About one third of patients with chronic hepatitis C (HCV) have normal levels of aminotransferases (ALT).1 Most of these patients have mild disease with lower histological fibrosis, although some may progress to advanced fibrosis and cirrhosis.2,3
ALT’s are still the more accessible and inexpensive tests, but less sensitive, to determine the activity of liver disease. A single measurement provides limited information and there is a weak association between elevation and severity of histopathological fin-dings.1,3 Although an undetectable viral load (HCV RNA) is the key indicator of response to antiviral therapy, biochemical resolution with normalization of ALT for many authors is an important indicator of successful response.2,4
The epidemiological characteristics found in patients with HCV and persistently normal transa-minases (PNALT) are: mainly women (59-72%), asymptomatic, genotype 2 (43-52%), normal (1520%) or low degree of histological activity (26-65%) and cirrhosis (1%).2,4,5 The definition of PNALT has changed in time, initially considered normal for 6, and then 12 or even 18 months in 3 or more consecutive values.2,4,6
From the beginning it was found that interferons can cause elevations of ALT known as flares and which in a non-negligible proportion of patients remained abnormal after therapy. Therefore watchful waiting was suggested.1,4,5 The treatment of patients with PNALT has shown sustained viral response (SVR) rates that are comparable to those achieved by patients with elevated ALT, so these patients were included as candidates for treatment.5-7
In a prospective clinical study we evaluated the biochemical and virological response to treatment with Peginterferon alfa 2a and Ribavirin (according to genotype and weight) in 24 previously untreated patients satisfying strict inclusion criteria for PNALT (defined as ≥ 3 ALT determinations in the past 24 consecutive months without any elevation greater than the upper normal limit, 40 IU/L). The primary efficacy endpoint was sustained virological response (SVR); a secondary endpoint was any detection of treatment-related flares in ALT activity. The exclusion criteria and treatment dose were the same as for patients with elevated ALT.8 Patients with genotype 1 received treatment for 48 weeks and patients with genotype non-1 for 24 weeks.
Between 2003 and 2005 we identified 350 consecutive patients with anti-HCV positive of whom 61 had normal ALT (17.4%). Of the 61 patients, 37 had undetectable viral load, so were excluded. Of these 24 remaining, 19 were women and 5 men, 18 (75%) had transfusion history. The mean age was 45.5 (23-65 years), mean weight 67 kilograms and mean BMI 26.5 (19.6-33.2). All patients were of Latino ethnicity (self-identified as “Latino or Hispanic”) with Spanish as their primary language. Nineteen biopsies were obtained, 17 reported chronic hepatitis (3 with Fibrosis-3 per Knodell index) and cirrhosis was reported in 1 (5.2%). Eleven were genotype 1 (45.8%), twelve genotype 2 (50%) and one genotype 3. All patients who received at least one dose of medication were included in the study analysis. One patient was lost to follow up and one patient suspended therapy before week 20 because of adverse events; the rest of the patients were adherent to therapy. Patients in whom HCV RNA values were missing at the end of follow up were classified as nonresponders.
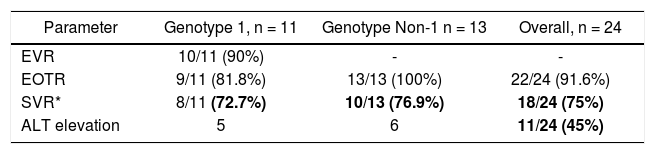
The early virological response (EVR) in genotype 1 was obtained in 10/11 patients (90%). The sustained virological response (SVR) was present in 72.7% (8/11) of genotype 1 patients and in 76.9% (10/13) of Non-1 genotype patients. The overall SVR was 75% (18/24) (Table 1). Eleven patients (45%) had elevated aminotransferase above 40 IU/L at some time during therapy. None of the elevations was greater than 100 IU/L. Of these eleven patients that elevated ALT, nine achieved an SVR and two relapsed.
Virological and biochemical response in patients with persistently normal ALT (PNALT).
| Parameter | Genotype 1, n = 11 | Genotype Non-1 n = 13 | Overall, n = 24 |
|---|---|---|---|
| EVR | 10/11 (90%) | - | - |
| EOTR | 9/11 (81.8%) | 13/13 (100%) | 22/24 (91.6%) |
| SVR* | 8/11 (72.7%) | 10/13 (76.9%) | 18/24 (75%) |
| ALT elevation | 5 | 6 | 11/24 (45%) |
* Not significant between genotype 1 vs. genotype non-1, Fisher exact test. EVR: early virological response. EOTR: end of treatment response. SVR: sustained virological response. ALT: alanine aminotransferase. PNALT: persistently normal aminotransferase.
Zeuzem, et al.9 in a multinational study described more than 400 patients with PNALT treated with PEG-INF + RBV in which SVR of 48 week arm (genotype 1) was 40 and 78% in genotype 2 or 3 without treatment-flares, results very similar to patients with pre-treatment abnormal ALT.
Puoti, et al.10 in a recent multicenter study report outcomes of 88 PNALT patients treated with PEG-INF + RBV, 52% were genotype 2, 60% were women, with mean BMI of 23.8 and the majority had grade 1 fibrosis. The overall SVR was 78% (62% in genotype 1 and 89% and 80% in genotypes 2 and 3 respectively). Any patient had elevated ALT during treatment. They concluded that treatment success in patients with PNALT is comparable or even better than that obtained in patients with elevated ALT.10
The results of this study are very similar to ours, both with the relevant feature that SVR of genotype 1 was better than the commonly reported (40-45%) in patients with elevated ALT in the U.S. and Euro-pe7 or Latino patients (39-42%).8 It is very likely that the cause of this outcome was a sub-selection of patients with better response factors such as lower BMI, female gender, good adherence, and overall less fibrosis. More intriguing could be the response in relation to IL-28B genotype, speculating that our group could be more of the C/C genotype who are more likely to achieved SVR, even though in Mexico is more prevalent (60%) the C/T genotype.11
AASLD guidelines12 indicate that regardless of the level of ALT, the decision to treat should be individualized and the regimen in PNALT should be the same as for patients with elevated ALT.12 Latin American guidelines13 recommend that treatment indications should be evaluated independently of ALT activity and treatment should be the same as with elevated ALT.13 The most recent European gui-delines14 briefly indicate that patients with repeatedly normal ALT should be evaluated to determine the severity of their disease and possible candidates could be considered regardless of baseline ALT level.14
Patients who are easy to treat or who have favorable prognostic factors may be treated for their high chance of success as well as patients with advanced fibrosis because their progression risk, regardless of the level of ALT. Finally, it is questioned if the previously established reference values to define “normal” levels of ALT are really accurate, in most laboratories the cutoff level is defined as twice the normal limit of high-normal range of healthy individuals being < 30 IU/L for men and < 19 IU/L for women.15 It would be of interest to determine whether use of these cutoffs have better sensitivity for identifying patients with true histo-logic activity.
AcknowledgementsWe appreciate the help of Dr. Raymond T. Chung, of the Gastrointestinal Unit of Massachusetts General Hospital (MGH), in revising this manuscript.
Not significant between genotype 1 vs. genotype non-1, Fisher exact test. EVR: early virological response. EOTR: end of treatment response. SVR: sustained virological response. ALT: alanine aminotransferase. PNALT: persistently normal aminotransferase.