Acute liver failure (ALF) is a severe condition secondary to a myriad of causes associated with poor outcomes. The prompt diagnosis and identification of the aetiology allow the administration of specific treatments plus supportive strategies and to define the overall prognosis, the probability of developing complications and the need for liver transplantation. Pivotal issues are adequate monitoring and the institution of prophylactic strategies to reduce the risk of complications, such as progressive liver failure, cerebral oedema, renal failure, coagulopathies or infections. In this article, we review the main aspects of ALF, including the definition, diagnosis and complications. Also, we describe the standard-of-care strategies and recent advances in the treatment of ALF. Finally, we include our experience of care patients with ALF.
Acute liver failure (ALF) is defined as the acute development of jaundice, synthetic failure and hepatic encephalopathy in patients without a previous history of liver disease. It is a severe and complex condition that results from acute and massive hepatocellular destruction [1]. Thus, ALF has a very poor prognosis, with an approximately 80% mortality rate in historical series [2]. A better understanding of the causes of ALF, specific treatments, improvements in intensive care management and the availability of liver transplantation (LT) have dramatically enhanced the prognosis for patients with ALF. Nowadays, those patients who receive a liver graft have a one-year survival of approximately 70–65% [3].
ALF is an infrequent condition, with an incidence of 1–8 cases per million inhabitants, and it is responsible for 6% of deaths due to liver disease and up to 7–8% of liver transplants [2]. ALF mainly affects young adults, with a peak age between 35 and 45 years. Women account for approximately 60% of cases [4]. The development of cerebral oedema, sepsis and multiple organ failure are the main causes of mortality [5].
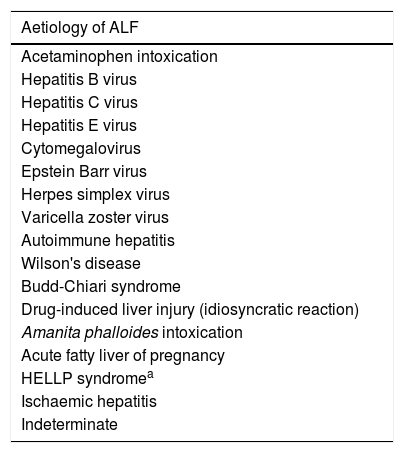
The aetiology of ALF differs significantly worldwide, and it is a very important aspect define treatment and prognosis (Table 1). Notably, indeterminate aetiology accounts for 20–45% of all ALF cases [1,4].
Main aetiologies of acute liver failure.
| Aetiology of ALF |
|---|
| Acetaminophen intoxication |
| Hepatitis B virus |
| Hepatitis C virus |
| Hepatitis E virus |
| Cytomegalovirus |
| Epstein Barr virus |
| Herpes simplex virus |
| Varicella zoster virus |
| Autoimmune hepatitis |
| Wilson's disease |
| Budd-Chiari syndrome |
| Drug-induced liver injury (idiosyncratic reaction) |
| Amanita phalloides intoxication |
| Acute fatty liver of pregnancy |
| HELLP syndromea |
| Ischaemic hepatitis |
| Indeterminate |
Thus, early identification of ALF, the administration of general and specific supportive treatments and the availability of LT are pivotal factors that can improve the outcomes for these patients.
2Definition and classificationIn the setting of acute hepatitis, ALF implies that there is evidence of abnormal liver synthetic function (international normalized ratio: INR>1.5) and the development of altered mental status (hepatic encephalopathy) within 26 weeks of the onset of illness in a patient without a history of liver disease [6]. Nonetheless, although the definition of ALF excludes the presence of previous underlying liver disease, there are some exceptions to this definition: an acute presentation of Wilson's disease, autoimmune hepatitis (AIH), Budd-Chiari syndrome or hepatitis B virus infection.
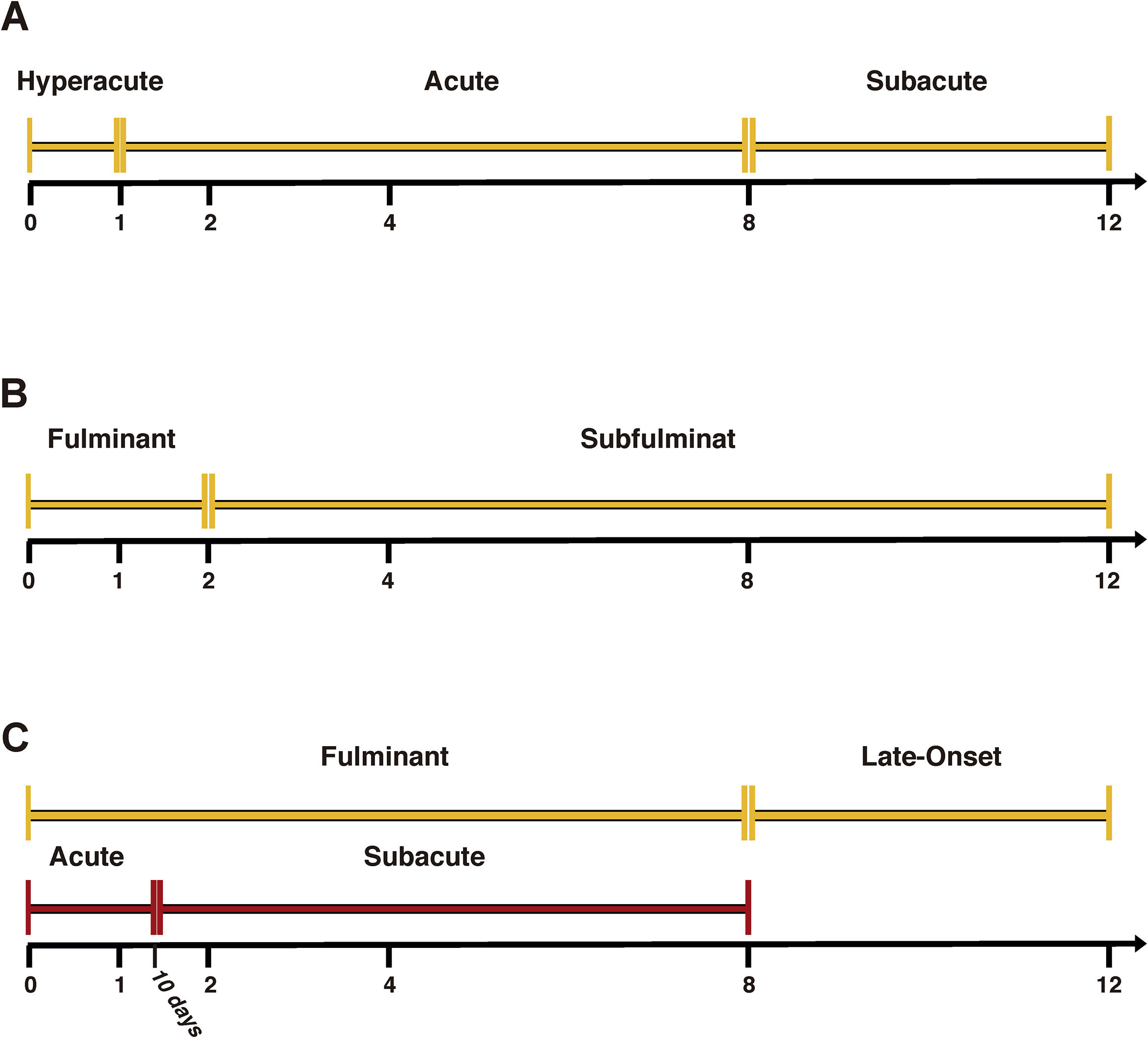
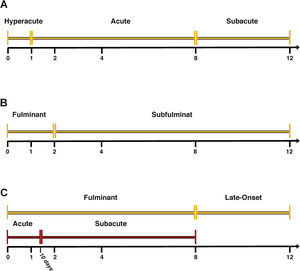
Hepatic encephalopathy (HE) is usually considered the hallmark of this disease and differentiates ALF patients from those with acute liver injury [6]. In ALF, several classifications have been described [5] (Fig. 1). O’Grady's classification possesses the advantage of having prognostic value [7]. Thus, the time between the presentation of jaundice and the onset of HE subdivides patients into three categories (hyperacute, acute and subacute), which are useful to define the prognosis. The hyperacute form has a better prognosis but a higher incidence of cerebral oedema. On the other hand, acute and subacute presentations have a worse prognosis, but a lower incidence of cerebral oedema [8].
3Role of liver biopsy in ALFLiver biopsy (LB) is an under-used resource. There are several histologic patterns with a limited specificity that need to be carefully considered based on the clinical picture. Furthermore, extensive necrosis is the common final stage of all ALF. There are two indications for LB: when a specific treatment or conduct can be decided (AH, drug-induced liver failure, viral infections, mushroom ingestions, Wilson's disease, malignancy) or if it is not clear if the patient has ALF or chronic liver disease [9]. On the other hand, in the most severe cases, LT should be considered and liver biopsy has no role in the treatment. A very common concern in ALF patients is the risk of bleeding. Thus, the potential harm of liver biopsy should be carefully evaluated. The most recommendable strategy is the use of global coagulation tests, such as thromboelastography (TEG) to evaluate the haemostasis (see coagulation section) AND a transjugular approach which is considered the safest and preferred route for liver biopsy in ALF patients, reducing the risk of liver bleeding although not excluding puncture related haemorrhagic complications (neck haematoma, haemothorax, retroperitoneal haematoma, etc.).
4TreatmentTreatments can be divided into those directed to treat the specific cause of ALF and those that provide supportive care for the clinical manifestations of ALF which are independent of the underlying aetiology.
5Specific treatments5.1Autoimmune hepatitisSteroids are the treatment of choice for AH AND a steroid trial in suspected severe AIH is an established practice and can be effective if given early [10]. However, although it can be useful in severe cases its role is not clear when ALF has developed [11]. In addition, its use has been associated with a significant risk of infections [12]. The presence of jaundice, encephalopathy and high INR are associated with null response to steroids [13]. We suggest a steroid trial administration carefully evaluated on a case by case basis considering the patient condition and the risks of infection. Nevertheless, clinicians should be aware to enlist for LT THOSE PATIENTS without improvement with no delay if LT seems to be a better option.
5.2Acute hepatitis BAcute liver failure secondary to hepatitis B virus (HBV) infection can be related to primoinfection or reactivation of a previous infection. Although there is no consensus related to the definition of ALF in HBV, it is clear that the diagnosis of ALF related to HBV excludes patients with cirrhosis [14]. However, excluding the presence of HBV is challenging considering that HBsAg and HBV DNA levels can decrease rapidly. There is retrospective evidence suggesting that antiviral treatment can improve the prognosis of patients with severe forms of acute hepatitis or reactivation, including ALF presentation [15]. Thus, the use of entecavir or tenofovir are proper alternatives due to its high efficacy [16]. The administration of immunosuppressive drugs (i.e., steroids, chemotherapy, or anti-CD20 antibodies) can induce A severe reactivation and ALF. Thus, the presence of HBV infection must be determined and antiviral nucleos(t)ide analogues (NA) must be administered before the onset of immunosuppressive treatment. Although hepatitis D virus (HDV) is a rare cause of ALF, HDV-HBV coinfection or superinfection are considered severe forms of viral ALF. Up to 20% of HDV-HBV coinfection cases develop ALF [17]. On the other hand, only 5% of HDV-HBV superinfection develops ALF [17]. Specific treatment options for HDV are scarce, considering that interferon-α is contraindicated in ALF, the lack of efficacy of NA and the fact that myrcludex B, nucleic acid polymers and lonafarnib has not been evaluated in this setting [18]. ALF secondary to HDV-HBV coinfection conferred a very poor prognosis and in severe cases liver transplantation remains as the treatment of choice [17].
5.3Amanita phalloidesThe manifestations of amanita intoxication are mainly gastrointestinal (abdominal pain, nausea, vomiting and cholera-like diarrhoea). This phase is followed by a marked increase in serum transaminases that eventually evolves into ALF. Amatoxins are transported through enterohepatic circulation and inhibit gene transcription, particularly in the most metabolically active cells, such as liver and kidney cells.
The recommended treatment includes gastric aspiration and lavage with a nasogastric tube and the administration activated charcoal [19]. A urinary output of 100–200ml/h has also been recommended to improve the urinary excretion of amatoxin [20]. Molecular adsorbent recirculating system (MARS®) has been useful in amanita-related ALF, but only in small cohorts of patients [21]. The fractionated plasma separation and adsorption system (FPSAS) has also been suggested to be useful [22]. Other strategies are the use of silibinin (20–50mg/kg/d intravenously for 48–96h or 1.4–4.2g/d orally). Penicillin G reduces the binding of amatoxin to plasma proteins thus increasing its excretion. The usual doses are 1,000,000IU/kg on the first day and 500,000IU/kg for the next two days. The combination of silibinin and penicillin seems to have similar efficacy to the use of silibinin alone [23]. The use of N-acetylcysteine has also been proposed at similar doses to those used for acetaminophen intoxication [24].
5.4Pregnancy-related liver diseaseAlthough uncommon severe liver dysfunction during pregnancy may have dismal prognosis [25]. Preeclampsia/eclampsia is the most common cause of liver disease during pregnancy (occurring after 20 weeks of pregnancy) and is caused by vasospasm of the hepatic vascular bed [26]. Serum aminotransferases can be elevated up to 20 times. However, jaundice occurs infrequently. HELLP syndrome (defined as haemolysis, elevated liver enzymes and a low platelet count) is a severe manifestation of preeclampsia and is associated to liver infarctions, large haematomas, hemoperitoneum and acute liver failure [27]. Acute fatty liver of pregnancy (AFLP) is a rare but potentially catastrophic liver disease usually diagnosed during the third trimester and associated to high foetal and maternal mortality. It seems to be associated with mitochondrial dysfunction secondary to defective long-chain fatty acid oxidation. Pregnancy interruption is the treatment of choice and considered mandatory for all these pregnancy-related liver diseases [25].
5.5Acetaminophen intoxication (AI)Acetaminophen intoxication is the most frequent cause of ALF in the US and the UK [5]. A recent series of 662 patients showed that AI has a good prognosis with transplant-free survival about 65% [28]. The effectiveness of N-acetylcysteine (NAC) for improving the prognosis of patients with AI induced ALF has been extensively evaluated in large series and one controlled trial that suggested a significant benefit in terms of survival [29]. NAC can be administered orally (loading dose of 140mg/kg and 17 subsequent doses of 70mg/kg every four hours) or intravenously (150mg/kg over 30min, followed by 70mg/kg over 4h, followed by 70mg/kg over 16h).
6Supportive management6.1HaemodynamicALF patients usually present hyperdynamic circulation (high cardiac output and low systemic resistance). The systemic inflammatory response plays a pivotal role in the pathogenesis of ALF [30]. Thus, proper fluid administration is necessary to improve tissue perfusion and vasopressor and inotropic drugs are required to maintain arterial pressure and cardiac output [31]. Haemodynamic monitoring is essential to choose the proper intervention with minimal adverse effects. At this regard, pulse pressure variability is a useful predictor of the response to fluid administration avoiding deleterious changes in intracranial pressure (ICP) [32]. There is scarce evidence about vasoactive drug choices. In 2007, Eefesen et al. published a single centre prospective study comparing noradrenalin and terlipressin in 10 ALF patients who had ICP monitoring and brain microdialysis. Terlipressin increased the CPP without changes in ICP along with a decrease in brain lactate, but the lactate/pyruvate ratio remained unchanged [33].
6.2Ventilatory supportVentilatory support may be necessary in patients with impaired consciousness or those who have developed of acute respiratory distress syndrome (ARDS). In ALF, approximately 21% of patients develop ARDS, characteristically late in the evolution of the disease and mainly associated with the occurrence of sepsis [32]. Although the development of ARDS implies more days on mechanical ventilation, there is no difference in the use of renal replacement therapies (RRT), ICU days or mortality [34]. Interestingly, the use of positive end–expiratory pressure can increase alveolar recruitment without raising ICP [35].
6.3Acute kidney injuryAcute kidney injury (AKI) is described in up to 70% of patients, and approximately 30% of the affected patients require RRT [36]. A myriad of causes can be involved in the aetiopathogenesis of AKI and is more frequently associated to ischaemic hepatitis, acute fatty liver of pregnancy, HELLP syndrome, heat stroke, hepatitis A virus infection and drug-induced liver injury [37]. Remarkably, it is independently associated with decreased survival [37]. Continuous RRT seems to be an appropriate treatment considering that intermittent therapies have been associated with a higher risk of haemodynamic instability and cerebral oedema [38].
6.4InfectionsPatients with ALF have an increased risk of infections, sepsis and septic shock, which lead to an increased risk of morbidity and mortality [39], mainly due to immune system dysfunction secondary to the systemic compensatory anti-inflammatory response [40]. Bacterial infections are common and have been reported in up to 80% of cases [39,41]. Its diagnosis can be very difficult and requires active surveillance due to its severe implications [42]. The mortality attributable to sepsis ranges between 10% and 52% [40]. The most common infections are pneumonia (50%), urinary tract infections (22%) and bloodstream infections (28%). Multi-resistant infections play a significant role in these patients and fungal infections affect up to one-third of cases [40].
Routine sampling for cultures and the administration of broad-spectrum antibiotics, particularly if the patient's clinical condition worsens, is recommendable [6]. A common regimen includes extended-spectrum β-lactam (e.g., piperacillin–tazobactam, ticarcillin–clavulanate) and vancomycin. Additionally, Candida treatment could be added if there are some risk factors such as diabetes, parenteral nutrition, previous exposure to broad-spectrum antibiotics therapy or abdominal surgery. However, the exact antimicrobial regimen should be based on local microbiological data. Antibiotic prophylaxis has been studied but the benefits are controversial and it cannot be widely recommended [43].
7Coagulation in ALF patientsDuring ALF, the liver diminishes the synthesis of coagulation factors (II, V, VII, IX, X), anti-coagulation factors (C and S proteins) and the fibrinolytic system. Thus, a new balance of the haemostasis is established [44]. Although INR increase is an important prognostic factor, it does not imply a higher risk of haemorrhage. In a study that employed TEG (see use of viscoelastic tests) in 20 patients with ALF a lack of correlation between INR and haemostasis was observed [45]. Indeed, only 20% of these patients had a hypocoagulable state based on the TEG assessment [45].
7.1Bleeding riskBleeding in ALF may occur spontaneously. Generally, it is from capillary origin and is associated with mucosal lesions. Indeed, the most common site of bleeding in patients with ALF is the gastric mucosa and prophylaxis with proton pump inhibitors has been recommended [46]. Procedure-associated bleeding is a life-threatening condition, and the evaluation of the haemostatic balance before an invasive procedure is mandatory [47].
The haemostatic profiles of ALF patients are characterized by a variable reduction in almost all of the coagulation, anti-coagulation and fibrinolytic factors [48]. Also, there is a reduction in platelet count and abnormal platelet function. The concept of rebalanced haemostasis points to a new equilibrium state between coagulant and anti-coagulant factors. In addition, there is a compensatory mechanism that involves the increase in platelet production of Von Willebrand's factor generating a new haemostatic balance without an increased risk of bleeding [49].
7.2Use of viscoelastic testsViscoelastic tests (VET), such as TEG and thromboelastometry, evaluate the coagulation phenotype through the different steps in clot formation. VET have been successfully used for guiding the administration of haemoderivatives before invasive procedures such as liver biopsy and LT [50]. Notably, in a recent systematic review the use of VET has been associated with a reduction in haemoderivative administration and decreased mortality [51].
7.3Treatment of bleeding episodesProphylactic transfusion of blood components based on conventional coagulation assays is no longer recommended and should be used only in cases of active bleeding [52]. Instead, we recommend monitoring VET, platelet counts and fibrinogen levels to evaluate the bleeding risk during invasive procedures (e.g., liver biopsy, intraparenchymal catheter placement for ICP, or liver transplantation) and implementing a rational administration protocol for blood components (i.e., fresh frozen plasma, prothrombin concentrate, cryoprecipitate or platelets).
Thus, the administration of fresh frozen plasma (FFP, 10ml/kg) is indicated to correct coagulopathy based on R time in TEG [53]. Prothrombin complex concentrates are a plausible alternative to FFP and recommended doses vary depending on the manufacturer. However, their efficacy during LT is under study in a randomized trial [54]. Cryoprecipitate (1 unit each 10kg of body weight) is used, when maximum amplitude (MA) of TEG is less than 50mm or plasma fibrinogen level <100mg/dL [53]. Platelet count below 50,000/mm3 is another cause of MA <50mm and should be corrected [53].
8Intracranial hypertension (ICH) in ALFICH (defined as an intracranial pressure above 20–25mmHg for more than 15min) [55] is one of the most severe complications of ALF and is associated with a poor prognosis [56]. Considering that ICH accounts for 20–35% of the mortality in ALF, its early recognition is critical to avoid permanent neurologic damage [5,57]. It is accepted that the development of ICH is related to a poor metabolization of ammonia by hepatocytes, which induces an accumulation of glutamine in astrocytes that has an osmotic effect and leads to cellular tumefaction, hepatic encephalopathy and eventually ICH [57]. Also, there is an impaired autoregulation of cerebral blood flow resulting in vasodilatation of cerebral arterioles increasing intracranial blood volume [57]. ICH diagnosis is challenging and requires continuous neurological assessments, the use of periodic neuroimaging and intracranial pressure monitoring in patients with brain oedema [57].
The presence of severe HE is associated with a high frequency of ICH (25–35% in grade III and up to 75% in grade IV) [8]. Other risk factors for ICH are female gender, younger age, severe liver failure (MELD>32), ammonium concentrations above 150–200μmol/L, and renal failure.
8.1Monitoring intracranial pressureIntracranial hypertension can produce death or serious sequelae precluding liver transplant and its monitoring allows the early detection and helps to determine cerebral perfusion pressure and evaluate the response to therapies. Besides, ALF patients have altered cerebrovascular autoregulation and are extremely sensitive to vasopressors [58], which can produce iatrogenic oedema secondary to vascular hyperperfusion. Continuous, real-time and accurate measurements of ICP can be obtained only through invasive methods. The rationale for ICP measurement is based on retrospective trials that showed a prevalence of over 50% of ICH in ALF patients and an association with elevated mortality risk [59]. Nonetheless, there is not randomized data available to evaluate the benefit of ICP monitoring.
Invasive ICP monitoring can be obtained through two different approaches: intraparenchymal microtransducers and direct catheters (intraventricular, subdural or epidural). Selection of the monitoring approach and placement is relevant considering the accuracy of the measurement and bleeding risks.
There is wide agreement that epidural transducers are a relatively safe method [60]. However, it has a less-than-optimal degree of precision compared with other methods due to the damping effect of the surrounding dura mater. Intracranial pressure evaluated by an intraparenchymal catheter has a good correlation with values obtained with intraventricular catheters [61]. The rates of infection from intraparenchymal catheters are minimal but are associated with a bleeding rate of 7% and bleeding-related deaths of approximately 3% [56,59,62]. A caveat in relation to parenchymal monitoring is that it may be not representative of true cerebrospinal fluid pressure because of intraparenchymal pressure gradients [63] and changes in zero values (atmospheric pressure) due to lost calibration.
Today, there are several indirect non-invasive approaches for the diagnosis of intracranial hypertension: transcranial Doppler (TCD), computed tomography (CT), magnetic resonance imaging (MRI) and optic nerve sheath diameter (ONSD) measurement. Intracranial hypertension affects the velocity profile of blood flow through external constriction of the blood vessels. Thus, an increase in systolic velocity along with a decrease in diastolic velocity increases the pulsatility index that is observed [64]. However, TCD remains as operator-dependent evaluation with intra- and inter-observer variations. CT and MRI may show radiological signs of brain oedema. However, the absence of these signs does not rule out ICH [65]. The optic nerve sheath (which has anatomic continuity to meninges) is distensible and the ICP influences its diameter [66]. Thus, literature suggests the utility of ONSD measurement as screening method to ICH diagnosis, with a cut-off of 5.7mm measured 3mm behind the globe [67]. However, there is scarce information in the setting of ALF. Considering the present state of knowledge, no definitive recommendations can be made at this regard.
8.2Prevention and treatment of cerebral oedemaConsidering the ominous outcomes associated to ICH [5], prophylactic measures are necessary. Thus, elevation of the patient's head at 30 degrees, reducing stimulation pain, Valsalva manoeuvres and proper sedation can effectively reduce intracranial pressure [57].
The use of lactulose has the potential to reduce cerebral ammonia levels, but its use has not been associated with an improvement in encephalopathy or mortality in ALF [57]. Serum sodium levels need to be strictly monitored. One trial randomized patients to receive hypertonic saline to increase serum sodium levels to 145–155mmol/L or standard care. Notably, a rapid decrease in intracranial pressure was seen in the active arm, followed by a significantly reduction in the cumulative incidence of ICH [68]. Mannitol boluses (0.5–1.0g/kg) are effective for reducing ICP [69] however, repeated boluses are associated with a risk of fluid overload and hyperosmolality (greater than 320mOsm/L). Therefore, a strict monitoring is necessary. Hyperventilation produces cerebral vasoconstriction secondary to cerebrospinal fluid alkalosis, reduces vascular inflow and eventually decreases intracranial pressure [70]. Decreasing PaCO2 to 25–30mmHg is recommended in cases of increased intracranial pressure [5] although its effect is short-lived and cerebral vasoconstriction can generate areas of cerebral ischaemia. Prophylactic hyperventilation does not provide a benefit in terms of reducing the incidence of cerebral oedema [70]. Indomethacin inhibits endothelial cyclooxygenase, produces cerebral vasoconstriction and decreases cerebral blood flow (CBF). In a small report, Toefteng et al. evaluated the effect of indomethacin on ICP in twelve patients with ALF. A significant reduction in ICP and an improvement in cerebral perfusion pressure (CPP) were observed. However, no additional clinical information is available today [71]. Hypothermia reduces CBF and the entry of ammonia into the brain, decreases the availability of glutamate in the cerebral extracellular space and diminishes anaerobic glycolysis [72]. In 1999, Jalan et al. showed that a temperature reduction to 32–33°C was associated with a significant decrease in ICP [73]. Interestingly, a recent retrospective study showed that therapeutic hypothermia had no impact on overall or LT-free survival [74]. However, the retrospective nature of the study warrants controlled prospective studies to evaluate the role of hypothermia on overall survival. Recently, the role of prophylactic hypothermia was evaluated in a randomized trial. This study included forty-six patients with intracranial pressure monitoring that were randomized to hypothermia (targeted temperature of 33–34°C) or normothermia (36°C) treatments. Interestingly, although the target temperature was consistently achieved in both groups, there was no difference in the incidence of sustained elevation of ICP (35% vs. 27% in the intervention group and the control group, respectively) or in overall survival [75].
9Extracorporeal support systemsDuring several years, the usefulness of extracorporeal liver support systems has been evaluated to improve the survival for patients on the waiting list for LT. Molecular adsorbent recirculating system (MARS®) was designed to eliminate inflammatory molecules by employing albumin dialysis, reducing nitric oxide levels and improving haemodynamic parameters [76]. Initial reports showed an improvement in systemic and cerebral haemodynamic variables [77]. However, no survival benefit was shown [78]. The same was true for HepatAssist which employs porcine hepatocytes contained within a hollow fibre bioreactor. In a randomized, controlled trial no advantage on 30-day survival was demonstrated [79].
Recently, a randomized controlled trial showed that high-volume plasma exchange can improve transplant-free survival [80]. The mechanisms involved seems to be reduced activation of the innate immune system secondary to the elimination of cytokines and toxic molecules and the administration of physiological substances contained in FFP. Notably, 54% of these patients suffered acetaminophen overdose. Thus, its usefulness in a different cohort of patients remains to be elucidated [81].
10Liver transplantation and prognostic scoresLT is the single most important intervention to improve survival in the most severe cases of ALF. However, performing LT has several restrictions including the following: organ shortage, severe clinical deterioration (i.e., uncontrolled sepsis or haemodynamic instability), severe comorbidities or substance abuse [29]. Nonetheless, when LT is feasible it offers excellent survival rates at one and five years (84% and 75%, respectively) [56]. However, these survival rates are lower in ALF than those achieved with LT for other indications. Based on the analysis of the United Network for Organ Sharing (UNOS), four factors associated with poor outcome were identified: recipient age >50 years, requirement for life support, body mass index ≥30kg/m2 and serum creatinine >2.0mg/dl. Other factors associated with a poor prognosis are the use of reduced-size organs, donor age >60 years, incompatible ABO group matching and donor liver steatosis [82].
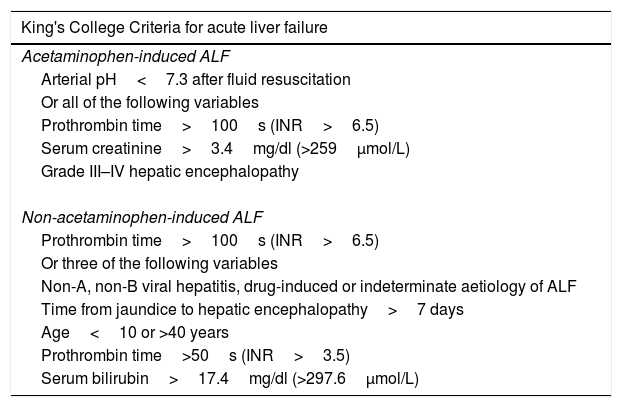
Several prognostic tools have been developed in order to predict mortality and the need for LT [83]. The King's College Criteria (KCC), the MELD score, the APACHE II score and the SOFA score are some of the most commonly used scoring systems.
Late in the 80s, O’Grady et al. described several variables that were independently related to survival in a cohort of 588 patients [84]. Based on this information, the authors generated two prognostic models for paracetamol-induced and non-paracetamol-induced ALF (Table 2). These models were validated in an independent retrospective cohort of 175 patients [85]. In a recently published meta-analysis, the diagnostic yield of the KCC was evaluated and compared to the MELD score [86]. For paracetamol-induced ALF (PIA), the sensitivity and specificity of the KCC and MELD score were 58% and 89%, vs. 80% and 53%, respectively. In cases of non-paracetamol-induced ALF (NPIA) the sensitivity and specificity of the KCC and MELD score were 58% and 74% vs. 76% and 73%, respectively. The overall area under the ROC curve (AUC) was 0.76 for the KCC and 0.78 for the MELD score. The authors concluded that the KCC seem to be more accurate in PIA and the MELD score can be a better predictor in NPIA. The SOFA score seems to be a useful prognostic tool in ALF. In 2012, Cholongitas et al. compared the diagnostic yield of the KCC, the MELD score, the APACHE II score and the SOFA score in a cohort of patients with PIA. Interestingly, the AUC of SOFA score (0.79) was similar to the AUC of the APACHE II score (0.72) and was significantly better than KCC and MELD scores (0.65 and 0.58, respectively) [87]. Additionally, in a previous study, the SOFA score was also independently related to the development of ICH [88]. Further studies are warranted, particularly studies that evaluate patients with NPIA, before a recommendation can be made.
King's College Criteria for establishing a poor prognosis and the need of liver transplantation.
| King's College Criteria for acute liver failure |
|---|
| Acetaminophen-induced ALF |
| Arterial pH<7.3 after fluid resuscitation |
| Or all of the following variables |
| Prothrombin time>100s (INR>6.5) |
| Serum creatinine>3.4mg/dl (>259μmol/L) |
| Grade III–IV hepatic encephalopathy |
| Non-acetaminophen-induced ALF |
| Prothrombin time>100s (INR>6.5) |
| Or three of the following variables |
| Non-A, non-B viral hepatitis, drug-induced or indeterminate aetiology of ALF |
| Time from jaundice to hepatic encephalopathy>7 days |
| Age<10 or >40 years |
| Prothrombin time>50s (INR>3.5) |
| Serum bilirubin>17.4mg/dl (>297.6μmol/L) |
The Clichy-Villejuif criteria (CVC) are mostly used in France (factor V level: <20% in patients <30 years of age, or <30% in patients ≥30 years of age) [89] and are based on a study by Bernuau et al., who evaluated patients with ALF secondary to hepatitis B virus [90]. In 1993, Pauwels et al. compared the KCC and the CVC with a cohort of patients with ALF mostly secondary to viral infections. On admission, the sensitivity and specificity of the KCC were superior to those of the CVC (86% and 79% vs. 73% and 58%, respectively) [91]. More recently, the CVC were re-evaluated on a cohort of 808 patients. The sensitivity and specificity of the CVC to predict mortality were 75% and 56% vs. 69% and 50% for PIA and NPIA, respectively [89]. Interesting, factor V levels were independently related to survival in PIA patients [89].
Lactate concentrations have been related to prognosis in ALF. Bernal et al. evaluated the diagnostic performance of lactate concentration in a cohort of 103 PIA patients. The results were validated in an independent cohort of 107 patients. A high sensitivity and specificity were shown for a lactate concentration (after fluid resuscitation) ≥3.0mmol/L (76% and 97%, respectively). Notably, incorporating lactate into KCC increased its sensitivity from 76% to 91% [92]. More recently, Cholongitas et al. reported an AUC of 0.8 in a cohort of 102 PIA patients with a cut-off of 4.7mmol/L [87]. In this study, the AUC for SOFA was 0.84. Interestingly, the addition of lactate to KCC, MELD or SOFA scores did not improve their diagnostic accuracy [87].
The dynamic evolution of 4 parameters (hepatic encephalopathy, serum bilirubin, INR and ammonia) over 3 days were evaluated in a recent study from India including 380 patients (mostly NPIA) in an attempt to predict survival. Interestingly, the worsening or lack of improvement in these parameters had high diagnostic accuracy when was validated in a different cohort (sensitivity 90% and specificity 80%) [93]. Thus, further studies including western centres seem to be warranted.
A recent study generated a prognostic index including coma grade, bilirubin level, phosphorus level, INR and M-30 (an apoptosis marker) from a cohort of 250 patients. This index was validated in a group of 250 different patients. Remarkably, the AUC for this index (named ALFSG) was significantly higher than those of KCC and MELD (0.84 vs. 0.68 and 0.71, respectively) [94]. Whereas promising, the usefulness of this index is limited by the low availability of M-30 determination.
Although uncommon in western transplant medicine, living donor liver transplantation (LDLT) is the only alternative of LT for ALF patients in several Asian countries. The first successful case was reported in 1992 [95] in one paediatric patient. Since then, appropriate outcomes have been reported compared to cases transplanted with deceased donors [96]. Interestingly, LDLT is also possible in adult recipients with good results [97,98]. In fact, Park et al., reported a cohort of forty adult to adult LDLT patients with an overall one-year patient survival of 85% [99]. Nonetheless, although feasible, significant expertise is necessary before systematically performing LDLT in ALF patients.
11Future directions11.1Cell transplantationHepatocyte transplantation (HT) is an alternative tested in several conditions with variable success. Hepatocytes can be harvested from liver grafts not suitable for LT or from neonatal donors. Next, they are isolated and directly administered through portal system or splenic artery injection. In metabolic diseases such as Crigler-Najjar or familial hypercholesterolemia, significant improvements have been reported. However, these metabolic improvements are transient and most patients would need LT as a definitive treatment [100]. In relation to ALF, several reports of HT have been published showing that it is a feasible procedure. However, recovery without LT is uncommon and further controlled studies are warranted [101].
Stem cell transplant (SCT) can be performed employing embryonic stem cells, induced pluripotent stem cells, hepatic progenitor cells, hematopoietic stem cells or mesenchymal stem cells (MSCs). MSCs do not present ethical conflicts and can be obtained from bone marrow, adipose tissue, umbilical cord or amniotic fluid and its differentiation to hepatic cells can be induced employing different methods [102]. MSCs inhibit T cells, dendritic cells and natural killers, they also reduce the activation of B cells and increase the production of T regulatory cells and levels of IL-10 and TGF-β modulating the immune response. These changes allow liver damage to be repaired and liver function to be improved. Thus, MSCs can improve liver function, decrease hepatocyte apoptosis and promote hepatocyte proliferation. Notably, MSCs improve the survival of rodent and pig ALF models. In patients with acute on chronic liver failure (ACLF) secondary to HBV, the infusion of MSCs has been associated with a significant improvement of liver function [103–105]. In a recent randomized controlled trial, including 110 patients with HBV-related ACLF, the infusion of MSCs was associated to an increased 24 weeks survival (73.2% vs. 55.6%) with no infusion-related side effects [106]. Thus, the infusion of MSCs is a promising alternative for the treatment of liver failure. However, controlled studies specifically designed to include ALF patients are warranted.
11.2Organ engineeringThe use of decellularized 3D extracellular matrix (ACM) scaffolds for whole organ engineering requires the decellularization of human or animal organ, which generates a scaffold to allow a recellularization process with parenchymal and non-parenchymal cells, i.e. hepatocytes, cholangiocytes, and endothelial cells. A successful alternative is the use of xenogeneic scaffold (porcine or murine). Several strategies are able to generate a structurally appropriate scaffold to be repopulated, maximizing the destruction of the native organ DNA thus avoiding the generation of a significant immune response [107]. Of course, the recellularization of the vascular and biliary network is also necessary. Very recently a successful repopulation of the vascular tree with endothelial cells has been achieved [108]. Several types of cells have been employed to repopulate the scaffold, i.e. adult human hepatocytes, foetal liver cells, hepatoblastoma-derived cells lines (HepG2), porcine-derived cells, each of them presents different limitations including: poor availability that limits the amount of cells, reduced ability to perform physiologic hepatocyte functions (foetal cells), the risk of malignant disease, immunogenicity or even zoonosis. Thus, different alternatives are being explored such as pluripotent stem cells or bipotent progenitor cells expanded as epithelial organoids [109]. Therefore, organ engineering could become a promising alternative to conventional LT for patients with severe liver diseases although several difficulties and technical shortcomings still need to be overcome before clinical trials can be performed.
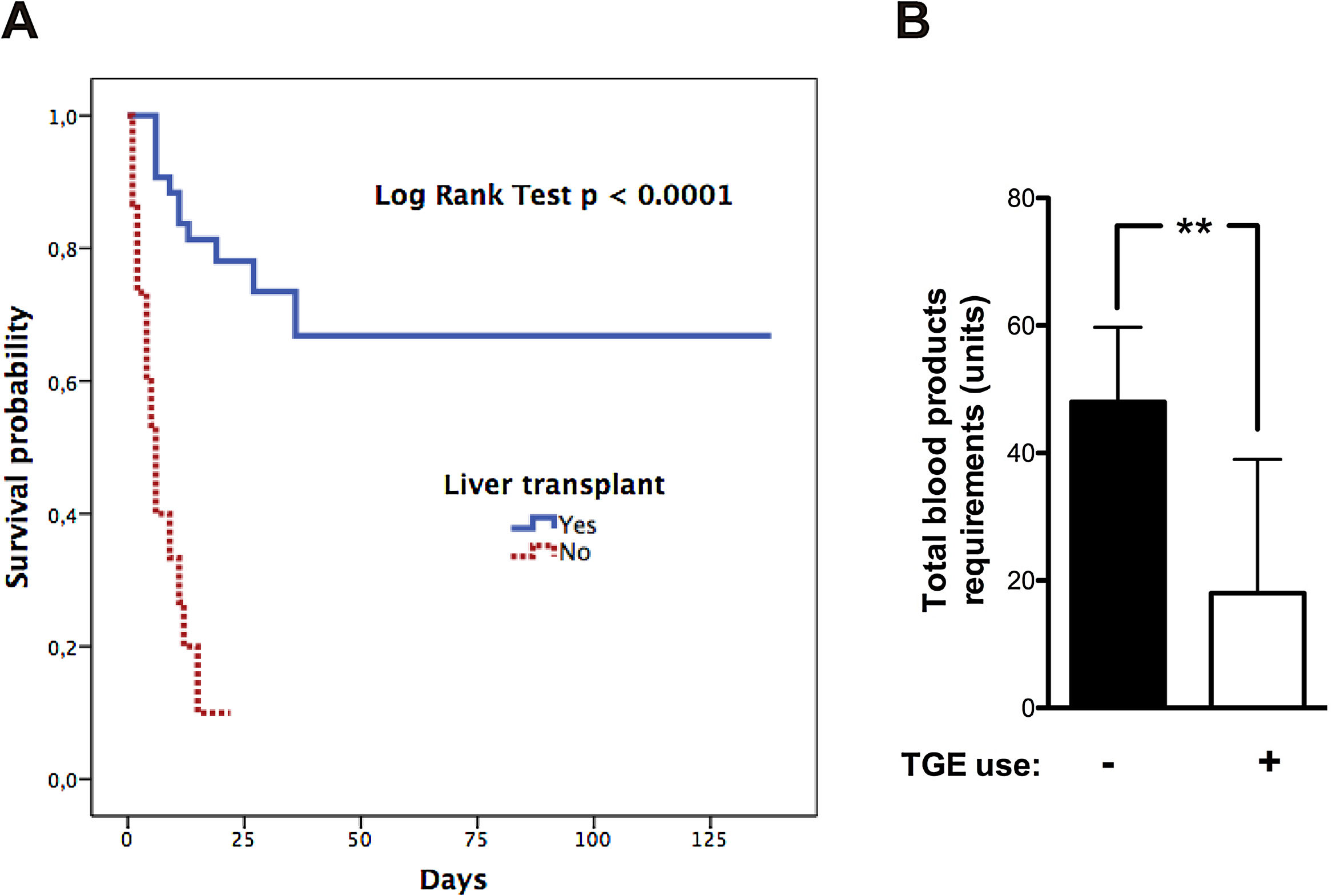
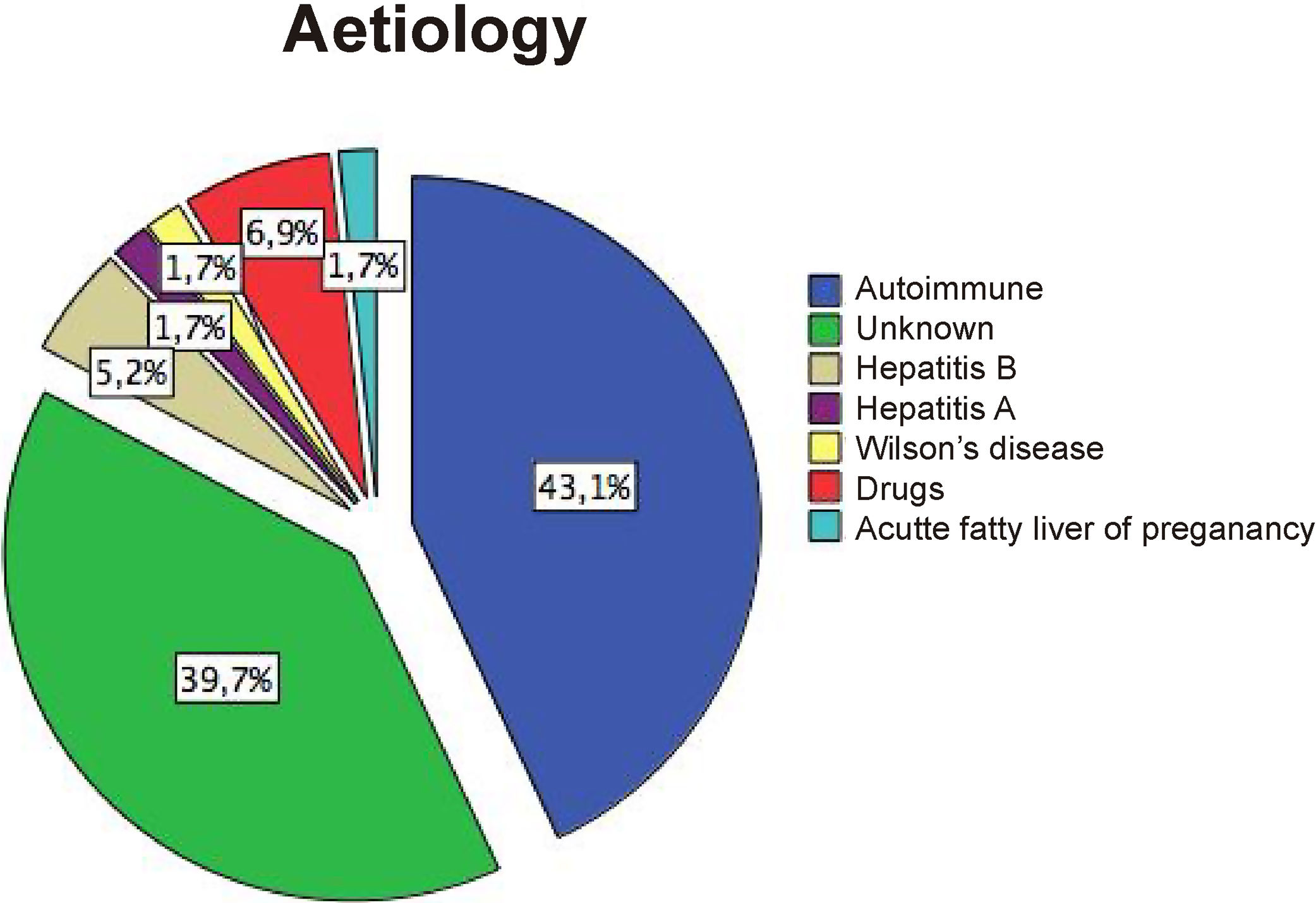
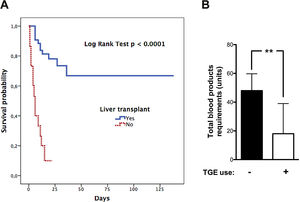
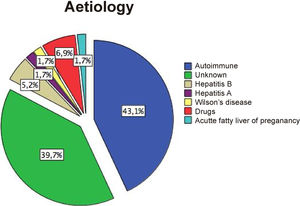
12Our experienceIn the last two decades, our hospital has become a national reference centre for LT. The shortage of grafts is a severe problem. Thus, patients can experience several complications during the waiting period. Since 2002 to date, we have treated 58 patients with ALF that meet the King's College Criteria. Most of them were caused by AH (Supplementary Fig. 1). Only two have undergone spontaneous recovery. Univariate analysis of the cohort showed a significant association between waiting time before LT (<3.5 days) and survival (Supplementary Table 1, LOS-LT: days elapsed before liver transplantation). Deaths occur early in the evolution of ALF as shown by survival curves (Fig. 2A). Monitoring of ICP was less frequent in those who survived and was performed employing microtransducers when patients exhibited brain oedema on CT. LT was the only intervention independently related to survival, in a multivariate analysis adjusted by age, sex, APACHE II, MELD score, and duration of waiting for a donor (OR 26.9, IC 2.67–271.8; p=0.005). Finally, we find an interesting relation between TEG assessment introduction in 2013 and transfusion requirements in our patients. We experimented a 63% reduction in blood products including FFP with TEG use (p=0.005 Mann–Whitney test, Fig. 2B).
13ConclusionsAcute liver failure is a very challenging disease that must be carefully managed by a multidisciplinary team that should include at least an intensivist, a hepatologist, a radiologist and transplant surgeons. The prompt diagnosis of ALF allows the administration of specific and general supportive therapies. Strict neurological monitoring is critical considering the high risk of cerebral oedema and intracranial hypertension in these patients. Considering that there are multiple causes of ALF with significant geographical variability, broad and rapid evaluation for the underlying aetiology is necessary. Identification of the aetiology of ALF allows the opportunity to administer specific treatments with the potential to change the initial prognosis of the patient. Special attention must be paid to the rational administration of hemoderivatives being the use of viscoelastic test a rational tool. Critical issues are proper monitoring and constant evaluation of prognostic factors to evaluate the need for LT considering that no liver support systems have consistently showed a survival benefit in this setting.AbbreviationsAFLP acute fatty liver of pregnancy acute kidney injury acute liver failure acute physiology and chronic health evaluation II autoimmune hepatitis cerebral perfusion pressure Clichy-Villejuif criteria fractionated plasma separation and adsorption system hepatic encephalopathy intracranial hypertension intracranial pressure King's College Criteria liver transplant model for end-stage liver disease molecular adsorbent recirculating system N-acetylcysteine non-paracetamol-induced acute liver failure optic nerve sheath diameter paracetamol-induced acute liver failure receiver operating characteristic curve renal replacement therapies sequential organ failure assessment thromboelastography transcranial Doppler viscoelastic tests
None declared.
Conflicts of interestNone of the authors have relevant conflict of interest present for any aspect of the submitted work.
Authors thank Dr. Glenn Hernández (Professor, Departamento Medicina Intensiva, Pontificia Universidad Católica de Chile) for his critical revision of manuscript.