This research aims to evaluate the efficacy and safety of prophylactic antibiotics in patients with alcohol-related liver disease (ALD).
Materials and MethodsWe systematically searched databases including PubMed, Embase, Cochrane, and Web of Science up to October 2023. Our scope encompassed the influence of prophylactic antibiotics on all-cause mortality, infection, variceal bleeding, hepatic encephalopathy (HE), hepatorenal syndrome (HRS), adverse events (AE), fungal infection, clostridioides difficile infection (CDI), and multidrug-resistant (MDR) bacterial infection. Additionally, total bilirubin, creatinine, platelet counts, and plasma endotoxin levels were also analyzed.
ResultsAfter comprehensive selection, 10 studies with 974 participants were included for further analysis. The study demonstrated that prophylactic antibiotic therapy was associated with reductions in infection rates, HE incidence, variceal bleeding, and all-cause mortality. The treatment did not increase the incidence of AE, fungal infection, and CDI, but it did raise the MDR bacteria infection rate. The analysis revealed no significant protective effect of antibiotic prophylaxis on total bilirubin and creatinine levels. Furthermore, the administration of antibiotics led to marginal increases in platelet counts, a minor reduction in endotoxin concentrations, and a subtle enhancement in HRS; however, these changes did not reach statistical significance.
ConclusionsProphylactic antibiotic therapy was an effective and safe treatment for advanced ALD. To mitigate the risk of MDR bacterial infections, a strategy of selective intestinal decontamination could be advisable. Future investigations should prioritize varied ALD patient populations with extended follow-up periods and assorted antibiotic regimens to solidify the efficacy and safety of ALD treatments.
In 2018, the World Health Organization estimated that alcohol consumption was a contributing factor in approximately three million fatalities worldwide [1]. Additionally, over 75 million individuals have been identified with alcohol use disorder, placing them at an elevated risk of developing alcohol-related liver disease (ALD) [2]. ALD encompasses a continuum of hepatic conditions that include fatty liver, steatohepatitis, progressive fibrosis, cirrhosis and its associated complications, and potentially hepatocellular carcinoma. Among those affected by ALD, a subset may experience alcoholic hepatitis (AH)—the most critical form of ALD, characterized by rapid onset jaundice and a precipitous decline in hepatic function, which notably escalates mortality risk [3]. The incidence of AH is on an upward trajectory, with a staggering 50% of patients succumbing within eight weeks of onset [4]. Thus, effective interventions are urgent to ameliorate adverse outcomes.
Excessive alcohol consumption can increase bacterial populations and cause gut permeability impairment, which facilitates the translocation of bacterial products such as lipopolysaccharides (LPS), endotoxins, bacterial DNA, and pathogen-associated molecular patterns (PAMPs) into the portal circulation [5]. Toxic metabolites such as PAMPs can stimulate Kupffer cells to elicit proinflammatory molecules and initiate inflammatory cascades, which leads to hepatocellular injury [6]. The relationship between gut and liver, or gut–liver axis, is pivotal in the pathogenesis of ALD. Therapeutic strategies, including the administration of antibiotics and probiotics, aim at modulating this axis to combat ALD. Antibiotics can reduce bacterial populations, decrease LPS release, and diminish the associated inflammatory responses [5]. Numerous studies in recent years have explored the efficacy of prophylactic antibiotics in reducing mortality, infection rates, and complications associated with ALD. For instance, rifaximin has been reported to lower endotoxemia and improve alcoholic cirrhosis-related thrombocytopenia [7], as well as decrease the incidence of infections, liver-related complications, and mortality rates [8]. In contrast, a study involving 284 patients with severe AH demonstrated that combining amoxicillin-clavulanate with prednisolone did not enhance two-month survival rates when compared to sole prednisolone treatment, although it did substantially decrease infection rates [9]. Therefore, the use of prophylactic antibiotics to treat ALD remains controversial.
Although a multitude of randomized controlled trials (RCTs) and cohort investigations have probed the role of prophylactic antibiotics in combating ALD, the field lacks a holistic, systematic review and meta-analysis focused on appraising the efficacy and safety profiles of such interventions in patients with ALD. To bridge this gap, we conducted an exhaustive systematic review and meta-analysis, incorporating the most recent scholarly contributions to furnish contemporary evidence-based insights for the management of ALD.
2Material and Methods2.1Literature searchThis study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (Supplementary Table S1). Our study's protocol garnered registration within the International Prospective Register of Systematic Reviews (PROSPERO), bearing the identifier CRD42024497645. Relevant articles were searched electronically by using Embase, PubMed, Cochrane Central Register of Controlled Trials, and Web of Science up to October 2023. The search harnessed both MeSH terms and text keywords, specifically: (“Hepatitis, Alcoholic” OR “Liver Diseases, Alcoholic”) AND (“Anti-Bacterial Agents”). A comprehensive presentation of our search strategy can be found in Supplementary Table S2. All included studies were evaluated independently by two researchers, Xiuyan Wang and Endian Zheng, with any contentions in study selection resolved by the arbitration of a third reviewer, Yi Huang.
2.2Identification and selection of eligible studiesThe inclusion criteria for this study were as follows: (1) studies adopting randomized controlled, cohort, or case-control designs; (2) patients aged over 18 years with a medical diagnosis of ALD, including AH, alcoholic cirrhosis (AC); (3) a confirmed diagnosis of AH or AC, substantiated by liver biopsy or clinical and laboratory assessments, coupled with a history of excessive alcohol consumption and the absence of mechanical or obstructive causes of cholestasis. Additionally, most of the candidates were screened for the presence of hepatitis B surface antigen or RNA of the hepatitis C virus, autoimmune liver diseases, and liver tumors; (4) intervention of antibiotics was primarily given before infection. (5) publications providing adequate data enabling the computation of odds ratios (ORs) or weighted mean differences (WMDs). (6) articles were published in the English language. We excluded non-English texts, reviews, case reports, editorials, letters, conference abstracts, and any works not formally published.
2.3Data extraction and quality assessmentAssessment of quality, level of evidence, and data extraction were carried out independently by two researchers, Xiuyan Wang and Endian Zheng, with any divergences reconciled through consultation with a third reviewer, Yi Huang. The following data were extracted: (1) study characteristics (first author, year of publication, research duration, country where the study was conducted, design of the study, population, sample size, age, registration number of RCTs, and mean follow-up); (2) intervention characteristics (type of drugs, duration of treatment, dosage, and any additional treatments given as an adjunct); (3) Outcomes of interest included all-cause mortality, infection, any adverse event, the change of the liver function, the incidence rate of each complication including variceal bleeding, HE, HRS between antibiotic group versus control group. All-cause mortality was the primary outcome; the others were secondary outcomes. Continuous variables presented as median with range, standard error, or interquartile range were converted into means ± standard deviations via statistical formulas [10,11]. The risk of bias within RCTs and cohort studies was appraised by employing the Cochrane [12] and Newcastle–Ottawa Scale (NOS) [13], respectively.
2.4Statistical analysisStatistical analysis was performed in the Review Manager 5.4 version (Cochrane Collaboration, Oxford, UK). Dichotomous outcomes were displayed as ORs and 95 % confidence intervals (CIs), while continuous variables were expressed as WMDs and 95 % CI. Statistical heterogeneity was evaluated by the chi-squared (χ2) test (Cochran's Q) and inconsistency index (I2) [14]. In cases where substantial heterogeneity was identified (χ2p value 〈 0.05 or I2〉 50 %), a random-effect model was utilized to aggregate WMDs or ORs. Conversely, the fixed-effect model was applied. Subgroup analyses were carried out to pinpoint potential sources of heterogeneity among the studies based on factors like study design, duration of follow-up, geographical region, the type of antibiotics and population. A leave-one-out sensitivity analysis was executed to assess whether any single study disproportionately influenced the aggregated results. Egger's regression tests (Stata 15 ver, Stata Corp College Station, TX, USA) [15] and funnel plots (Review Manager 5.4 version) were applied to assess publication bias. A p-value threshold of <0.05 was set for determining statistical significance.
2.5Ethical declarationsAll analyses were based on previously published studies; thus, no ethical approval or patient consent is required.
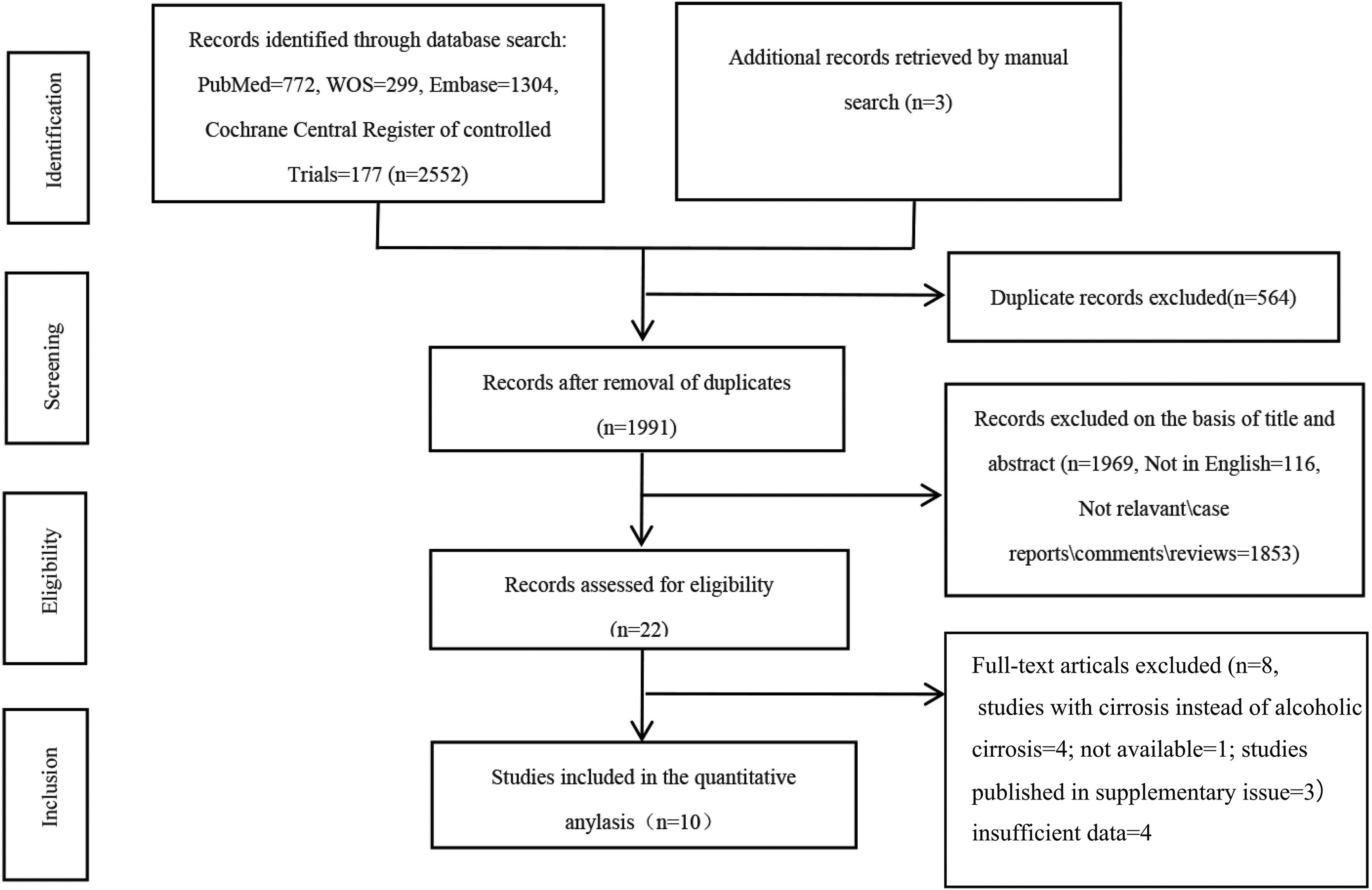
3Results3.1Study search and selectionThe flow of study identification and selection is illustrated in Fig. 1. Our initial search yielded a total of 2555 articles, from which 2552 were collected from four databases— PubMed (n = 772), Web of Science (n = 299), Embase (n = 1304), Cochrane Central Register of Controlled Trials (n = 177)— and three additional records retrieved by manual search. After duplicates were eliminated (n = 564), we meticulously screened 1991 titles and abstracts. This led to a full-text review of 22 articles, culminating in the inclusion of 10 studies encompassing 974 participants for the subsequent meta-analysis.
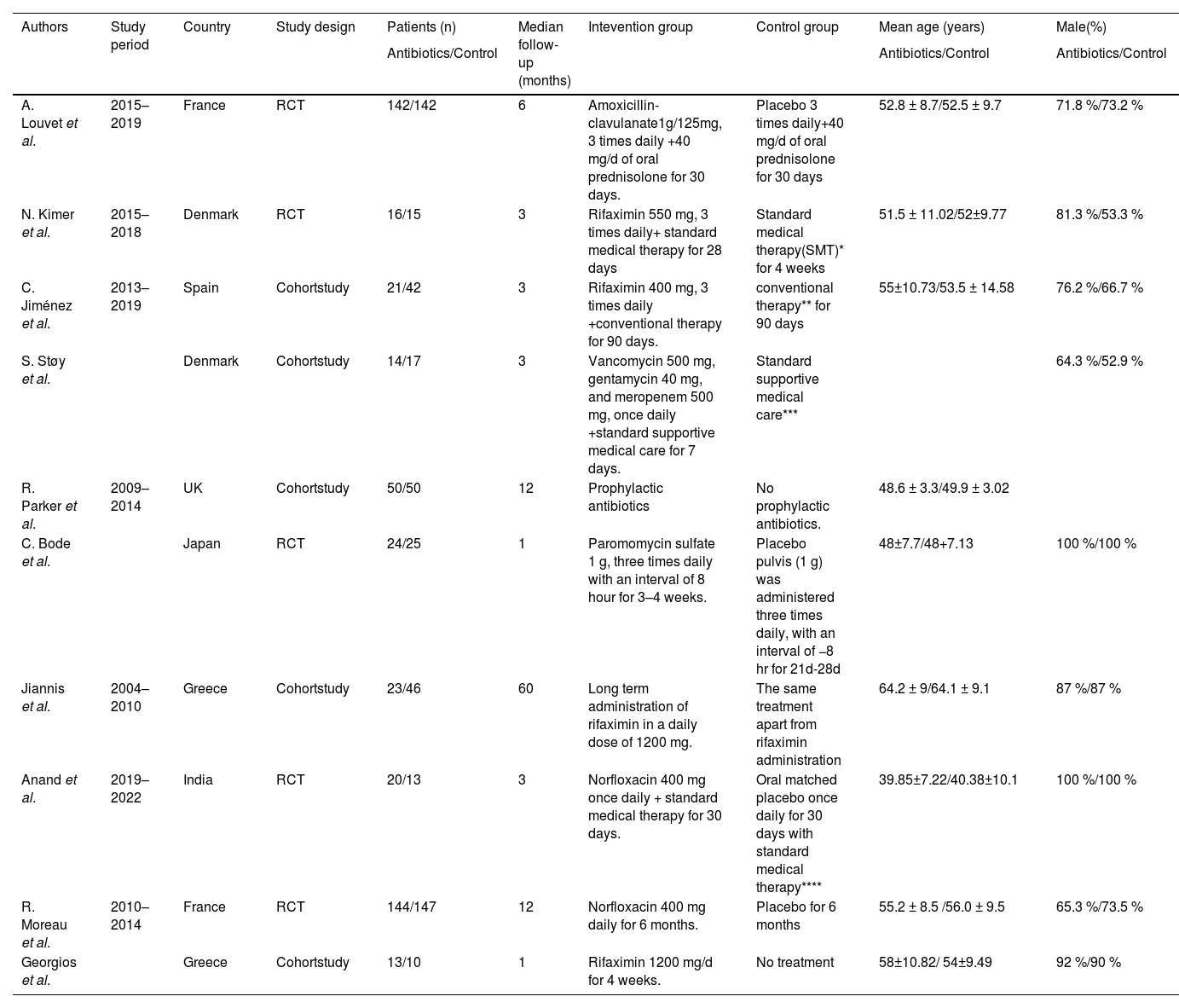
3.2Study characteristics and baseline characteristicsThe study demonstrated that half of the studies [7,8,16-18] were regarded as high quality. The details of the quality assessment are presented in Supplementary Table S3 and Supplementary Table S4. Regarding the study type, five articles were cohort studies [7,8,16-18], whereas the other five studies were RCTs [9,19-22]. In terms of antibiotic therapy, four studies used rifaximin [7,8,18,19], two studies used norfloxacin [21,22], four studies used another type of antibiotics including amoxicillin-clavulanate [9], multiple combined antibiotics (vancomycin, gentamycin and meropenem) [16], paromomycin sulfate [20], and non-defined antibiotics [17]. As for the study region, two studies were conducted in Asia [20,21], and others in Europe. The baseline characteristics are detailed in Table 1. No significant differences in sex (male/total, OR: 0.92; 95 % CI: 0.67, 1.27; p = 0.63) and age (WMD: −0.69; 95 % CI: −1.57, 0.19; p = 0.12) between the antibiotic group and control group were found.
Baseline characteristics of include studies.
SMT* included pentoxifylline 400 mg x 3 daily for 2–4 weeks. and nutrition, fluid therapy, systemic antibiotics and possibly Vitamin K, if suggested by the treating physician. In case of improvement, prednisolone 40 mg per day was administered. Conventional therapy ** included support nutrition, preventive therapy for withdrawal syndrome and corticosteroids if they did not exhibit any contraindications at the time of admission. Standard supportive medical care*** included nutrition therapy and prednisolone 40 mg/d if Glasgow AH Score ≥9. Standard medical therapy**** included diuretics, steroid therapy for alcohol-associated hepatitis (AAH), beta-blockers, lactulose, and nutritional support.
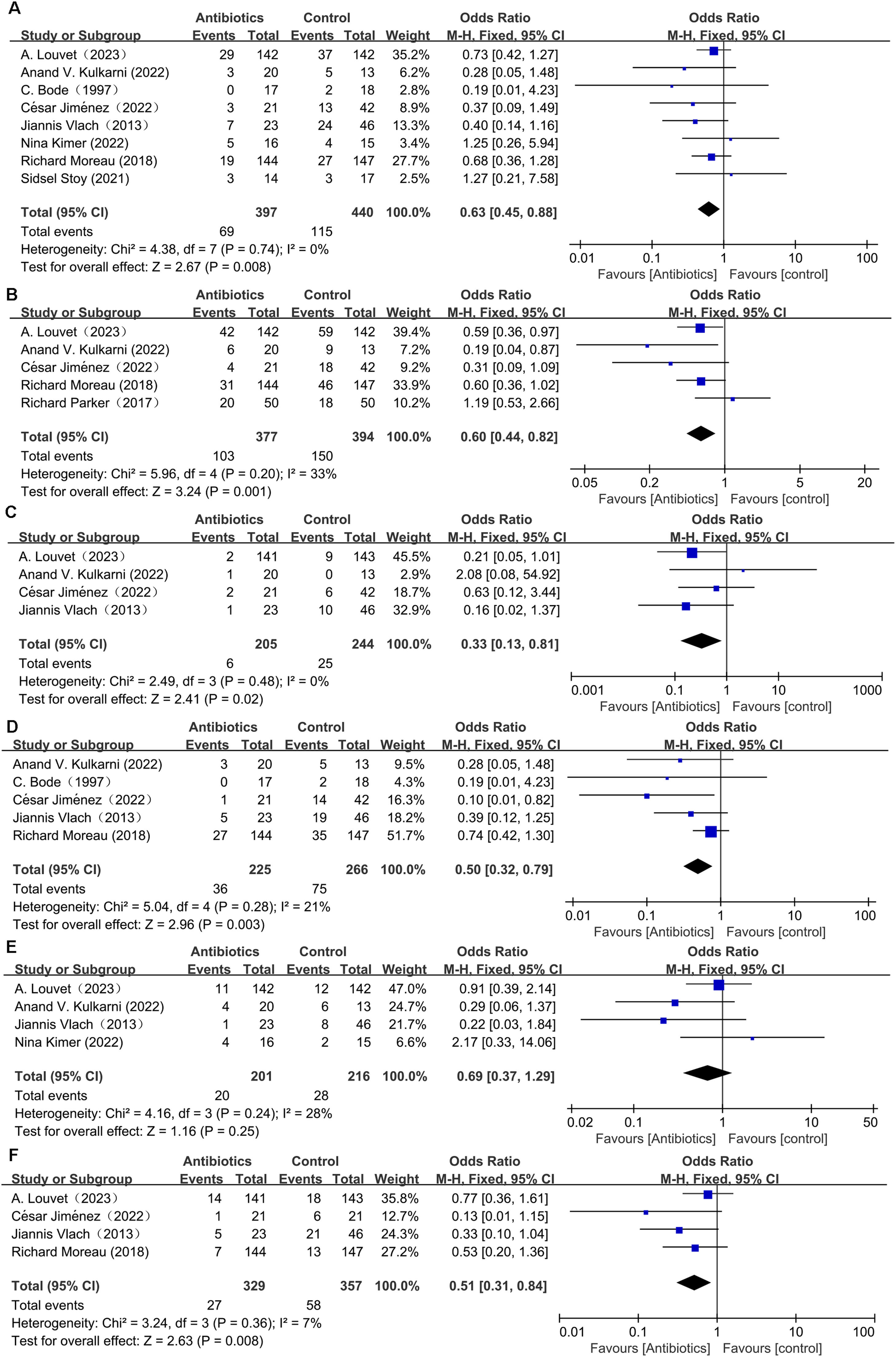
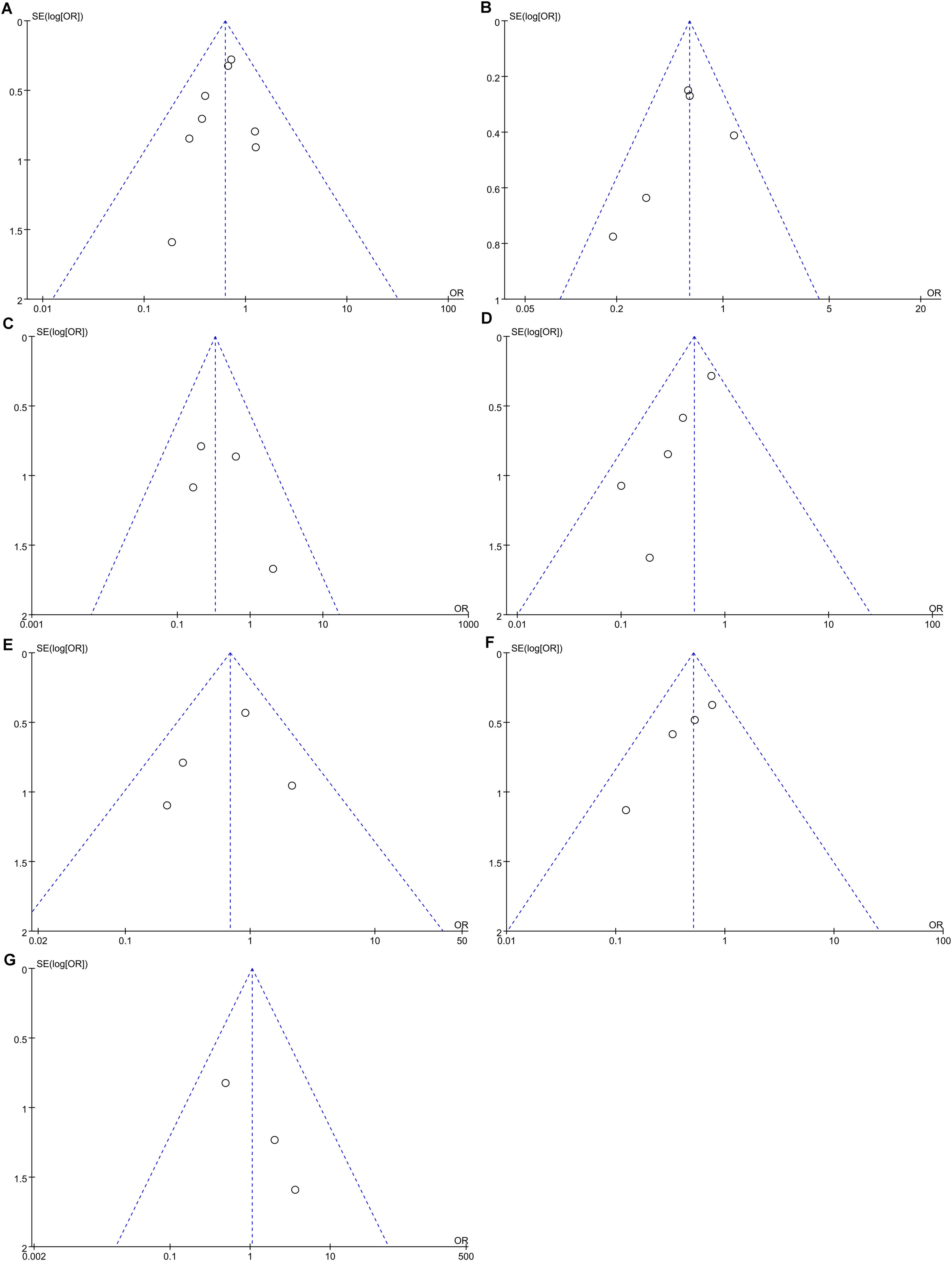
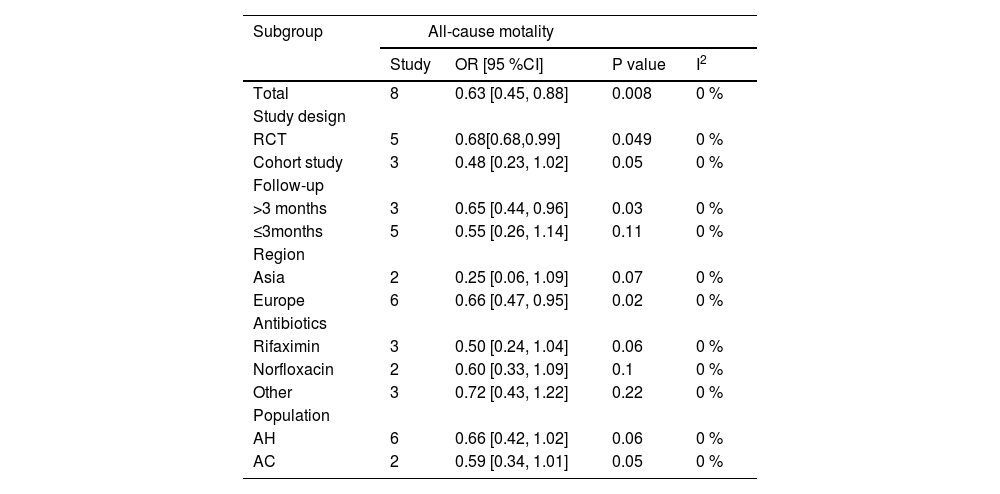
Data pertaining to all-cause mortality was aggregated from eight studies [8,9,16,18-22]. The synthesis revealed that prophylactic antibiotics treatment was associated with a significant decrease in the all-cause mortality rate (OR: 0.63; 95 % CI: 0.45, 0.88; p = 0.008). There was an absence of heterogeneity among these studies (I2 = 0 %, p = 0.74) as depicted in Fig. 2A, and no publication bias was detected as evidenced by the funnel plot (Fig. 3A) and Egger's test (p = 0.381). Subgroup analyses supported the primary outcome, indicating a statistically significant reduction in all-cause mortality within the RCT subgroup (OR: 0.68; 95 % CI: 0.48–0.99; p = 0.049), in studies with a follow-up duration greater than three months (OR: 0.65; 95 % CI: 0.44–0.96; p = 0.03), and within the European cohort (OR: 0.66; 95 % CI: 0.47–0.95; p = 0.02). Each of these subgroups exhibited negligible heterogeneity (Table 2). Conversely, no significant mortality benefit was observed in the cohort studies, those with a follow-up duration of three months or less, or within the Asian population. Furthermore, stratification by antibiotic type or specific populations such as AH or AC did not yield any substantial divergence in mortality outcomes between the antibiotic group and the control group (Table 2). Forest plots depicting the subgroup analyses based on study design, follow-up duration, geographical region, the type of antibiotics, and population demographics are presented in Supplementary Figures S1-S5.
Subgroup analysis of antibiotics versus control for alcoholic liver disease.
OR, odds ratio; CI, confidence interval; AH, alcoholic hepatitis;AC, alcoholic cirrhosis.
Pooled analysis from five studies[8,9,17,21,22] indicated that prophylactic antibiotics significantly diminished infection rate (OR: 0.6; 95 % CI: 0.44, 0.82; p = 0.001) (Fig. 2B) with mild-to-moderate heterogeneity (I2 = 33 %, p = 0.2). The evidence of publication bias was not detected by the funnel plot (Fig. 3B) and Egger's test (p = 0.425). In addition, prophylactic antibiotics also reduced the incidence rate of spontaneous bacterial peritonitis (SBP) significantly [8,9,18,21] (OR: 0.33; 95 % CI: 0.13, 0.81; p = 0.02), with no heterogeneity (I2 = 0, p = 0.48) (Fig. 2C) and no publication bias (Fig. 3C and Egger's test, p = 0.455).
3.4.2Hepatic encephalopathyThe cumulative analysis of hepatic encephalopathy (HE) incidence, drawn from a total of five studies [8,18,20-22], delineated that the occurrence of HE was notably reduced in the antibiotic group compared to the control group (OR: 0.50; 95 % CI: 0.32–0.79; p = 0.003). This outcome indicates a substantial protective effect of prophylactic antibiotics against the development of HE. Additionally, the studies exhibited low heterogeneity (I2 = 21 %, p = 0.28), as illustrated in Fig. 2D. Nevertheless, funnel plot (Fig. 3D) and Egger's test (p = 0.023) identified the presence of publication bias in these studies.
3.4.3Hepatorenal syndromeThe analysis from four studies [9,18,19,21] showed that prophylactic antibiotics could not control the incidence of hepatorenal syndrome (HRS) (OR: 0.69; 95 % CI: 0.37, 1.29; p = 0.25). Furthermore, heterogeneity was not detected in this study (I2= 28 %, p = 0.24) (Fig. 2E). Publication bias was not detected by funnel plot (Fig. 3E) or Egger's test (p = 0.634).
3.4.4Variceal bleedingThe data on variceal bleeding rate were synthesized from three studies [8,9,18,22]. Pooled results revealed that prophylactic antibiotics reduced variceal bleeding incidence significantly when compared with the control group (OR: 0.51; 95 % CI: 0.31, 0.84; p = 0.008), without significant heterogeneity (I2 = 7 %, p = 0.36) (Fig. 2F). However, publication bias was identified by funnel plot (Fig. 3F) and Egger's test (p = 0.036).
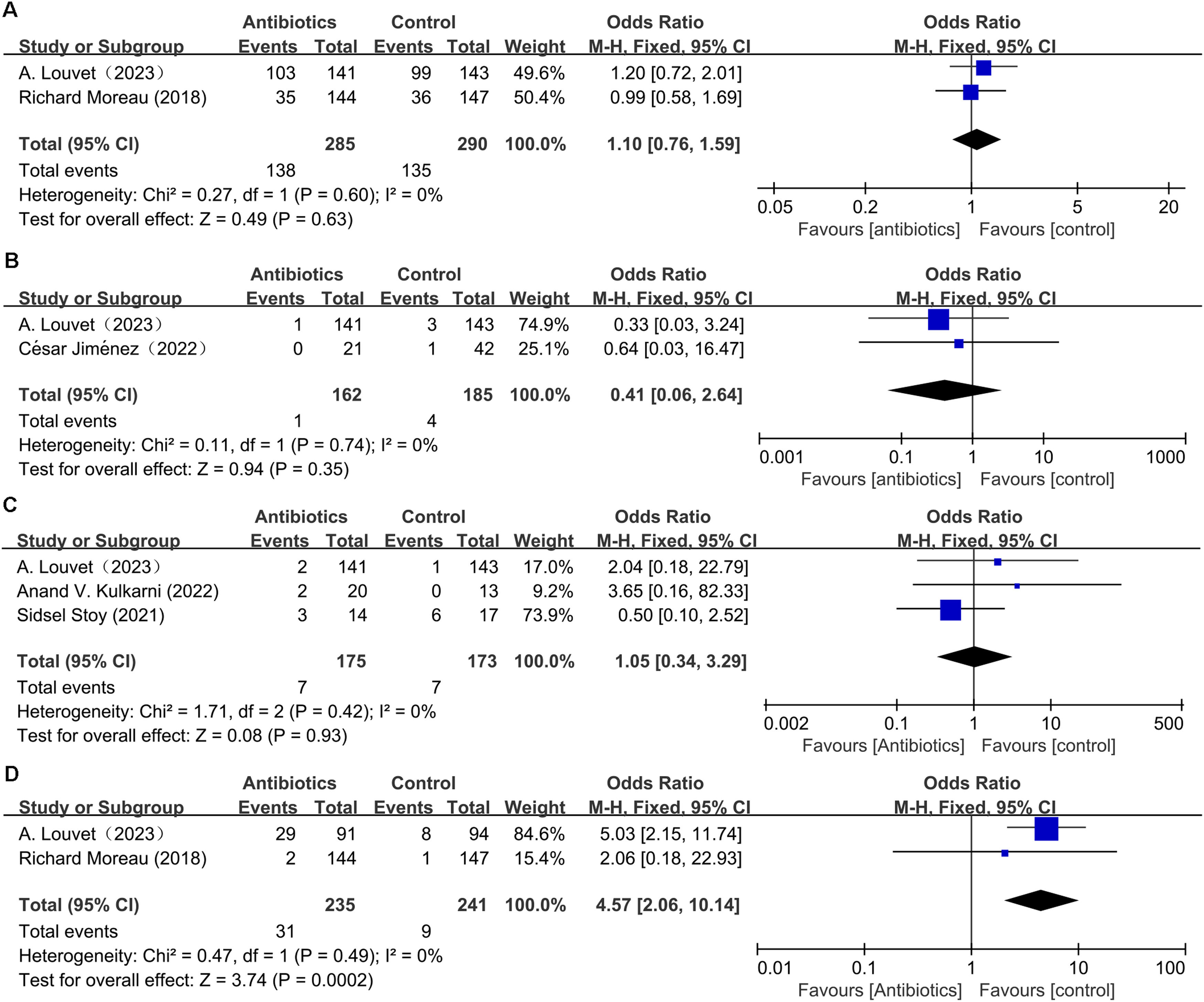
3.5Adverse eventAdverse events considered included not only serious adverse events that resulted in death, were life-threatening, required in-patient hospitalization or an extension of existing hospitalization, led to persistent or significant disability or incapacity, were congenital anomalies/birth defects, or were deemed clinically significant by the investigator [9,22], but also nonserious adverse drug reactions. All prespecified liver-related complications were categorized as serious adverse events. Adverse events were classified using the Medical Dictionary for Regulatory Activities (www.meddra.org). The adverse event from two articles [9,22], was similar between the prophylactic antibiotic group and control group (OR: 1.1; 95 % CI: 0.76, 1.59; p = 0.63). Further analysis showed that heterogeneity was not observed in this study (I 2 = 0 %, p = 0.6) (Fig. 4A). Moreover, the meta-analysis showed that the incidence of clostridioides difficile infection (CDI) in the prophylactic antibiotic group was not significantly different from the control group (OR: 0.41; 95 % CI: 0.06, 2.64; p = 0.35). Heterogeneity was not observed (I 2 = 0 %, p = 0.74) (Fig. 4B).
The effect of prophylactic antibiotics on fungal infection was examined in three clinical trials [9,16,21]. Pooled results indicated that antibiotics increased fungal infection slightly, with no significant difference (OR: 1.05; 95 % CI: 0.34, 3.29; p = 0.93), and no heterogeneity was observed (I2 = 0 %, p = 0.42) (Fig. 4C). Publication bias was not detected by funnel plot (Fig. 3G) and Egger's test (p = 0.113).
Regarding the effect of prophylactic antibiotics on MDR bacteria infection, the analysis from two articles [9,22] demonstrated that MDR bacteria infection rate increased significantly in the antibiotic group (OR: 4.57; 95 % CI: 2.06, 10.14; p = 0.0002). Heterogeneity was not observed in this study (I2 = 0 %, p = 0.49) (Fig. 4D).
3.6Change in plasma endotoxin concentrationsThe influence of prophylactic antibiotics on alterations in plasma endotoxin concentrations was assessed across two clinical investigations [7,20]. Synthesis of the evidence revealed comparable decreases in plasma endotoxin levels between the antibiotic group and the control group (WMD: 0.03; 95 % CI: −0.33, 0.38; p = 0.89), with no significant heterogeneity (I2 = 0 %, p = 0.67) (Supplementary Figure S6).
3.7Change in platelet levelChanges in platelet counts were analyzed in two studies [7,20], with pooled data showing parallel increases in platelet levels between subjects receiving antibiotics and controls (WMD: 14.17; 95 % CI: −36.35, 64.69; p = 0.58). No significant heterogeneity was identified in this study (I2 = 0 %, p = 0.75) (Supplementary Figure S7).
3.8Total bilirubin and creatinine levelTwo publications addressed fluctuations in serum total bilirubin [8,20] and creatinine concentrations [19,20]. Meta-analysis indicated that antibiotic-treated subjects exhibited comparable differential values of bilirubin and creatinine levels to those in the control group (WMD: 1.41; 95 % CI:−2.21, 5.03; p = 0.45). Heterogeneity assessments for both bilirubin (I² = 0 %, p = 0.99) (Supplementary Figure S8) and creatinine metrics (WMD: 4.94; 95 % CI: −32.10, 41.98; p = 0.79) (I² = 0 %, p = 0.32) demonstrated no significant variances (Supplementary Figure S9).
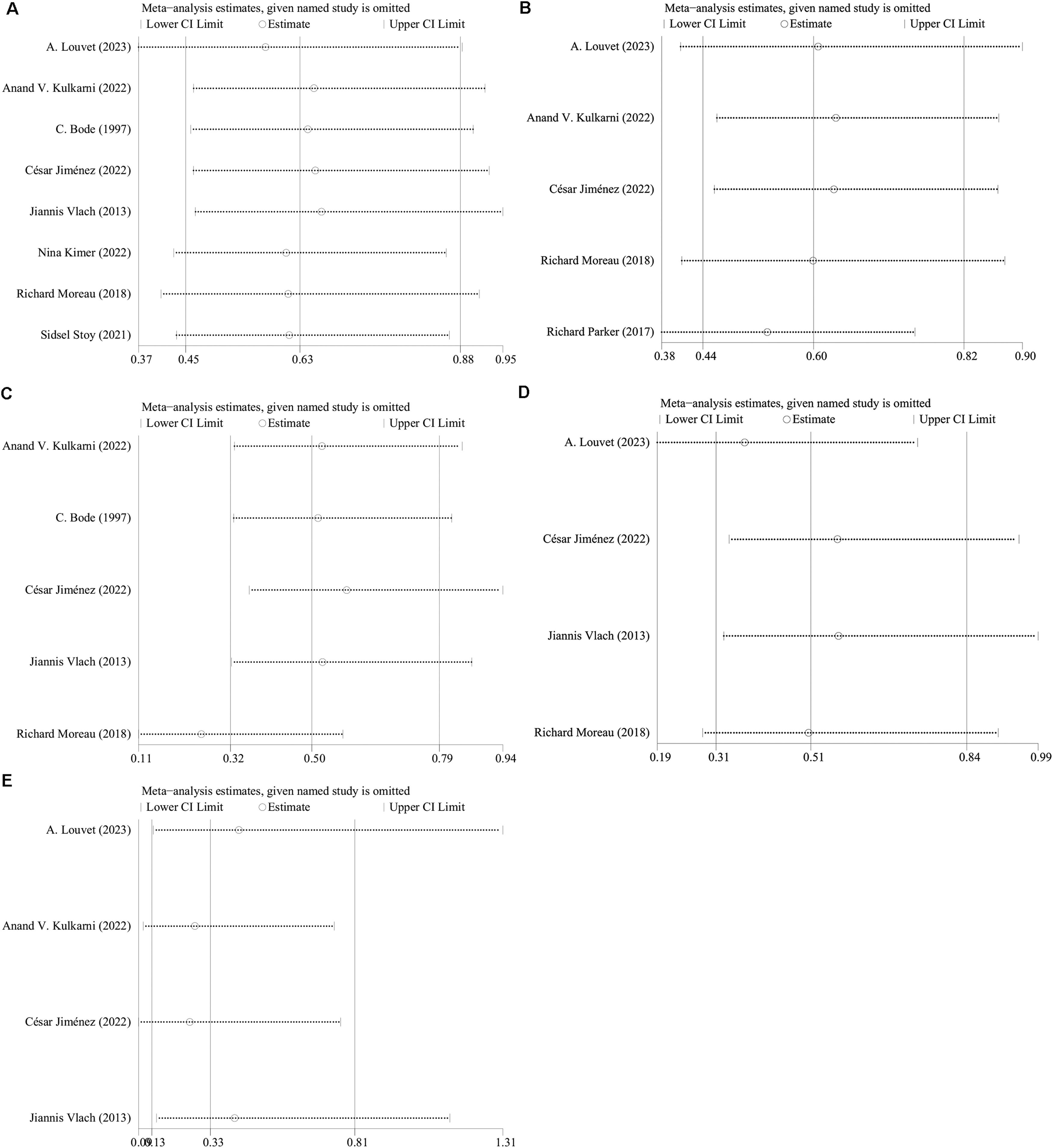
3.9Sensitivity analysisLeave-one-out sensitivity analysis procedures were employed to ascertain the influence on the cumulative OR of all-cause mortality, infection prevalence, HE, SBP, and variceal bleeding within each included study. The outcomes indicated that the exclusion of individual studies did not markedly impact the rates of all-cause mortality, infection prevalence, HE occurrences, or variceal bleeding (Fig. 5A, 5B, 5C, 5D). Contrarily, the significance of the aggregated OR for SBP incidence shifted from noteworthy to non-significant upon the sequential omission of either A. Louvet et al.'s or Jiannis et al.'s studies, signaling instability in these findings (Fig. 5E).
4DiscussionThe international debate surrounding the preventative use of antibiotics in patients with ALD is both vigorous and ongoing, as reflected by numerous studies [7-9,16,19]. In an effort to contribute to this conversation, we executed a comprehensive systematic review and meta-analysis, examining data from 974 participants across 10 studies, which spanned various ALD stages, such as AH and AC, and incorporated disparate antibiotic interventions.
The study demonstrated that prophylactic antibiotics were associated with a lower number of infections, non-infection complications (e.g., HE, variceal bleeding), and all-cause mortality. In addition, prophylactic antibiotics didn't increase the incidence rate of all adverse events, fungal infection, and CDI, but they raised the multidrug-resistant bacteria infection rate. Based on differential values of total bilirubin and creatinine levels, prophylactic antibiotics had no protective effect on the liver and renal function. Moreover, while there was a marginal improvement in HRS, a slight elevation in platelet counts, and a minor reduction in endotoxin levels attributed to prophylactic antibiotics, these alterations did not achieve statistical significance. Prophylactic antibiotic in ALD treatment act as selective intestinal decontamination agents to eliminate or inhibit the gut flora, modify the microbiome, inhibit the bacterial overgrowth, reduce bacterial translocation, lessen the infection resulting from pathogens from gastrointestinal flora, reduce systemic inflammation, and eventually alleviate susceptibility to liver injury.
Our findings, in concordance with other research [8,9,22], indicated that prophylactic antibiotic administration diminished infection rates among individuals with ALD. Nevertheless, Richard Parker et al.'s study [17] presented conflicting evidence. Potential reasons for these divergent outcomes could stem from distinct methodological approaches, with Parker's team utilizing a retrospective design and potentially inadequate control of disease severity variables. Additionally, our analysis suggested that the incidence of SBP was reduced in the prophylactic antibiotics group compared to controls. This aligns with several reports highlighting the beneficial impact of norfloxacin and rifaximin in managing primary and secondary SBP [23,24]. Another meta-analysis underscored the efficacy of rifaximin, showcasing a 47 % reduction in SPB risk versus no antibiotic regimen for primary prophylaxis, and a 74 % decrease when compared with systemic antibiotics for secondary prophylaxis [25]. However, the variable outcomes from our leave-one-out sensitivity analyses regarding SBP call into question the robustness of these conclusions.
Our pooled analysis of non-infection complications revealed that prophylactic antibiotics could significantly lower the risk of variceal bleeding and HE. Prophylactic antibiotics have been shown to potentially decrease infection in variceal bleeding with cirrhosis [26], presumably by reducing hepatic venous pressure gradient (HVPG) [27]. A reduction of portal pressure by >20 % of baseline values or <12 mmHg could effectively protect against the risk of initial or recurrent variceal bleeding [28]. Current disease management guidelines recommend antibiotic prophylaxis in the management of upper gastrointestinal bleeding in cirrhosis [29]. A similar benefit of antibiotic prophylaxis was also confirmed in overt hepatic encephalopathy treatment [30]. In a pilot study [8], mere one out of 21 subjects undergoing rifaximin treatment developed mild HE, whereas the control arm saw 14 out of 42 participants affected. This suggests that rifaximin, when deployed in severe AH management, may be associated with a lower HE occurrence rate. Furthermore, rifaximin has been approved for use as a first-line therapy for prophylaxis of HE.
The intervention of prophylactic antibiotic in ALD patients significantly reduced the mortality rate caused by complications such as infection, variceal bleeding, and HE. Nevertheless, this observation stands in contrast to the findings reported by A. Louvet [9] and S. Stoy [16], with potential discrepancies arising from various factors, including the distinct influence of different antimicrobials on gut microbiota. Subgroup analyses yielded consistent results supporting the efficacy of prophylactic antibiotics on all cause mortality within RCTs, in studies with follow-up duration exceeding three months, and within European subgroup. Conversely, no significant differences were detected between the two groups in the cohort studies, studies with duration of follow up ≤ 3months, and Asian population. In terms of study design, the nonrandomized studies typically displayed more favorable findings and exhibited higher risks of bias. However RCTs represent the most robust and rigorous research methods, providing higher-quality evidence to evaluate the efficacy and safety of interventions [31]. Consequently, the positive outcomes derived from RCTs are considered more reliable. Regarding follow-up duration, our analysis indicated that longer follow-up periods were associated with more pronounced reductions in mortality rates due to prophylactic antibiotics. Intriguingly, studies with a follow-up duration of ≤ 3months pertained exclusively to the AH population. In contrast, studies with longer follow-up periods primarily involved patients with AC. This divergence might be attributed to several factors: the variation in disease severity between AH and AC, the prevalent use of corticosteroids for severe AH which increases the risk of infectious complications due to defective lymphocyte signaling [32], and the potential for infections to act as a precipitator of acute-on-chronic liver failure, thereby elevating mortality risk in AH. The smaller sample size in the ≤3-month subgroup also contributed to less reliable outcomes. In terms of the types of antibiotics, the efficacy of various antibiotic treatments in reducing mortality rates was inconsistent, indicating variable outcomes across studies. Although the results were not statistically significant, rifaximin appeared more effective than norfloxacin in reducing mortality, as surported by a lower OR 0.5 [0.24,1.04] vs. 0.6 [0.33,1.09]) in subgroup analysis (Table 2). This might be due to the increasing prevalence of gram-positive, quinolone-resistant, and multi-drug-resistant (MDR) bacterial in recent years [23], and the extensive, long-term use of prophylactic quinolines exacerbating this trend. Gut microbiota dysbiosis and disruption of the gut–liver axis are implicated in the pathogenesis of various chronic liver diseases including ALD, contributing to liver inflammation, fibrosis, and portal hypertension [33]. While antibiotics generally have detrimental effects on the gut microbiota—including reduced species diversity, altered metabolic activity, and screening of antibiotic-resistant organisms—rifaximin has unique effects. It appears to increase populations of beneficial intestinal bacteria without significantly altering the overall composition of the gut microbiota, and is thought to aid in restoring the intestinal barrier, thereby potentially mitigating bacterial translocation and systemic endotoxemia in individuals with cirrhosis [33-36]. Besides, a RCT [37] comparing rifaximin and norfloxacin for secondary prophylaxis of SBP reported threefold higher encephalopathy-related deaths in the norfloxacin group, with fewer side effects observed in the rifaximin group. Therefore, rifaximin, a gut-selective antibiotic with low microbial resistance and broad antibacterial spectrum, has been suggested as an effective oral alternative to norfloxacin.
Drug safety is the primary concern in medical therapy and significantly influences the choice of medication for patients. In this study, prophylactic antibiotics have been shown not to significantly increase the incidence of all adverse effects, fungal infections, or CDI, yet they were associated with an increased rate of MDR infections. One RCT [9] identified a higher occurrence of MDR bacteria infection (29/91 or 31.9 %) in the amoxicillin-clavulanate group with 31.9 % (29/91) of participants affected, as opposed to only 8.5 % (8/94) in the placebo group. Moreover, systemic administration of amoxicillin-clavulanate is linked to systemic side effects, including hepatotoxicity, gastrointestinal disturbances, allergic reactions, enhanced bacterial resistance, and potential drug interactions [38]. J. Fernandez et al [39] reported that prolonged norfloxacin prophylaxis was an independent predictor of MDR bacteria infections. Thus, patients should receive the right drug and dose for the right duration to reduce or avoid the MDR bacteria infection. Nevertheless, we did not carry out subgroup analysis for the rate of MDR bacteria infection based on different types of antibiotic owing to the insufficient data.
In this study, we present a comprehensive and the most up-to-date meta-analysis by including publications from 1997 to 2023, which makes our study more credible. We also acknowledge several limitations in this study. Firstly, the research was exclusively conducted in Asian and European countries, which may introduce selection bias, thus the conclusion cannot be generalized to a wider population. Secondly, the research was exclusively conducted in individuals with advanced ALD. Future studies should enroll more patients from different ethnic backgrounds with long-term follow-up in different types antibiotics treatments, to validate the efficacy and safety of ALD therapy. Lastly, the number of our meta-analyses was limited, which may impact the reliability of the I2 statistic in detecting study heterogeneity. Paul T. von Hippel et al. have highlighted that the widely utilized heterogeneity statistic, I2, can be imprecise and biased when used in smaller meta-analyses. They suggested that calculating confidence intervals around I2 could potentially mitigate this issue [40].
5ConclusionsProphylactic antibiotic was an effective and safe treatment for advanced ALD with improvements in all-cause mortality, infection, variceal bleeding, and HE, without additional risk of any adverse events, fungal infection, and CDI. The non-absorbable antibiotics and selective systemic antibiotics for bowel decontamination can be opted to reduce MDR bacterial infection. In future studies, it is essential to consider diversity in ALD patient recruitment with long-term follow-up and more antibiotic treatments to solidify the efficacy and safety of ALD therapy.
FundingThis study was funded by the Wenzhou Bureau of Science and Technology (Grant Number Y2020948).
Availability of dataAll data from this study are included in this published article and its supplementary files.
Not applicable.
Supplementary Figure S1. Effect of prophylactic antibiotics on all-cause mortality stratified by study design. Supplementary Figure S2. Effect of prophylactic antibiotics on all-cause mortality stratified by duration of follow-up. Supplementary Figure S3. Effect of prophylactic antibiotics on all-cause mortality stratified by geographical region. Supplementary Figure S4. Effect of prophylactic antibiotics on all-cause mortality stratified by the type of antibiotics. Supplementary Figure S5. Effect of prophylactic antibiotics on all-cause mortality stratified by population demographics. Supplementary Figure S6. Forest plot of plasma endotoxin concentrations Supplementary Figure S7. Forest plot of platelet level Supplementary Figure S8. Forest plot of total bulirubin level Supplementary Figure S9. Forest plot of creatinine level