Abstracts Asociación Mexicana del Hígado (AMH) 2023
More info: Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, there are approximately 71 million infected individuals worldwide, up to 40% of them will have spontaneous resolution and60% will develop chronic infection with risk of developing cirrhosis and hepatocellular carcinoma.In Mexico it is the fourth cause of death and one of the main causes of disability. Current HCV treatment with direct-acting antivirals (ADDs) has a high rate of sustained viral response that ensures the cure of the infection, decreases the progression of liver fibrosis and decompensation rates in patients with cirrhosis. This study aimed to describe the demographic and clinical characteristics of patients with chronic HCV infection, treated at a third-level care hospital in Mexico City.
Materials and PatientsA retrospective cohort study in a part of the patients from the HCV clinic of an IMSS third-levelhospital, which included cases with confirmed HCV infection, who received treatment with direct-acting antivirals +/- ribavirin, in the period of 2017-2022.
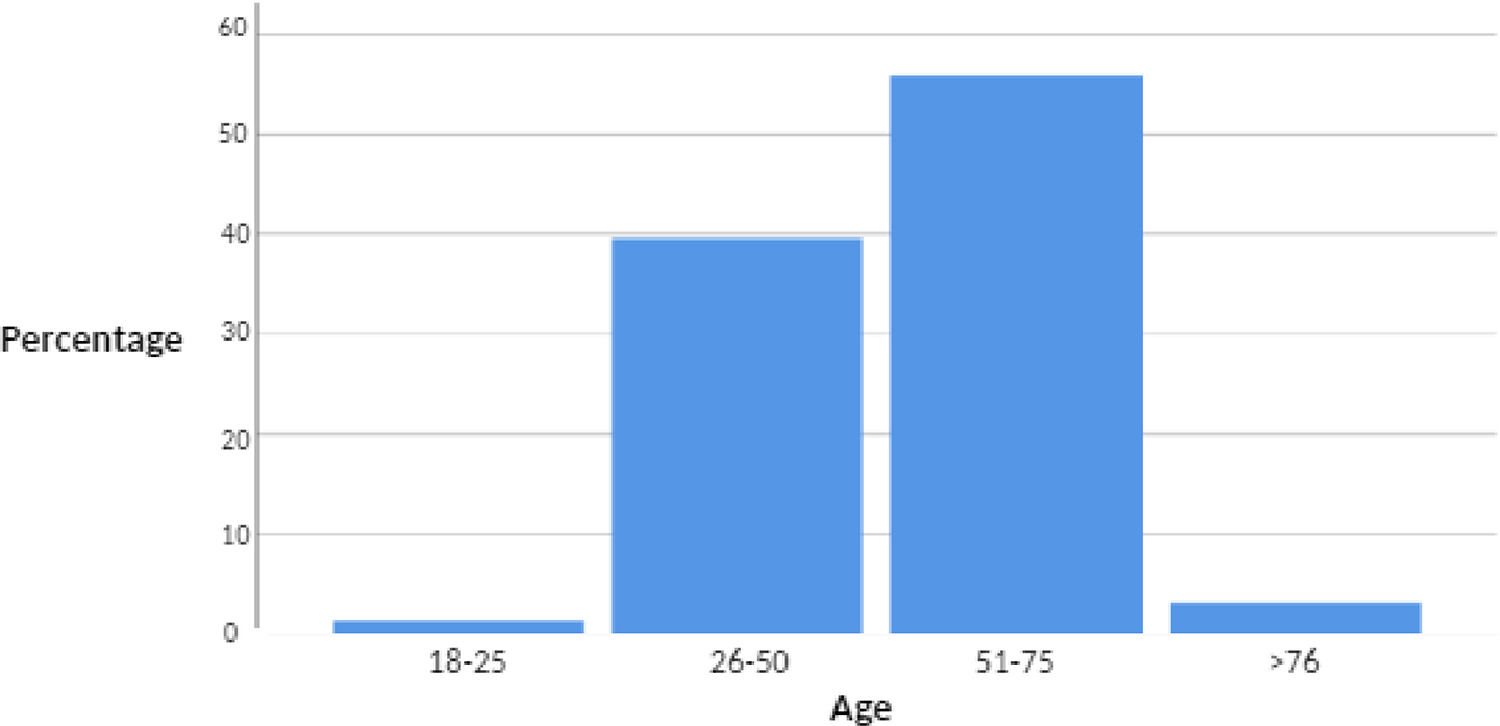
ResultsData from 222 treated patients was collected; a mean age of 53 years was reported, with a male-female ratio of 1:1. Among candidates for treatment with direct-acting antivirals 50.5% had advanced chronic liver disease at the time of diagnosis. Of these patients 76.1% were classified as a compensated chronic liver disease with a stage of Child Pugh A and 86.7% had a MELD-Na score of less than 14 points. The sustained virologic response rate in this population was 99%.
ConclusionsIt was observed that the collected treated population was on average in the sixth decade of life, with no gender predilection. Half of this population had advanced chronic liver disease at the time of diagnosis and initiation of treatment with direct antivirals. The majority of patients were in a compensated stage by Child Pugh, and showed a low MELD-Na score which was favorable for follow-up and subsequent management.
Ethical statement
The protocol was registered and approved by the Ethics Committee. The identity of the patients is protected. Consentment was obtained.
Declaration of interests
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Table 1. Prevalence of ages.
Table 2. Prevalence of Child-Pugh stage.