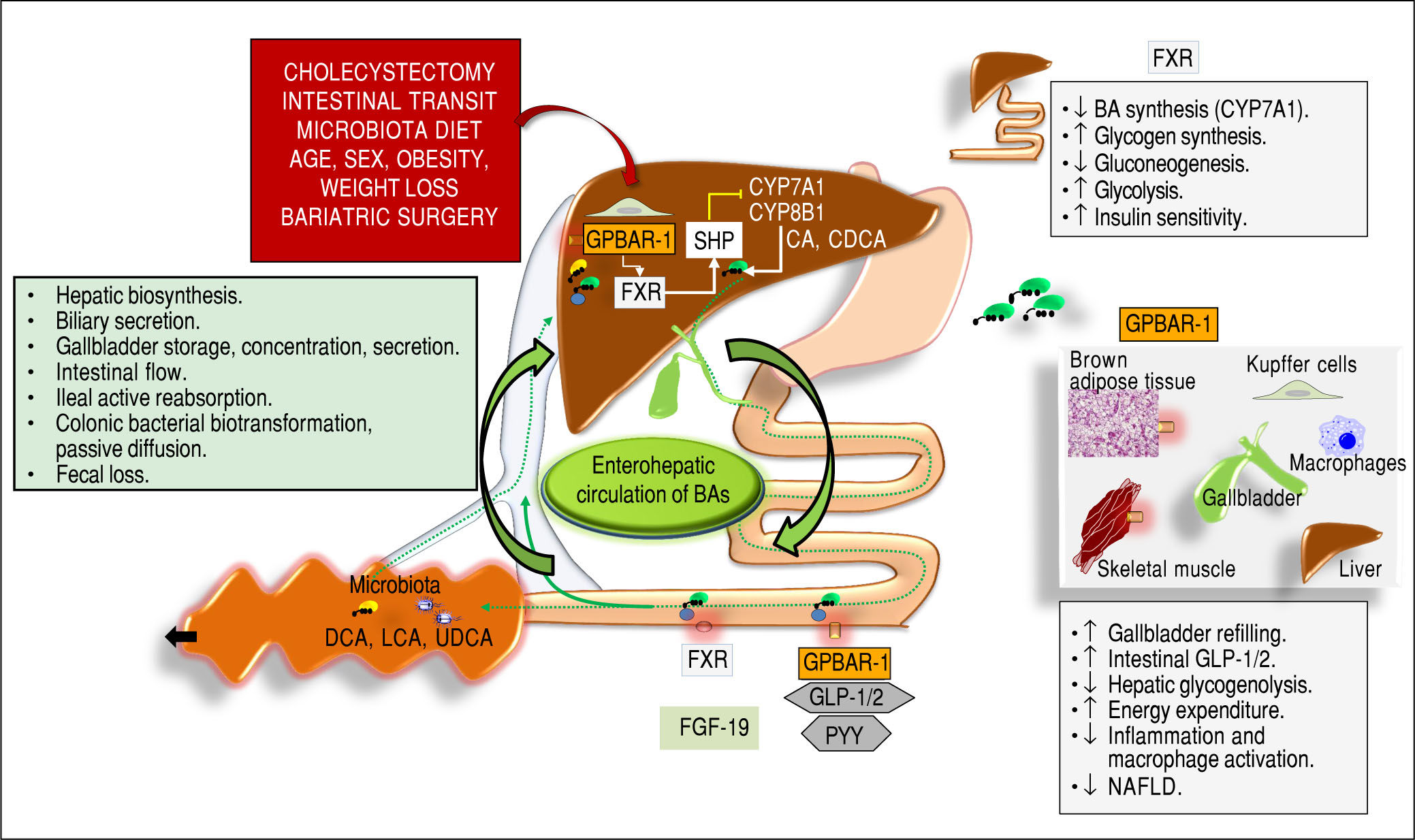
Factors affecting the BA compositionSeveral metabolic conditions may have a pathophysio-logical impact on the pool size and enterohepatic circulation of BA. Such conditions act as potential confounding factors for altering the overall BA profile after bariatric surgery when major metabolic changes are driven by anatomical and functional manipulations of the gastrointestinal tract. Cholecystectomy is associated to a decrease in the BA pool size110 and BA fluctuations, likely due to the missing function of the gallbladder acting as a reservoir of concentrated hepatic bile109,111-113 and effect on FXR-me-diated inhibition of BA synthesis.114 Changes of the intestinal transit time may also influence both the pool size and the enterohepatic circulation of BA.115-117 A prolonged co-lonic transit is associated with increased proportion of de-oxycholic acid.118,119 Qualitative and/or quantitative and/or topographic changes of the intestinal microbiota in the small and/or large intestine, due to bariatric surgery and the resulting “starvation” condition, might also influence BA composition.120-123 Changes of BA composition might be the consequence of dietary modifications124,125 that impact the microbioma profile.126 Obese patients often display decreased circulating concentrations of BAs compared to lean subject,100,127 as well as decreased expression of hepatic bile salt export bump (BSEP) and the sodi-um-taurocholate co-transporting polypeptide.128 It could be speculated that the defective gallbladder motility that is often observed in obese patients129-135 might assimilate this condition to that of an “endogenous” cholecystectomy. By contrast, insulin resistance induces an increase in the synthesis, composition and recirculation of BAs.128,136,137 Body weight loss decreases plasma BA concentrations14 and also improves insulin resistance138 status, which is able, in turn, to influence BA pool.139
Bariatric surgery and BAsBariatric surgery often changes BA composition14 and the novel status quo might promote BA-mediated metabolic effects29,100,140 which may be independent of body weight loss. This may explain why gastric bypass leads to the remission of T2DM.141
Bariatric procedures like RYGB, VSG, and BPD certainly increase the delivery of bile and pancreatic fluid to the distal small intestine100 and this greatly enhances BA absorption.142
Both RYGB29,30,100,141,143-151 and BPD144,149 are associated with an increase in fasting and post-prandial total BA concentrations, while studies on VSG have shown either in-creased30,150,152 or no change149,153,154 in BA concentrations. Gastric banding has no influence on BA concentra-tions.141,143
Caution is necessary when the findings connecting BAs and metabolic effects after the surgery are interpreted. Studies concerning the overall changes of BAs following bariatric surgery may show fluctuations and differ with respect to surgical procedures, study model (human or animal), type of patients and methodology, postoperative follow-up (either single141,144,146,151,155 or multiple measurements before and after the surgery30,100,156), and metabolic outcomes.
Bariatric surgery and metabolic effects of BAsThe recognition of BAs as metabolically active agents has raised the interest of research involving bariatric surgery. The increased plasma BA levels following bariatric surgery might induce GLP-1 secretion in the intestine and possibly other incretins157 and activate thyroid hor-mones.23,28 Few animal studies support this view although caution is necessary when translating these results to the human conditions. GPBAR-1 knockout mice on high fat diet show a higher increase in body fat mass compared with wild-type mice. In contrast, by increasing GPBAR-1 expression with the GPBAR-1 agonist INT-777, the HFD-induced obesity is blunted and energy expenditure increased.158,159 Notably, at the level of brown adipose tissue and skeletal muscle, BAs induce energy expenditure by promoting local activation of the type II iodothyronine deiodinase (DIO2), which transforms the inactive thyrox-ine (T4) to active thyroid hormone (T3), a key regulator of metabolism and energy homeostasis.160 The food-mediated increase of BAs might activate the BA-GPBAR-1-cAMP-DIO2 (type II iodothyronine deiodinase) signaling pathway to improve metabolic condition. BA treatment of brown adipocytes and myocytes increases their oxygen consumption rate, but this thermogenic effect is lost in DIO2 knockout mice.23 Obese Zucker rats display increased levels of the circulating BAs after ileal interposition with or without VSG. The intestinal adaptation after bariatric surgery results in increased recycling of BAs, which protects against obesity-related comorbidities.24,161 Similar effects are observed in rats with diet-induced obesity.25 An electro-physiologic experimental model (Ussing chamber) shows that BAs work on the GPBAR-1 that is expressed in the basolateral membrane of the GLP-1 secreting L-cells. The effect on GLP-1 release, therefore, would require the initial BA absorption across the intestinal epithelium,28 suggesting that the circulating levels of BAs effectively stimulates GPBAR-1. A translational study performed in rats and patients has shown that endothelial dysfunction is rapidly reversed by RYGBP, which is able to restore the endothelium-protective properties of HDL via a GLP-1 mediated mechanism.162 The surgery improves diabetes and liver steatosis by enhancing GLP-1 secretion through increasing serum BAs and the proliferation of L cells in the ileum.
In humans, as already mentioned, RYGBP, BPD, and VSG are certainly followed by increased circulating levels of BAs. VSG is associated with decreased gene expression of GPBAR-1 receptor in the white adipose tissue, suggesting that levels of serum BAs increase after bariatric surgery, independently from dietetic variations.150 Morbidly obese subjects display a bimodal rise of total fasting BAs after RYGB: ursodeoxycholic acid (UDCA) and its gly-cine and taurine conjugates increase at 1 month (with insulin sensitizing effects) while primary unconjugated BAs as well as dexoxycholic acid and its glycine conjugate increases after 24 months.14,100,101 Thus, the increase in the circulating BA levels after bariatric surgery might also increase the energy expenditure and the reduction of body weight through a thyroid hormone-stimulated pathway involving GPBAR-1.23 Activation of ileal GPBAR-1 increases levels of peptide YY (PYY) with anorexigenic effect (i.e., appetite reduction), as well as GLP-1 and GLP-2.163 Another mechanism might therefore involve the activation of the BA-GPBAR-1-GLP-1 axis. GLP-1, in particular, promotes insulin release, lowers glucagon secretion from the liver and inhibits gastrointestinal motility and food in-take.164 Yu, et al.165 found that levels of chenodeoxycholic acid (CDCA) in obese patients with type 2 diabetes undergoing RYGBP are a prognostic marker of diabetes remission after RYGBP. Notably, these results might be, at least in part, explained by the other BA signaling involving FXR. Shen, et al.166 found that CDCA increases intracellu-lar glucose transport in adipocyte cell lines by activating GLUT4 transcription via the FXR-FXR response element (FXRE) signaling.
As mentioned earlier, BAs also act as signaling agents of the nuclear receptor FXR in the liver and intestine. After bariatric surgery, this condition might also explain several metabolic effects at the level of gluco-lipidic homeostasis (Figure 2)33. Dufer, et al. showed an acute action of BA on β-cell function and insulin secretion, mediated by FXR.167 The gut microbiota might be also involved in this re-spect.14,123 In obese FXR knockout mice, the beneficial effects of VSG are secondary to both increased circulating levels of BAs and associated changes of gut microbiota, rather than to a mechanical restriction of the stomach per se. Furthermore, in the absence of FXR, the surgery-induced reduction of body weight and the improvement in glucose tolerance are blunted.33
Overall events involved in the function of bile acids (BAs) acting as signaling molecules and receptor ligands of farnesoid X receptor (FXR) and the G protein-coupled receptor 1 (GPBAR-1). In the left box, the steps involved in BA biosynthesis, secretion, storage, intestinal flow, absorption, colonic biotrans-formation and fecal loss are listed, as part of the enterohepatic circulation of BAs. The location of FXR and GPBAR-1 are shown in the liver, intestine, and several other tissues with respect to the control of BAs on: - Hepatic biosynthesis of primary BAs via the rate-limiting enzyme 7α-hydroxylase (CYP7A1) and CYP8B1 controlled negatively by the small heterodimer partner (SHP): Cholic acid (CA) and chenodeoxycholic acid (CDCA).169-171 -Intestinal release of FXR-mediated secreton of the fibroblast growth factor 19 (FGF19; known as FGF15 in mice), which circulates to the liver and reduces the expression of CYP7A1 to inhibit hepatic BA synthesis through FXR;172,173 -Intestinal GPBAR-1-mediated release of peptide YY (PYY), glucagon-like peptide 1 (GLP-1) and glucagon-like peptide 2(GLP-2).28 Several metabolic functions (boxes on the right).23,152,174-178 Adapted from Garruti, et al.,179 and Di Ciaula, et al. in this issue of Ann Hepatol 2017.
Bacterial biotransformation (i.e., deconjugation, oxidation, sulfation, and dehydroxylation) of the primary BAs to the secondary BAs is of key importance in the overall metabolism of BAs and resident intestinal microbiota. The possibility that the modified gut microbiota following bari-atric surgery may modify the BA composition, kinetics and metabolic functions, should be taken into account. In an animal model, the transfer of the gut microbiota from mice with gastric bypass to germ-free mice resulted in body weight loss, implying that the gut microbiota may play a role in the beneficial effects observed after RYGBP.168
The overall mechanisms responsible for the increase in plasma BA concentrations after bariatric surgery remains elusive. Moreover, not all studies on bariatric surgery are consistent with the overall beneficial effect of BAs on glucose homeostasis or energy metabolism. Kohli, et al.143 found that RYGBP is indeed associated with increased circulating BAs and GPBAR-1 signaling (i.e., increased postprandial peak of GLP-1 and decreased serum TSH). However, these changes do not correlate with in insulin-response to a meal, insulin sensitivity, or resting energy expenditure. BAs do not appear to be key mediators of the early increment in GLP-1 and gut peptide secretion in obese subjects undergoing bariatric surgery (gastric bypass or VSG). A significant increase in GLP-1 and PYY levels appears 1 week and 3 months after the surgery, but the basal and postprandial levels of BAs increase more slowly and progressively, reaching significant rises only one year after the surgery.30 It has been noted that beneficial metabolic effects on glucose tolerance might occur without intestinal resection but simply with ileal interposition.169-174 In this procedure, a part of the terminal ileum is interposed into the proximal jejunum, and this allows a premature contact of nutrients with the ileal mucosa, thereby leading to a subsequent stimulus of the L cells to produce GLP-1 and PYY. The increased delivery of BAs to the distal L cells and the altered GPBAR-1 receptor activation, however, do not seem to contribute to the early and substantial increases in intestinal secretion of GLP-1 and PYY, as seen after bariatric surgery.30 Indeed, the contact of undigested food with the L cells of the mucosa of the interposed ileum induces an early delivery of GLP-1 with normalization of the first phase of insulin secretion occurring 30 min after food intake. The effects of GLP-1 have been extensively studied in both humans and animal models. GLP-1 inhibits gastric motility and acid secretion, decreases food intake and enhances satiety, as well as increases postprandial suppression of glucagon secretion. In addition, GLP-1 increases insulin secretion in a dose-dependent manner, induces differentiation of progenitor duct cells into pancreatic β cells and preserves them from apoptosis.175,176
Conclusions and PerspectivesOverweight and obesity are exponentially increasing worldwide, and a significant proportion of the population already live or will live with one or more metabolic disorders which have an impact on the overall survival rate. Bariatric surgery, in selected groups of patients, is based on effective surgical strategies leading to body weight loss combined with a global improvement of metabolic abnor-malities177 and co-morbidities that are linked with excessive fat storage and insulin resistance. The beneficial effect of bariatric surgery is not simply secondary to the mechanical restriction of the stomach or to malabsorption of nutrients, since bariatric surgical procedures are able to positively modify more complex key pathways. Several lines of evidence show that BAs, beside their fat solubiliz-ing properties in the digestive system, also act as distinctive signaling molecules and activate two main sensors in the body, i.e., the nuclear receptor FXR and the cell surface receptor GPBAR-1. In health, in disease and following bariatric surgery, BAs are emerging as regulators of some complex homeostatic pathways at a systemic level, which involve cholesterol, triglyceride, glucose and energy metabolisms. After bariatric surgery, in particular, the increased intestinal and systemic availability of BAs seems to initiate and perpetuate significant and beneficial metabolic effects. Beside the already available studies in the animal models, growing evidence is also accumulating in the human setting. Further systematic studies, however, are needed to fully explore the novel and beneficial function of BAs on lipid, glucose and energy metabolism, acting as beneficial metabolic signaling agents in the body.
Conflicts of InterestWe declare that we have no conflicts of interest.