Background. The evaluation of liver disease in HIV patients is cumbersome because may result from a number of different causes. The aim of this retrospective study was to estimate the incidence of severe drug induced liver injury (DILI) in a group of HIV inpatients and investigate potential risk factors.
Material and methods. We performed a retrospective analysis of data from HIV-infected patients hospitalized between August 2010 and August 2011 in a tertiary hospital in São Paulo, Brazil. Severe hepatotoxicity was defined as grade 3 (5.1 to 10 × ULN) or 4 (> 10 × ULN) of ALT and AST levels. Factors analyzed included demographics, infection with hepatitis viruses, alcohol history and use of hepatotoxic drugs prior to or during hospital admission.
Results. A total of 149 patients with HIV were hospitalized during the study period. The majority were male over 42 years of age and 82 (55%) were taking HAART initiated prior to admission. Mean CD4 counts were 164 cells/mm3. Thirty three patients (22.1%) developed severe DILI during hospital stay, which had a mean duration of 26 days. Factors associated with severe DILI in the multivariate analysis were abnormal baseline ALT levels [OR 2.02 (95%CI 1.13-3.59); p = 0.017] and tuberculosis therapy [OR 2.31 (95% CI 1.27-4.19); p = 0.006]. In conclusion, in this group of HIV patients admitted to a tertiary hospital in Brazil, we found a high incidence (22.1%) of severe DILI. The use of anti-tuberculosis drugs and baseline liver injury were independent factors associated with severe DILI during hospital stay.
Since the introduction of HAART in 1996,1 life highly active antiretroviral therapy expectancy of HIV patients has increased and the causes of morbidities observed in these patients have changed over the years. It has been observed that hepathopaties, cardiovascular diseases, metabolic disturbances, drug associated toxicity, cancer, and kidney diseases, among others, have become more frequent among these patients.2–5
Liver injury, as reflected in elevation of liver enzymes, is not an uncommon occurrence in the HIV-infected patient. There are numerous studies on DILI after initiation of antiretroviral therapy, which primarily takes place in the ambulatory setting.6 However, little attention has been paid to the development of DILI in the patients with advanced HIV infection who are admitted to the hospital. As multiple factors potentially leading to liver enzyme elevation concur in this setting causality assessment can be very challenging. Thus, opportunistic and non-opportunistic infections, hepatitis A, B (HBV) or C (HCV), use of alcohol and recreational drugs, infiltrating tumors and infections, sepsis, parenteral nutrition, hepatotoxic drugs (statins, anticonvulsants, psychiatric drugs, antibiotics, antiretrovirals, etc.) and metabolic, auto-immune and hepatobilliary diseases, are among processes possibly causing liver injury.7–9
Abnormal levels of transaminases in hospitalized HIV-infected patients lead to additional diagnostic studies and increase in lengths of stay and costs. Moreover, an association between elevated transaminases and death has been previously reported in HIV-infected patients.10,11
The first objective of this study was to determine the incidence of severe DILI in a group of HIV/AIDS inpatients, most of them with advanced HIV disease, admitted to the Infectious Diseases Service at a tertiary hospital in São Paulo, Brazil. The second objective was to identify the factors associated with severe DILI in this setting.
Material and MethodsStudy design and patient selectionThis is a retrospective study in which hospital admission records were reviewed. The charts of all patients with a diagnosis of HIV infection as defined by anti-HIV antibody positive confirmed by Western Blot, who were hospitalized for at least two days in Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo between 1 August 2010 and 1 August 2011 were reviewed. This study was conducted with the approval of the local Ethics Committees.
Clinical data recordedData recorded included demographics and HIV data: age, gender, HIV risk factor, estimated duration of HIV infection, use of HAART, treatment compliance, and HIV RNA levels and CD4 lymphocyte counts at the time of admission.
We also recorded: pertinent co-morbidities such as HBV and/or HCV infection, metabolic diseases (comprises interrelated disease states including obesity, insulin resistance and type 2 diabetes, dyslipidemia, and hypertension), use of hepatotoxic drugs prior to or during hospital stay, history of alcohol abuse consumption (defined as ingestion of quantities > 50 g of alcohol per day within six months prior to admission), discharge diagnosis, abdominal image findings (ultrasound, computed tomography or magnetic resonance imaging).
HBV chronic infection was defined by the presence of HBs antigen and HCV chronic infection by the presence of HCV RNA by PCR.
We considered as potentially hepatotoxic drugs those which have high risk of liver damage according to the literature: rifampicin, isoniazid, pyrazinamide, sulfa drugs, statins, imidazole, anticonvulsant, nonsteroidal anti-inflammatory drugs, and macrolides.7,8,12 All antiretroviral drugs were also considered potentially hepatotoxic.
Laboratory data and definition of DILILiver tests including ALT (alanine aminotransferase), AST (aspartate aminotransferase) and bilirubin were requested by attending physician according to clinical judgment. Usually, enzymes were taken every two or three days and in some cases daily, especially when patient experienced elevations in transaminases.
Baseline ALT and AST were defined as ALT and AST measurements at the time of hospital admission.
The analysis and levels of ALT elevations and AST above the ULN (upper limit of normal) followed the classification:13
- •
Grade 1 (1.25 to 2.5 x ULN).
- •
Grade 2 (2.6 to 5 x ULN).
- •
Grade 3 (5.1 to 10 x ULN).
- •
Grade 4 (> 10 x ULN).
Changes in total bilirubin were outlined as:13
- •
Grade 1 (1.1 to 1.5 x ULN).
- •
Grade 2 (1.6 to 2.5 x ULN).
- •
Grade 3 (2.6 to 5.0 x ULN).
- •
Grade 4 (> 5.0 x ULN).
Severe DILI was defined as ALT or AST levels increased to grade 3 or 4 during treatment.7,14
We adopted the upper normal limits established by the local laboratory: 37 AST U/L for men and 31 U/L for women; ALT at 41 g/dL for men and 31 U/L for women; and total bilirubin at 1 mg/dL.
Statistical analysisData were reported as absolute values and percentages for categorical and as mean and range for continuous variables. For identification of DILI risk factors we first performed the analysis of the frequency distribution of each of the studied variables. We then estimated prevalence ratio (PR) of different variables and confidence intervals (95%). The variables with p value < 0.25 by the Wald test were selected for multivariate analysis. Regardless of statistical significance, the variable “use of hepatotoxic drugs during hospitalization” was included in the final model because of its clinical relevance. Cox regression model with robust variance was adopted. Variables with p value < 0.05 in multivariate analysis were considered statistically significant. Data were analyzed using Stata 11.0 (StataCorp LP, College Station, Texas, United States).
ResultsA total of 149 patients with HIV infection were hospitalized during the studied period to the Infectious Diseases Service for any reason.
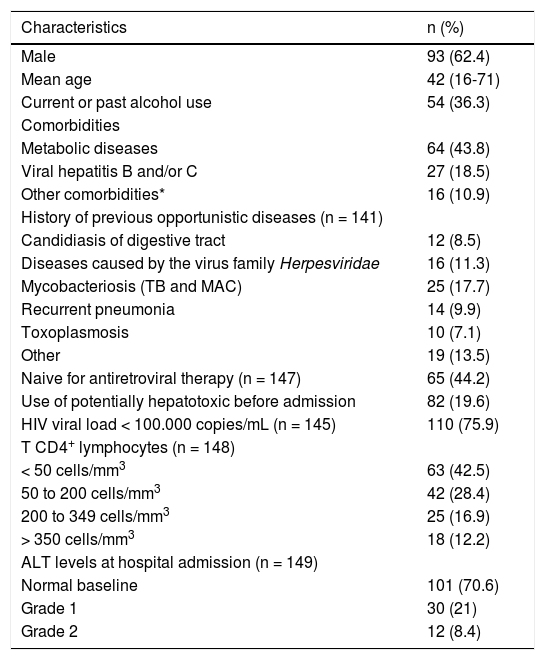
The mean age was 42 (16-71) years and the majority of the patients were male over 40 years of age, with very advanced HIV infection. Thirty-four (23.3%) patients had no previous diagnosis of HIV and were diagnosed while hospitalized. The characteristics of the patients at the time of hospital admission are summarized in table 1.
Characteristics of the 149 HIV/AIDS patients at the time of hospital admission.
| Characteristics | n (%) |
|---|---|
| Male | 93 (62.4) |
| Mean age | 42 (16-71) |
| Current or past alcohol use | 54 (36.3) |
| Comorbidities | |
| Metabolic diseases | 64 (43.8) |
| Viral hepatitis B and/or C | 27 (18.5) |
| Other comorbidities* | 16 (10.9) |
| History of previous opportunistic diseases (n = 141) | |
| Candidiasis of digestive tract | 12 (8.5) |
| Diseases caused by the virus family Herpesviridae | 16 (11.3) |
| Mycobacteriosis (TB and MAC) | 25 (17.7) |
| Recurrent pneumonia | 14 (9.9) |
| Toxoplasmosis | 10 (7.1) |
| Other | 19 (13.5) |
| Naive for antiretroviral therapy (n = 147) | 65 (44.2) |
| Use of potentially hepatotoxic before admission | 82 (19.6) |
| HIV viral load < 100.000 copies/mL (n = 145) | 110 (75.9) |
| T CD4+ lymphocytes (n = 148) | |
| < 50 cells/mm3 | 63 (42.5) |
| 50 to 200 cells/mm3 | 42 (28.4) |
| 200 to 349 cells/mm3 | 25 (16.9) |
| > 350 cells/mm3 | 18 (12.2) |
| ALT levels at hospital admission (n = 149) | |
| Normal baseline | 101 (70.6) |
| Grade 1 | 30 (21) |
| Grade 2 | 12 (8.4) |
ALT: alanine aminotransferase. MAC: Mycobacterium avium complex. ULN: upper limit of normal. TB: tuberculosis. *Other comorbidities: immune diseases, neoplasia, mental health pathologies.
Twenty eight (19.6%) patients were using potentially hepatotoxic drugs prior to hospitalization.
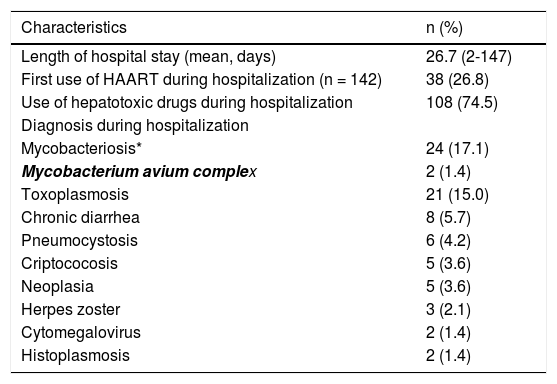
Results related to the hospital course are summarized in table 2.
Data related to the hospital course of the 149 HIV/AIDS patients included in the study.
| Characteristics | n (%) |
|---|---|
| Length of hospital stay (mean, days) | 26.7 (2-147) |
| First use of HAART during hospitalization (n = 142) | 38 (26.8) |
| Use of hepatotoxic drugs during hospitalization | 108 (74.5) |
| Diagnosis during hospitalization | |
| Mycobacteriosis* | 24 (17.1) |
| Mycobacterium avium complex | 2 (1.4) |
| Toxoplasmosis | 21 (15.0) |
| Chronic diarrhea | 8 (5.7) |
| Pneumocystosis | 6 (4.2) |
| Criptococosis | 5 (3.6) |
| Neoplasia | 5 (3.6) |
| Herpes zoster | 3 (2.1) |
| Cytomegalovirus | 2 (1.4) |
| Histoplasmosis | 2 (1.4) |
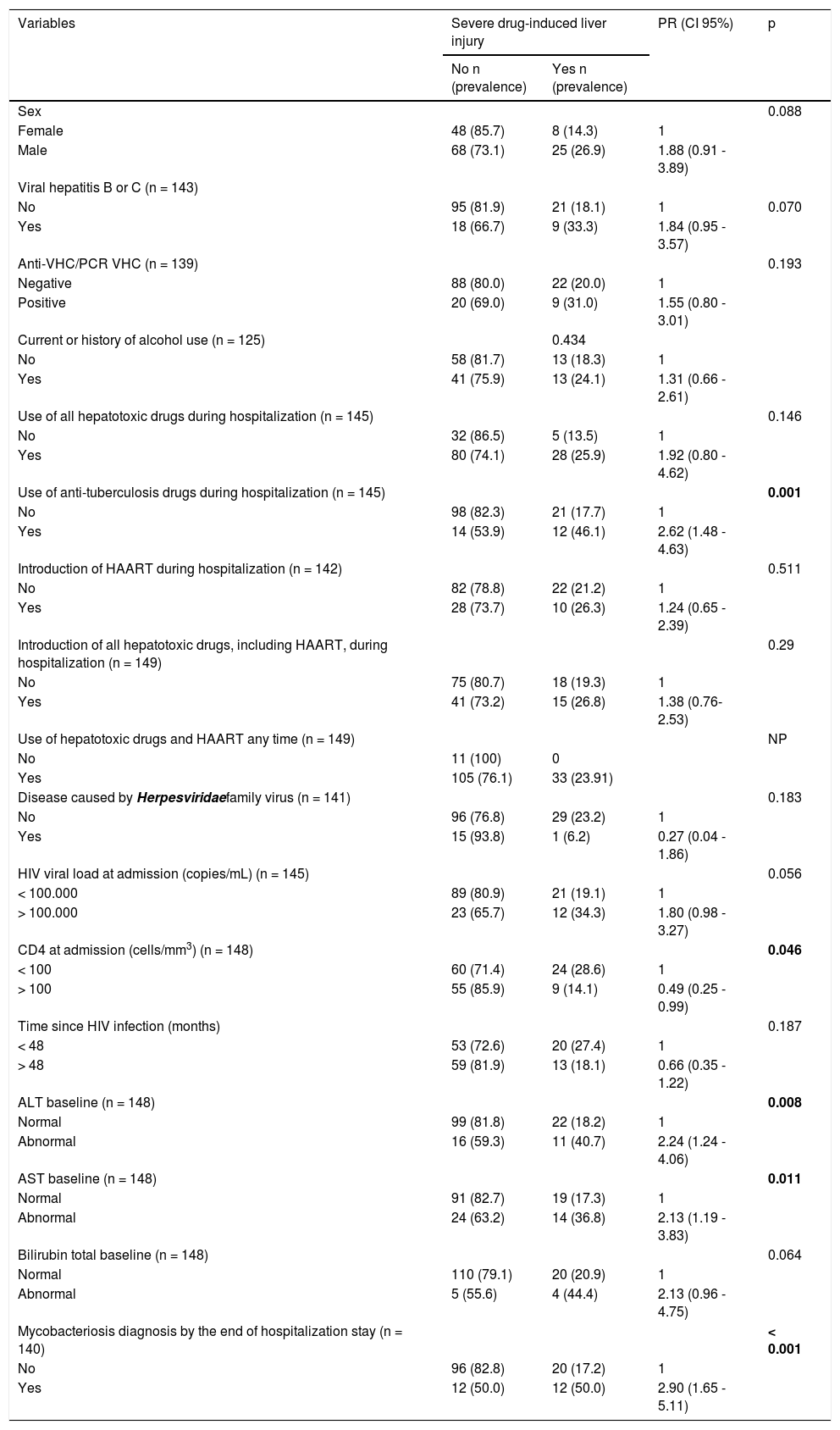
Thirty-three patients (22.1%; CI 95%: 15.8-29.7) developed severe DILI during hospitalization.The majority of cases of DILI was diagnosed during the first two weeks of hospitalization (range 2-40 days). In the univariate analysis, a higher incidence of severe DILI was observed among patients with abnormal ALT and AST levels at hospital admission (p = 0.008 and p = 0.011, respectively), patients with diagnosis of mycobacterial infection and the use of anti-tuberculosis drugs during hospitalizations (p < 0.001 and p = 0.001, respectively), CD4 < 100 cells/mm3 (p = 0.046), patients of male sex (p = 0.09), with HIV RNA > 100,000 copies/mL (p = 0.056), with HBV or HCV infection (p = 0.07) and exposed to hepatotoxic medication during hospitalization (p = 0.1) (Table 3).
Factors associated with severe liver damage during hospitalization. Univariate analysis.
| Variables | Severe drug-induced liver injury | PR (CI 95%) | p | |
|---|---|---|---|---|
| No n (prevalence) | Yes n (prevalence) | |||
| Sex | 0.088 | |||
| Female | 48 (85.7) | 8 (14.3) | 1 | |
| Male | 68 (73.1) | 25 (26.9) | 1.88 (0.91 - 3.89) | |
| Viral hepatitis B or C (n = 143) | ||||
| No | 95 (81.9) | 21 (18.1) | 1 | 0.070 |
| Yes | 18 (66.7) | 9 (33.3) | 1.84 (0.95 - 3.57) | |
| Anti-VHC/PCR VHC (n = 139) | 0.193 | |||
| Negative | 88 (80.0) | 22 (20.0) | 1 | |
| Positive | 20 (69.0) | 9 (31.0) | 1.55 (0.80 - 3.01) | |
| Current or history of alcohol use (n = 125) | 0.434 | |||
| No | 58 (81.7) | 13 (18.3) | 1 | |
| Yes | 41 (75.9) | 13 (24.1) | 1.31 (0.66 - 2.61) | |
| Use of all hepatotoxic drugs during hospitalization (n = 145) | 0.146 | |||
| No | 32 (86.5) | 5 (13.5) | 1 | |
| Yes | 80 (74.1) | 28 (25.9) | 1.92 (0.80 - 4.62) | |
| Use of anti-tuberculosis drugs during hospitalization (n = 145) | 0.001 | |||
| No | 98 (82.3) | 21 (17.7) | 1 | |
| Yes | 14 (53.9) | 12 (46.1) | 2.62 (1.48 - 4.63) | |
| Introduction of HAART during hospitalization (n = 142) | 0.511 | |||
| No | 82 (78.8) | 22 (21.2) | 1 | |
| Yes | 28 (73.7) | 10 (26.3) | 1.24 (0.65 - 2.39) | |
| Introduction of all hepatotoxic drugs, including HAART, during hospitalization (n = 149) | 0.29 | |||
| No | 75 (80.7) | 18 (19.3) | 1 | |
| Yes | 41 (73.2) | 15 (26.8) | 1.38 (0.76-2.53) | |
| Use of hepatotoxic drugs and HAART any time (n = 149) | NP | |||
| No | 11 (100) | 0 | ||
| Yes | 105 (76.1) | 33 (23.91) | ||
| Disease caused by Herpesviridaefamily virus (n = 141) | 0.183 | |||
| No | 96 (76.8) | 29 (23.2) | 1 | |
| Yes | 15 (93.8) | 1 (6.2) | 0.27 (0.04 - 1.86) | |
| HIV viral load at admission (copies/mL) (n = 145) | 0.056 | |||
| < 100.000 | 89 (80.9) | 21 (19.1) | 1 | |
| > 100.000 | 23 (65.7) | 12 (34.3) | 1.80 (0.98 - 3.27) | |
| CD4 at admission (cells/mm3) (n = 148) | 0.046 | |||
| < 100 | 60 (71.4) | 24 (28.6) | 1 | |
| > 100 | 55 (85.9) | 9 (14.1) | 0.49 (0.25 - 0.99) | |
| Time since HIV infection (months) | 0.187 | |||
| < 48 | 53 (72.6) | 20 (27.4) | 1 | |
| > 48 | 59 (81.9) | 13 (18.1) | 0.66 (0.35 - 1.22) | |
| ALT baseline (n = 148) | 0.008 | |||
| Normal | 99 (81.8) | 22 (18.2) | 1 | |
| Abnormal | 16 (59.3) | 11 (40.7) | 2.24 (1.24 - 4.06) | |
| AST baseline (n = 148) | 0.011 | |||
| Normal | 91 (82.7) | 19 (17.3) | 1 | |
| Abnormal | 24 (63.2) | 14 (36.8) | 2.13 (1.19 - 3.83) | |
| Bilirubin total baseline (n = 148) | 0.064 | |||
| Normal | 110 (79.1) | 20 (20.9) | 1 | |
| Abnormal | 5 (55.6) | 4 (44.4) | 2.13 (0.96 - 4.75) | |
| Mycobacteriosis diagnosis by the end of hospitalization stay (n = 140) | < 0.001 | |||
| No | 96 (82.8) | 20 (17.2) | 1 | |
| Yes | 12 (50.0) | 12 (50.0) | 2.90 (1.65 - 5.11) | |
ALT: alanine aminotransferase. AST: aspartate aminotransferase. PR: prevalence ratio. VHC: hepatitis C virus. NP: not possible.
All variables with p value < 0.25 by the Wald test in the univariate analysis were selected for multivariate analysis: diagnosis of mycobacterial infection (p < 0.001), abnormal ALT and AST levels (p = 0.008), CD4 < 100 cells/mm3 (p = 0.046), HIV viral load < 100.000 copies/mL (p = 0.056), viral hepatitis B and/or C (p = 0.070), male sex (p = 0.088), use of potentially hepatotoxic before admission (p = 0.089), use of potentially hepatotoxic any time (p = 0.115) and diseases caused by the virus family Herpesviridae (p = 0.183).
In the multivariate analysis, after adjusting for the use of hepatotoxic drugs and HAART during hospitalization [OR 1.12 (95% CI 0.62-2.03); p = 0.705], we found that development of severe DILI, was independently associated only with abnormal levels of ALT at hospital admission [OR 2.02 (95% CI 1.13-3.59); p = 0.017] and with use of anti-tuberculous drugs during hospital stay [OR 2.31 (95% CI 1.27-4.19); p = 0.006].
Eleven (7.4%) patients died, but all from causes unrelated to hepatotoxicity.
DiscussionThere are multiple studies that have evaluated DILI in HIV-infected patients in the ambulatory setting, but to our knowledge, this is the first study of its kind, where DILI of new onset was assessed in HIV-infected after admission to the hospital for any reason. We observed a high incidence of DILI in the studied population, above a fifth of the patients analyzed. This is relevant as among individuals negative for markers of viral hepatitis B and C, elevated ALT above normal limits was associated with liver mortality in a large death certificate-based study.15
The leading causes of hospitalization in this group of patients were opportunistic infections, and among the invasive ones, bacterial were the most common followed by tuberculosis and toxoplasmosis. The high frequency of tuberculosis as cause of hospitalization in our population is in line with other reports coming from countries where the prevalence this infection in the general population is moderate or high.16,17
As shown in studies evaluating DILI in HIV patients treated with HAART,6,18,19 we observed that patients with abnormal enzyme levels at baseline had a higher risk of developing severe DILI during hospitalization. However, in those reports, HCV-coinfection was the most frequent cause of abnormal ALT at baseline, while the prevalence of this infection in our population was relatively low. Therefore, the cause of abnormal transaminases in our report could be explained by other causes, including liver infiltration of mycobacteria. Supporting this is the high frequency of hepatomegaly and splenomegaly. Additionally, alcohol consumption could have also contributed to increase liver enzyme levels.
Also consistent with studies performed in the ambulatory setting, concomitant use of tuberculosis therapy was independently associated with severe DILI.20
However, our severe DILI rate is higher than that estimated in patients with HIV seen in the ambulatory setting from areas with high tuberculosis prevalence21,22 and similar to that reported in HIV patients treated for tuberculosis at the hospital.23 Several studies suggest that liver injury induced by anti-tuberculosis drugs develops more frequently in HIV-coinfected patients.23,24 It is unknown if it is due to HIV-related immunosuppression or to other comorbidities that are often present in patients with advanced HIV. Noteworthy is the fact that the diagnosis of HIV infection was made during hospitalization in more than one fifth of the patients, and that the majority of the patients were profoundly immunosuppressed with a very high HIV load. But while in univariate analysis we observed an association between DILI and CD4 ≤ 100 cells/mm3 and high HIV viral load, they lost statistical significance in multivariate analysis. However, other authors have observed higher incidence of DILI among patients with low CD4 counts.25,26
In our study, we did not find a significant association between the introduction of HAART during hospitalization and elevation of liver enzymes. Maybe the explanation for this is the fact that nowadays we are using less hepatotoxic drugs than we did in the past. Another point that should be taken into consideration is our sample size. It could be reasonable to think that the small number patients included in our series was not adequate to identify a possible association between the introduction of HAART and elevation of liver enzymes.
Unlike what other authors have previously reported,27,28 in our study, decompensated liver disease, hepatocellular carcinoma, and viral hepatitis were not the main causes of hospitalization among these patients. Rather, we found mycobacteriosis and toxoplasmosis, which are classic opportunistic infections commonly diagnosed in advanced stages of immunosuppression, as the main causes of hospitalization. This finding may suggest that despite the great availability of drugs and care to people living with HIV in Brazil, the control of the epidemics is still inadequate, overshadowing these other hepatic morbidity.
When analyzing our data, it should be taken in consideration that our service is a tertiary and reference hospital for these patients and therefore, the population attended at our service may not be representative of the HIV population living in our country.
In our study, the high frequency of DILI was associated with presence of mycobacteriosis with use of anti-tuberculosis drugs in patients with very HIV advanced disease and elevated liver enzymes at the time of admission.
We believe that we could improve outcome through early diagnosis and treatment of HIV infection and tuberculosis. While consistent with other studies there were no deaths related to hepatotoxicity itself,23 the elevation of liver enzymes led to additional diagnostic tests and increases in length of stay, therefore increasing the costs.
ConclusionsIn this group of HIV patients in Brazil, we found a high prevalence (22.1%) of DILI. We found that the use of anti-tuberculosis drugs at admission and altered ALT levels at baseline were independently associated with the risk of DILI.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
DILI: drug induced liver injury.
- •
HAART: highly active antiretroviral therapy.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
NP: not possible.
- •
PCR: polymerase chain reaction.
- •
PR: prevalence ratio.
- •
ULN: upper limit of normal.
This manuscript was reviewed by a professional science editor and by a native English-speaking copy editor to improve readability.
The authors declare that they have no competing interests related to this study.
AcknowledgementsWe thank Patricia Braga for assistance during the statistical analysis.
Author’S ContributionLGMMT, MCMC and MN designed the study. LGMMT and MCMC contributed to clinical data. LGMMT and MCMC checked the clinical data for patients. LGMMT participated in data collection. LGMMT, MCMC and MN wrote the manuscript. All authors reviewed the draft and approved the final version.
Financial SupportThis article was prepared without financial support from institutions for research funding.