Non-alcoholic fatty liver disease (NAFLD) is one of the most common diseases in the United States. Metabolic distress (obese diabetes) is the main causative element of NAFLD. While there is no cure for NAFLD, endurance exercise (EEx) has emerged as a therapeutic strategy against NAFLD. However, mechanisms of EXE-induced hepatic protection especially in female subjects remain unidentified. Thus, the aim of the study is to examine molecular mechanisms of EXE-induced hepatic protection against diet-induced NAFLD in female mice.
Material and methodsNine-week-old female C57BL/6J mice were randomly divided into three groups: normal-diet control group (CON, n=11); high-fat diet/high-fructose group (HFD/HF, n=11); and HFD/HF+EEx group (HFD/HF+EEx, n=11). The mice assigned to HFD/HF and HFD/HF+EEx groups were fed with HFD/HF for 12 weeks, after which the mice assigned to the EEx group began treadmill exercise for 12 weeks, with HFD/HF continued.
ResultsEEx attenuated hepatic steatosis, reduced de novo lipogenesis (reduction in ATP-Citrate- Lyase and diacylglycerol-O-acyltransferase 1), and enhanced mitochondrial biogenesis and fatty-acid activation (oxidative phosphorylation enzymes and Acyl-CoA synthetase1). Also, EEx prevented upregulation of gluconeogenic proteins (glyceraldehyde-3-phosphate dehydrogenase, glucose-6-phosphatase, and phosphoenolpyruvate-carboxykinase1), premature senescence (suppression of p53, p22, and p16, tumor-necrosis-factor-α, and interleukin-1β, and oxidative stress), and autophagy deficiency. Furthermore, EXE reversed apoptosis arrest (cleaved cysteine-dependent-aspartate-directed protease3 and Poly-(ADP-ribose)-polymerase1).
ConclusionEEx-mediated reparations of metabolic and redox imbalance (utilization of pentose phosphate pathway), and autophagy deficiency caused by metabolic distress critically contribute to preventing/delaying severe progression of NAFLD. Also, EEx-induced anti-senescence and cell turnover are crucial protective mechanisms against NAFLD.
Non-alcoholic fatty liver disease (NAFLD), featured as excessive fat accumulation (hepatic steatosis), is one of the most common types of liver disease in the United States, affecting over 25% of the population [1, 2]. NAFLD has emerged as a primary health concern due to its close link to obesity, affecting over 40% of the population [1]. Thus, the prevention of NAFLD or search for a cure has been a central topic. In this regard, regular endurance exercise (EEx) has been considered a potent non-pharmacological strategy against NAFLD [3]; however, its molecular mechanisms remain unclear and are thus the focus of this study.
Fat quantity in the cell is balanced by lipogenesis and lipolysis [4]. The importance of lipolysis in preventing NAFLD has been reported in recent studies where a defect in lipolysis promotes NAFLD [5, 6], and reinforced lipolysis confers hepatic protection against NAFLD [7–10]. Similarly, EEx-reinforced lipolysis protects the liver against NAFLD [8, 11]. Besides lipolysis, fatty acid transportation to mitochondria is a crucial initial step in fatty oxidation because downregulation of carnitine palmitoyltransferase-1A (CPT-1A), a liver-specific rate-liming enzyme of fatty acid transportation, contributes to NAFLD [12]. Furthermore, mitochondrial biogenesis and respiratory function (e.g., the activities of electron transport chains: Complex I-V, referred to as oxidative phosphorylation: OXPHOS) play a prime role in modulating the rate of fatty acid oxidation. In this regard, a recent study has shown that high-fat diet-induced defects in mitochondrial biogenesis are associated with NAFLD [13]. The concept of mitochondrial impairment in the pathogenesis of NAFLD has provided insight into potential mechanisms of EEx in conferring protection against NAFLD in that EEx is a potent inducer of mitochondrial biogenesis, rejuvenates mitochondrial function, and promotes mitochondrial remodeling [14, 15].
A hypercaloric state facilitates de novo lipogenesis [16, 17]. ATP-citrate lyase (ACLY) is a critical enzyme involved in de novo lipogenesis by generating cytosolic acetyl-CoA from cytosolic citrate [18]. Recent studies have demonstrated that ACLY plays an essential role in hepatic steatosis, as its abrogation prevents NAFLD in diabetic mice [18–20]. Diacylglycerol O-acyltransferase 1 (DGAT1) synthesizing triglyceride (TG) from diacylglycerol and fatty acids (FAs) contributes to NAFLD; for instance, in mice models, inhibition of DGAT1 reduces lipogenesis and protect against HFD-associated lipogenesis [21, 22]. Currently, whether EEx-induced hepatic protection against NAFLD is associated with the reversal of lipogenesis remains controversial. Aside from dysregulating fat metabolism, NAFLD also contributes to hyperglycemia [23], whereas EEx attenuates it [24]. However, the molecular mechanisms that cause these differences remain unknown.
Cellular senescence is a state of irreversible cell-cycle arrest (e.g., prohibition of apoptosis), leading to secretion of pro-inflammatory cytokines such as interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) and oxidative stress, known as senescence-associated secretory phenotype [2]. Recent studies have shown that senescence plays a causative role in the pathogenesis of NAFLD [25–27]. Thus, it is important to examine if EEx-mediated anti-senescence is a potential protective mechanism against NAFLD. In addition to cell turnover, autophagy (a lysosome-dependent catabolic process) plays a substantial role in sustaining normal cell functions in the liver by disposing of damaged proteins, lipids, and small organelles such as mitochondria [28]. A seminal study has demonstrated that autophagy is essential for lipid metabolism, as autophagy inhibition expands lipid droplets [29]. A recent study has reported that EEx promotes autophagy in the liver [30]; however, it is still unclear if autophagy is required to prevent NAFLD because studies have shown contradictory results.
Antioxidant capacity such as manganese superoxide dismutase (MnSOD), copper-zinc SOD (CuZnSOD), and CATALASE have been reported to increase in the various tissues in EEx-trained animals [30, 31]. Moreover, EEx-mediated upregulation of glutathione peroxidase (GPx) and downregulation of reduced nicotinamide adenine dinucleotide phosphate oxidase (NADPHOx) has been shown to protect various tissues [30, 31]. However, the effect of exercise on GPx and NADPHOx in NAFLD has not been extensively studied. Especially, given that the oxidative pentose phosphate pathway (PPP, also known as a hexose monophosphate shunt) uses nicotinamide adenine dinucleotide phosphate (NADP) to generate a reduced form of NADP (NADPH), which is the prime source of redox regulation (e.g., reduction of glutathione after being oxidized via GPx and generation of superoxide anions via NADPHOx, the examination of this pathway is important to reveal a novel redox mechanism linked to EEx-induced hepatic protection against NAFLD.
Despite growing evidence of EEx-mediated protection against NAFLD, its molecular mechanisms remain scarce and unidentified, especially in female populations. Thus, the aim of the study is to examine EEx-mediated modulations of integrative molecular signaling nexus involved in metabolism, senescence, redox balance, and apoptosis, using a female mouse model of NAFLD induced by a long-term high-fat/high-fructose diet.
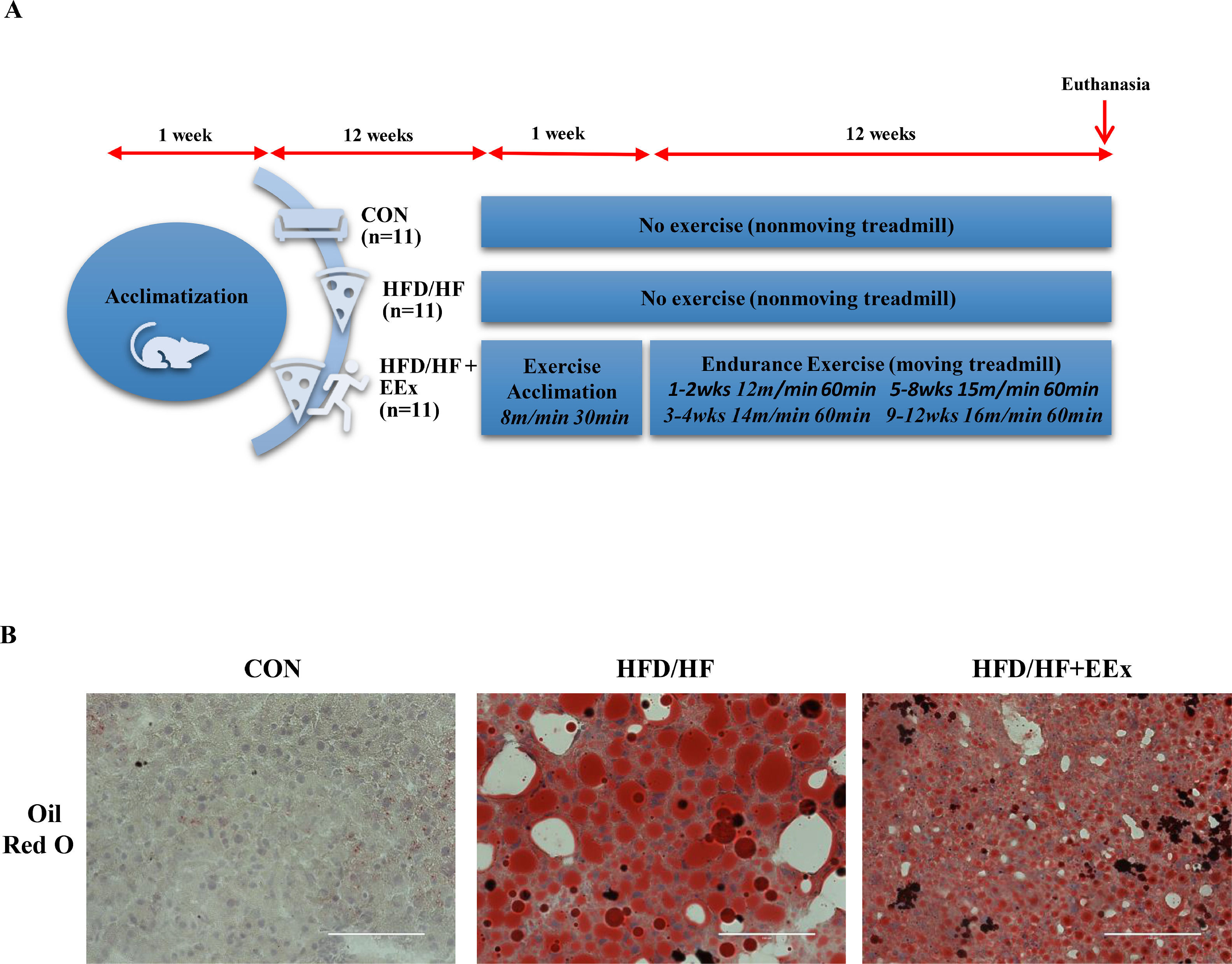
2Material and methods2.1Animals and dietsNine-week-old female C57BL/6J mice (n = 33) were purchased from Envigo (Indianapolis, IN) and housed at a 12-h dark–12-h light cycle at 22°C ± 2°C with 50% relative humidity with food and water ad libitum. As depicted in Fig. 1A, after one-week acclimatization to the animal housing facility environment, mice were fed with high-fat (FHD) diet (protein: 20 kcal %; fat: 60 kcal %; and carbohydrate: 20 kcal %, Cat. no. D12392, Research Diet, New Brunswick, NJ) with tap water containing 20% high fructose (HF) (HFD/HF, n= 22) or normal diet (protein: 28.5 kcal %, fat: 13.5 kcal %, carbohydrate: 58 kcal %; no. 5001, LabDiet, St. Louis, MO) with tap water (CON, n=11) for 12 weeks. Afterwards, the mice assigned to the HFD/HF diet were divided into three groups: HFD/HF (n=11), HFD/HF + exercise (HFD/HF + EEx, n = 11), and CON (n=11). Then the designated diets continued for additional 13 weeks (a total of 25 weeks of HFD/HF diet). Animal experimental procedures were approved by the Institutional Animal Care and Use Committees (approval number 2017006) at the University of West Florida. All animal experiments complied with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Experimental design and lipid droplet imaging. A. Illustration of experimental design. Nine-week-old female C57BL/6 J mice were acclimated for one week and then assigned to three different groups: Control (CON), high-fat + high fructose (HFD/HF), high-fat + high fructose + exercise (HFD/HF + EEx). HFD/HF and HFD/HF + EEx groups were fed with a diet high in fat and fructose for 24 weeks. EXE started 12 weeks after HFD/HF initiation, and one weeks after familiarization with treadmill running exercise, the main EEx program continued for 12 weeks. B. Representative images of lipid droplets in the liver tissues, stained with Oil Red O. HFD/HF diet displayed a substantial increase in the size of lipid droplets, evidenced by increased lipid quantities (red) per section compared to the CON group; however, EEx treatment significantly reduced the size of lipid droplets (red) compared to the HFD/HF group (n=3 per group). The scale bars indicate 100 μm.
After 12 weeks of HFD/HF diet, the mice assigned to EEx were acclimated with treadmill running for five days (8 m·min−1, 30 min·d−1) at 0% grade and then performed a 12 week-EEx training program, with a running speed progressively increased. For instance, the mice ran at 12 m·min−1 (60 min·d−1, 5 d·wk−1) for two weeks, and the speed was increased to 14 m·min−1 in the following two weeks. Thereafter, the speed was increased to 15 m·min−1 (60 min·d−1, 5 d·wk−1) in the following four weeks followed by a 16 m·min−1 speed for the following four weeks. To exclude possible confounding factors that environmental stresses may cause (e.g., treadmill machine noise and enclosure inside the treadmill) rather than exercise effects, the mice assigned to the sedentary control group stayed in the nonmoving treadmill for the same duration. The intensity (75%–80% V˙ O2max) of this treadmill exercise was selected according to a previous study [32]. In the present study, we did not apply an electric shock typically used to force mice to run because it may cause unknown physiological responses that may interrupt a critical data interpretation. Instead, bristle brushes were placed at the end of each lane to encourage mice to run until the designated training period. All mice successfully completed the training program.
2.3Tissue samplingMice were euthanized by cervical dislocation twenty-four hours after the last session of EXE training. We chose a cervical dislocation procedure because various methods of euthanasia such as CO2, sodium pentobarbital, and isoflurane have been reported to affect liver metabolism, compared to the immediate euthanasia by cervical dislocation [33]. Then, the liver samples were excised, immediately frozen with liquid nitrogen, and stored at -80°C. Liver samples (n = 3 per group) were covered with optimum cutting temperature compound and submerged into precooled isopentane in liquid nitrogen for 30 seconds for Oil Red O staining. Then the tissues were stored at -80°C.
2.4Western blottingThe liver tissues were homogenized with a glass homogenizer in T-PER® tissue protein extraction reagent (ThermoFisher Scientific, USA) containing a HaltTM Protease and Phosphatase inhibitor cocktail (ThermoFisher Scientific, USA), incubated on ice for 20 min, and centrifuged at 14,000 RPM for 20 min to extract total proteins. The extracted proteins (30 μg) were separated by BoltTM 10% Bis-Tris Gel (ThermoFisher Scientific, USA) and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk dissolved in Tris-buffered saline solution containing 0.1% Tween-20 (TBS-T) for non-phosphorylated proteins, or 5% bovine serum albumin for phosphorylated proteins for one hour at room temperature. The membranes were incubated over night at 4 ˚C with designated primary antibodies as follows: FABP1 (13368, 1:1000), p-AMPK (2535, 1:1000), AMPK (2532, 1:1000), p-AKT (9271, 1:1000), AKT (9272, 1:1000), GAPDH (2118, 1:1000), and PARP1 (9532, 1:1000), PCK1 (12940S, 1:1000), G6PD (12263S, 1:1000), LC3A/B (12741, 1:1000), ATG7 (2631, 1:1000) from Cell Signaling Technology; FAT/CD36 (sc-7309, 1:500), ACSS2 (sc-398559, 1:500), DGAT1 (sc-271934), p-IRβ (sc-81500, 1:500), IRβ (sc-373975, 1:500), p-GSK3β (sc-373800, 1:500), GSK3β (sc-53931, 1:500), gp91phox (sc-74514, 1:500), p22phox (sc-130550, 1:500), p47phox (sc-17845, 1:500), p67phox (sc-374510, 1:500), GPx1/2 (sc-133160, 1:500), TNF-α (sc-52746, 1:500), IL-1β (sc-12742, 1:500), p53 (sc-393031, 1;500), p21 (sc-817, 1:500), p16INK4a (sc-56330) from Santa Cruz Biotechnology; ATGL (55190-1-AP, 1:1000), ABHD5 (12201-1-AP, 1:1000), ACSL1 (13989-1-AP, 1:1000), ACLY (15421-1-AP, 1:1000), CPT-1A (15184-1-AP, 1:1000), GLUT2 (20436-1-AP, 1:1000), CASPASE 3 (19677-1-AP, 1;1000), PGD (14718-1-AP, 1:1000) from Protein Tech; HSL (PA1-16966, 1:1000) from ThermoFisher Scientific; and OXPHOS (ab110413, 1:1000) from Abcam; G6PC (NBP1-80533, 1:1000). After the overnight incubation, the membranes were washed with TBS-T and incubated with designated secondary antibodies: horseradish peroxidase-conjugated goat anti-rabbit (#1148960) or rabbit anti-goat (#811620) from ThermoFisher, or goat anti-mouse (sc-2005) from Santa Cruz Biotechnology at 1:5000 dilution for one hour at room temperature. Then, the membrane was washed with TBS-T, and digital blot images of target proteins were acquired using the ECL Western blotting detection substrates (GE Healthcare, USA) and a ChemiDoc XRS imaging system (Bio-Rad, USA). The intensity of target proteins was analyzed and quantified with the Bio-Rad Image Lab Software. Each target protein intensity was normalized by the intensity of Ponceau-stained total proteins. All protein levels were presented as a percentage.
2.5Oil red O stainingTo determine hepatic steatosis, oil red O staining was used. Briefly, liver tissue sections were fixed in 4% paraformaldehyde fixing solution for 15 min and rinsed with 60% isopropanol five times. After fixation, the samples were stained with 0.5% Oil Red O (ThermoFisher, USA) for 10 min and washed with distilled water. Then, the sections were mounted on cover slides with a glycerin jelly mounting medium. The images of hepatic lipid content were captured with an EVOS high-resolution light microscope (ThermoFisher Scientific, USA).
2.6Statistical analysisAll values were presented as mean ± standard error of the mean (SEM), and statistical significance was set at p < 0.05. Data presented in bar graphs were based upon percentage changes compared to the CON group. One-way Analysis of Variance (ANOVA) was used to determine the statistical significance using the Prism 9 software (San Diego, CA, USA). Tukey's honestly significant difference (HSD) post hoc test was selected to determine group differences if a statistical significance was found.
3Results3.1EEx intervention reduces HFD/HF diet-induced hepatic steatosisTo explore whether EEx would reduce TG storage in the liver, we first examined the size of lipid droplets in the cross-sectioned liver tissues from each group, using an Oil-Red O lipid staining technique. Our data showed that HFD/HF diet displayed a substantial increase in the size of lipid droplets (red), whereas EEx treatment significantly reduced the size of lipid droplets (red) compared to the HFD/HF group. (Fig 1B).
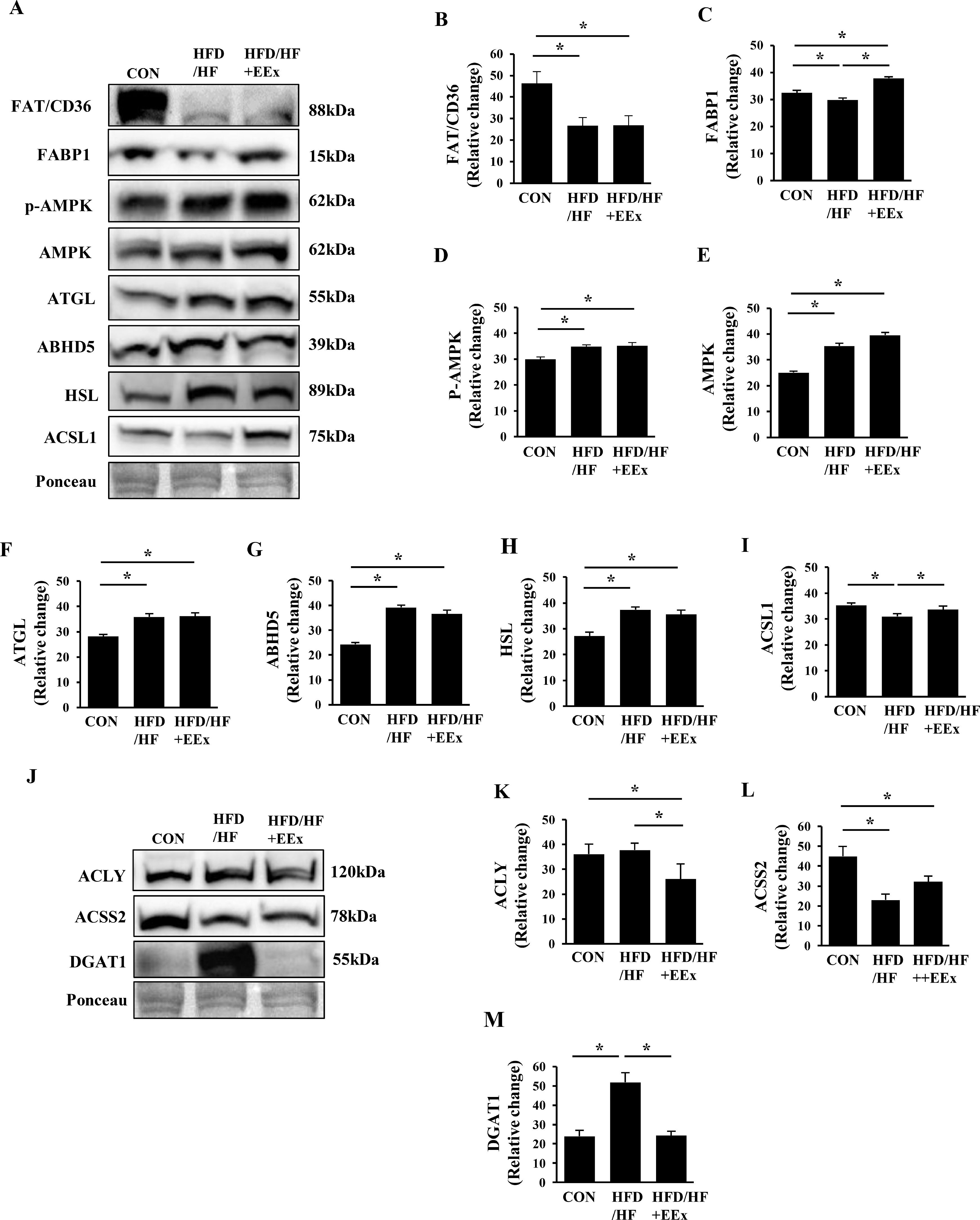
3.2Both HFD/HF and EEx upregulate lipolysis-related proteins, but EEx suppresses de novo lipogenic proteinsTo explore how EXE attenuates HFD/HF diet-induced lipid accumulation, we examined several proteins involved in fatty acid uptake and transportation, lipolysis, and lipogenesis. The plasma membrane-bound fatty acid translocator (FAT/CD36) levels were markedly reduced in both HFD/HF and HFD/HF+EXE groups, compared to the CON group (Fig. 2A and B). Next, we assessed cytosolic fatty acid-binding protein 1 (FABP1) levels. The HFD/HF group suppressed FABP1 levels compared to the CON and HFD/HF + EEx groups. Of note, EEx intervention upregulated the protein levels even at greater levels than the CON group (Fig. 2A and C).
Assessment of hepatic lipolysis and lipogenesis. A. Representative Western blot images of lipolysis markers in the liver: FAT/CD36, FABP1, P-AMPK, AMPK, ATGL, ABHD5, HSL, and ACSL1. Ponceau staining was used as a loading control. B-I. Quantification of the lipolysis markers. Both HFD/HF and EEx upregulate lipolysis-related proteins. J. Representative Western blot images of lipogenesis markers (ACLY, ACSS2, and DGAT1) in the liver, showing EEx-induced suppression of lipogenic proteins. Ponceau staining was used as a loading control. K-M. Quantification of lipogenesis markers (n=7-8 per group). An asterisk (*) indicates significant differences at p <0.05. FAT: fatty acid translocase, FABP1: fatty acid binding protein 1, AMPK: AMP-activated protein kinase, ATGL: adipose triglyceride lipase, ABHD5: alpha-beta hydrolase domain-containing 5, HSL: hormone sensitive lipase, ACSL1: Acyl-CoA synthetase long chain family member 1, ACLY: ATP Citrate Lyase, ACSS2: Acyl-CoA Synthetase Short Chain Family Member 2, DGAT1: diacylglycerol O-acyltransferase 1.
Lipolysis is an essential process to hydrolyze stored triglyceride into free fatty acids, which can then be used in the mitochondria. Given the possibility that enhanced lipolysis would be linked to the reduced size of lipid droplets, we examined lipolysis-related proteins. Surprisingly, the HFD/HF diet itself elevated all major lipolysis proteins (AMPK, p-AMPK, ATGL, ABHD5, and HSL) without any synergic effects of EEx, compared to the CON group (Fig. 2A, D-H). By contrast, the levels of acyl-CoA synthetase long-chain family member 1 (ACSL1), a cytosolic free fatty acid activation protein was significantly reduced in the HFD/HF group. However, EEx maintained its levels comparable to the CON group (Fig. 2A and I).
We next examined lipogenesis-related proteins (ACLY, ACSS2, and DGAT1). HFD/HF + EEx administration significantly reduced ACLY levels compared to both CON and HFD/HF groups (Fig. 2J and K). ACSS2 is also associated with lipid biosynthesis by converting acetate to acetyl-CoA. Since HFD has been reported to increase acetate levels in the blood and emerged as a potential factor for developing obesity, we measured ACSS2 levels. Both HFD/HF and HFD/HF + EEx treatments significantly downregulated ACSS2 expression (Fig. 2J and L). Intriguingly, DGAT1, another enzyme involved in lipogenesis, was markedly upregulated in only HFD/HF group, while EEx intervention completely reversed its upregulation (Fig. 2J and M).
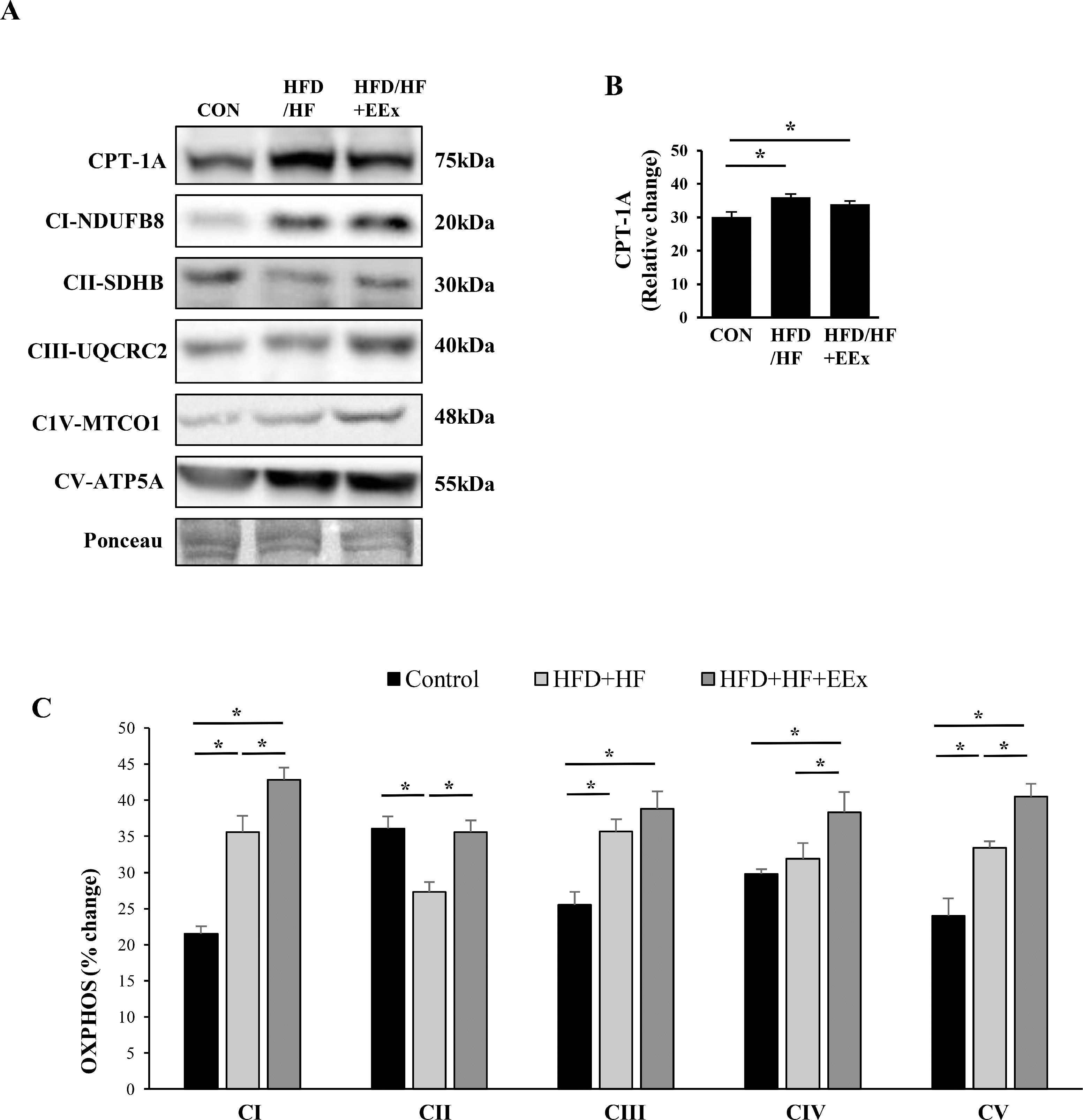
3.3EEx synergizes HFD/HF-mediated mitochondrial biogenesisTo examine if EEx -induced improvement in mitochondrial fatty acid transportation and mitochondrial biogenesis is associated with reducing a lipid droplet size, we measured mitochondrial fatty acid transportation protein CPT-1A and five crucial mitochondrial proteins localized in the electron transport chain complexes (complex I through V, referred to as OXPHOS). Both HFD/HF and HFD/HF + EEx treatments upregulated CPT-1A levels compared to the CON group (Fig. 3A and B). Interestingly, HFD/HF diet per se caused upregulation of most OXPHOS, and EEx synergized its effect. Specifically, in HFD/HF group, CI, CIII, and CV levels were elevated, but CII and CIV levels were downregulated and unaltered, respectively, compared to the CON group (Fig. 3A and C). In contrast, EEx intervention increased CI, CIII, CIV, and CV, compared to the CON; more importantly, EEx synergized HFD/HF-induced OXPHOS upregulation in three complex protein levels (e.g., CI, CIV, and CV) (Fig. 3A and C). Of note, while complex II levels were significantly downregulated in the HFD/HF group, EEx intervention restored the protein levels equivalent to those in the CON group (Fig. 3A and C).
Assessment of mitochondrial fatty acid transport protein (CPT-1A) and mitochondrial biogenesis (OXPHOS). A. Representative Western blot images of fatty acid transport marker (CPT-1A) and mitochondrial biogenesis (OXPHOS: CI, CII, CIII, CIV, and CV) in the liver. Ponceau staining was used as a loading control. B-C. Quantification of the CPT-1A and OXPHOS. Data show that both HFD/HF and HFD/HF + EEx improves CPT-1A and OXPHOS; however, HFD/HF downregulates CII and causes no changes in CIV (n=7-8 per group). An asterisk (*) indicates significant differences at p <0.05. CPT-1A: carnitine palmitoyl transferase 1A.
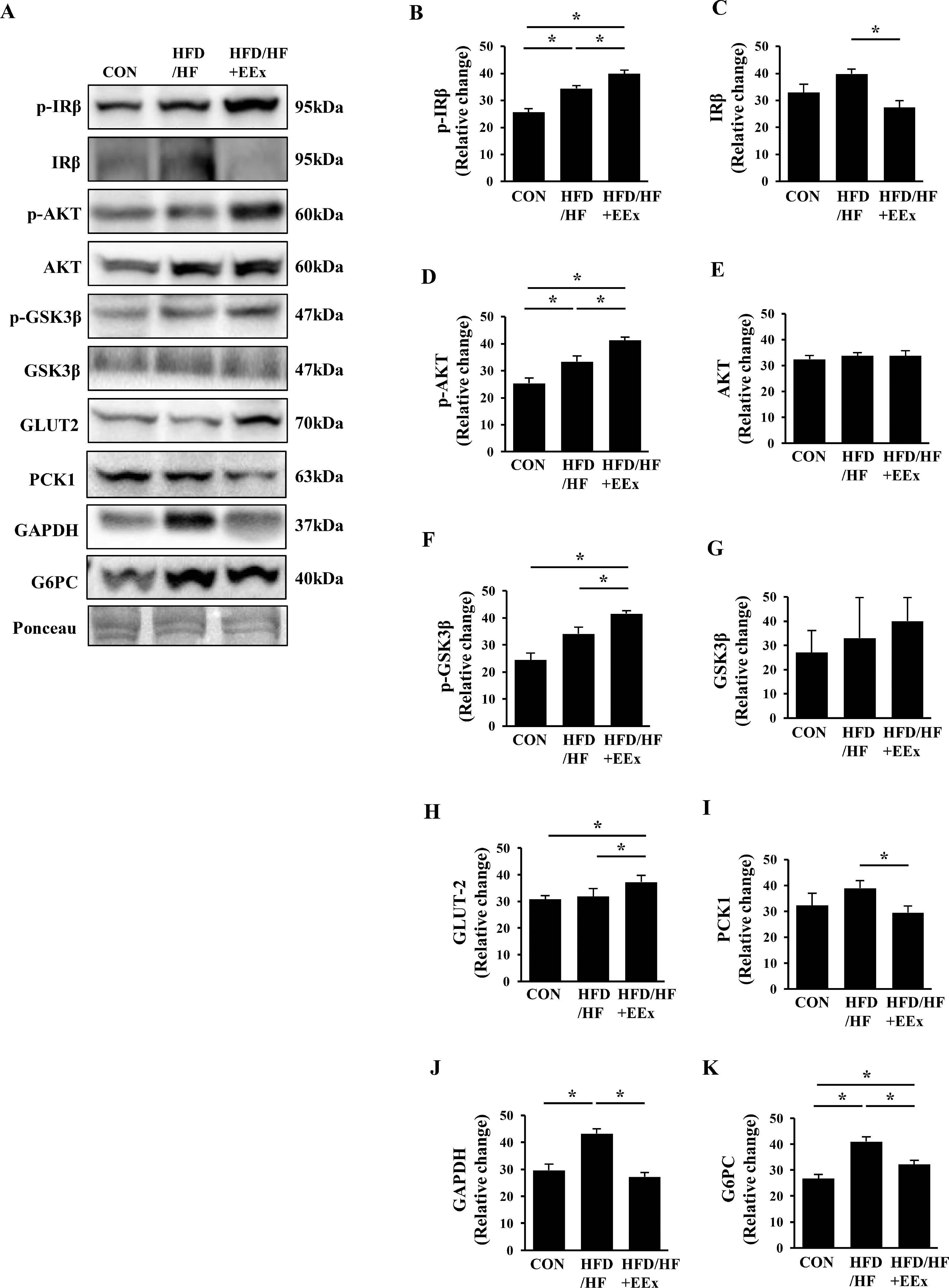
We assessed proteins involved in the insulin signaling cascade as well as glucose transporter 2 (GLUT2). Surprisingly, as opposed to our postulation, both HFD/HF and HFD/HF + EEx groups exhibited a significant rise in phosphorylation levels of insulin receptor beta (p-IRβ), compared to the CON group (Fig. 4A and B); interestingly, HFD/HF + EEx downregulated total (IRβ) levels (Fig. 4A and C) compared to other groups. In parallel with p-IRβ data, AKT phosphorylation levels were elevated in both HFD/HF and HFD/HF + EEx groups without changes in total AKT levels compared to the CON group (Fig. 4A, D, and E). We further examined the phosphorylation state of GSK3β, a downstream target of AKT and found that both HFD/HF and HFD/HF + EEx groups also augmented phosphorylated GSK3β levels, compared to the CON group; of note, GSK3β phosphorylation levels were higher in HFD/HF + EEx group without changes in total GSK3β levels, compared to those in the HFD/HF group (Fig. 4A, F, and G). Despite the enhanced insulin signaling cascade in both groups, only HFD/HF + EEx group showed GLUT2 upregulation (Fig. 4A and H).
Assessment of hepatic glucose regulation. A. Representative Western blot images of glucose uptake regulation markers (p-IRβ, p-AKT, p-GSKβ, and GLUT2) and gluconeogenesis markers (PCK1, GAPDH, and G6PC) in the liver. Ponceau staining was used as a loading control. B-K. Quantification of the glucose regulation markers. Data show that EEx improves glucose uptake signaling and prevents gluconeogenesis signaling (n=7-8 per group). An asterisk (*) indicates significant differences at p <0.05. IRβ: insulin receptor β, AKT: protein kinase B, GSK; glycogen synthase kinase, GLUT2: glucose transporter 2, PCK1: phosphoenolpyruvate carboxykinase1, GAPDH: glyceraldehyde-3-phosphate dehydrogenase, G6PC: glucose-6-phosphatase.
Since enhanced hepatic gluconeogenesis contributes to blood glucose dysregulation, we next examined if an HFD/HF diet modulates gluconeogenic protein expressions and whether EEx reinstates them back to normal levels. EEx maintained lower levels of phosphoenolpyruvate carboxykinase 1(PCK1) compared to the HFD/HF group (Fig. 4A and I) and prevented HFD/HF-induced upregulation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fig. 4A and J). More importantly, EEx attenuated HFD/HF-induced upregulation glucose-6-phosphatase (G6PC) (Fig. 4A and K).
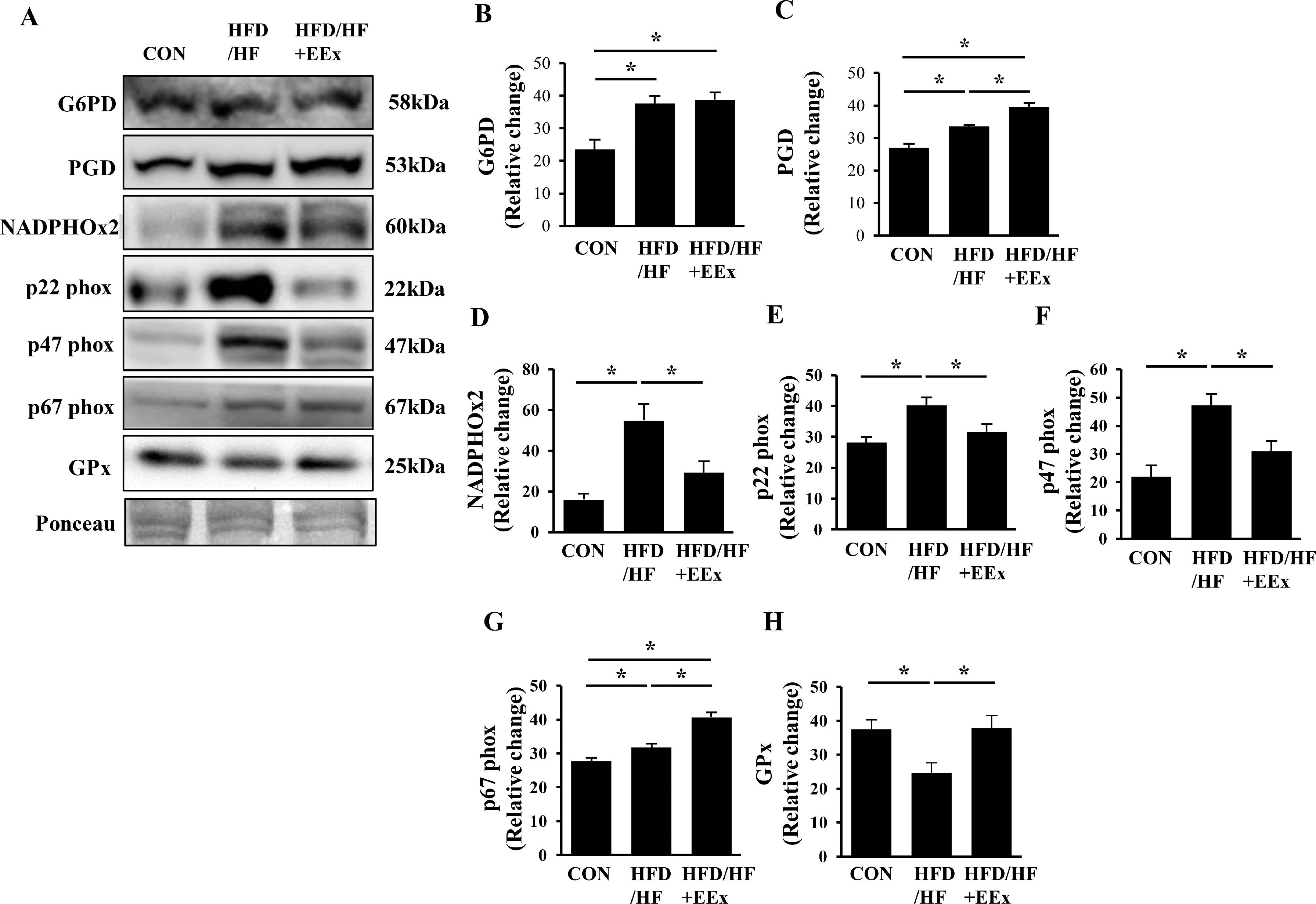
3.5EEx potentiates antioxidative capacity via the pentose phosphate pathway and reduces inflammation against HFD-HF diet-induced NAFLDNADPH can be used oxidatively (e.g., superoxide anion production) or anti-oxidatively (e.g., reduction of oxidized glutathione), or biosynthetically (e.g., lipogenesis). Since glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (PGD) are critical enzymes that produce NADPH through the pentose phosphate pathway, we measured these two proteins levels. Both HFD/HF and HFD/HF + EEx groups significantly upregulated G6PD and PGD, compared to the CON group; however, PGD levels were higher in the HFD/HF + EEx group (Fig. 5A, B, and C), indicating more production of NADPH in HFD/HF-treated groups. To examine the potential usage of NADPH, we next measured NADPH oxidase 2 (NADPHOx2) and its subunits p22 phox, p47 phox, and p67 phox because these NADPHOx2 complexes use NADPH as a substrate to produce superoxide anions. HFD/HF diet increased NADPHOx2, p22 phox, p47 phox, and p67 phox compared to those in the CON group, while EEx prevented those proteins from rising except p67 phox (Fig 5D-G). GPx transfers a thiol group from glutathione to oxidizing molecules to mitigate oxidative stress. While an HFD/HF diet significantly suppressed GPx levels, EEx restored its levels comparable to the CON group (Fig. 5A and H).
Assessment of oxidative stress potentials associated with the pentose phosphate pathway (PPP). A. Representative Western blot images of the primary enzymes in PPP (G6PD and PGD) and pro-oxidative enzyme (NADPHOx2 and its subunits p22phox, p47phox, and p67phox) and antioxidative enzyme (GPx). Ponceau staining was used as a loading control. B-H. Quantification of NADPHOx2 and its subunits p22phox, p47phox, and p67phox), and GPx. Data show that while both HFD/HF and HFD/HF + EEx promote the PPP, EEx improves antioxidant potentials (n=7-8 per group). An asterisk (*) indicates significant differences at p <0.05. G6PD: glucose-6-phosphate dehydrogenase, PGD: 6-phosphogluconate dehydrogenase, NADPHOx2: NADPH oxidase 2, p22phox: a membrane-bound protein and the final electron transporter from NADPH, contributing to superoxide generation from NADPHOx2, p47phox: a critical cytosolic protein of the superoxide-generating NADPHOx2, p67 phox: a critical cytosolic protein of the superoxide-generating NADPHOx2, GPx: glutathione peroxidase.
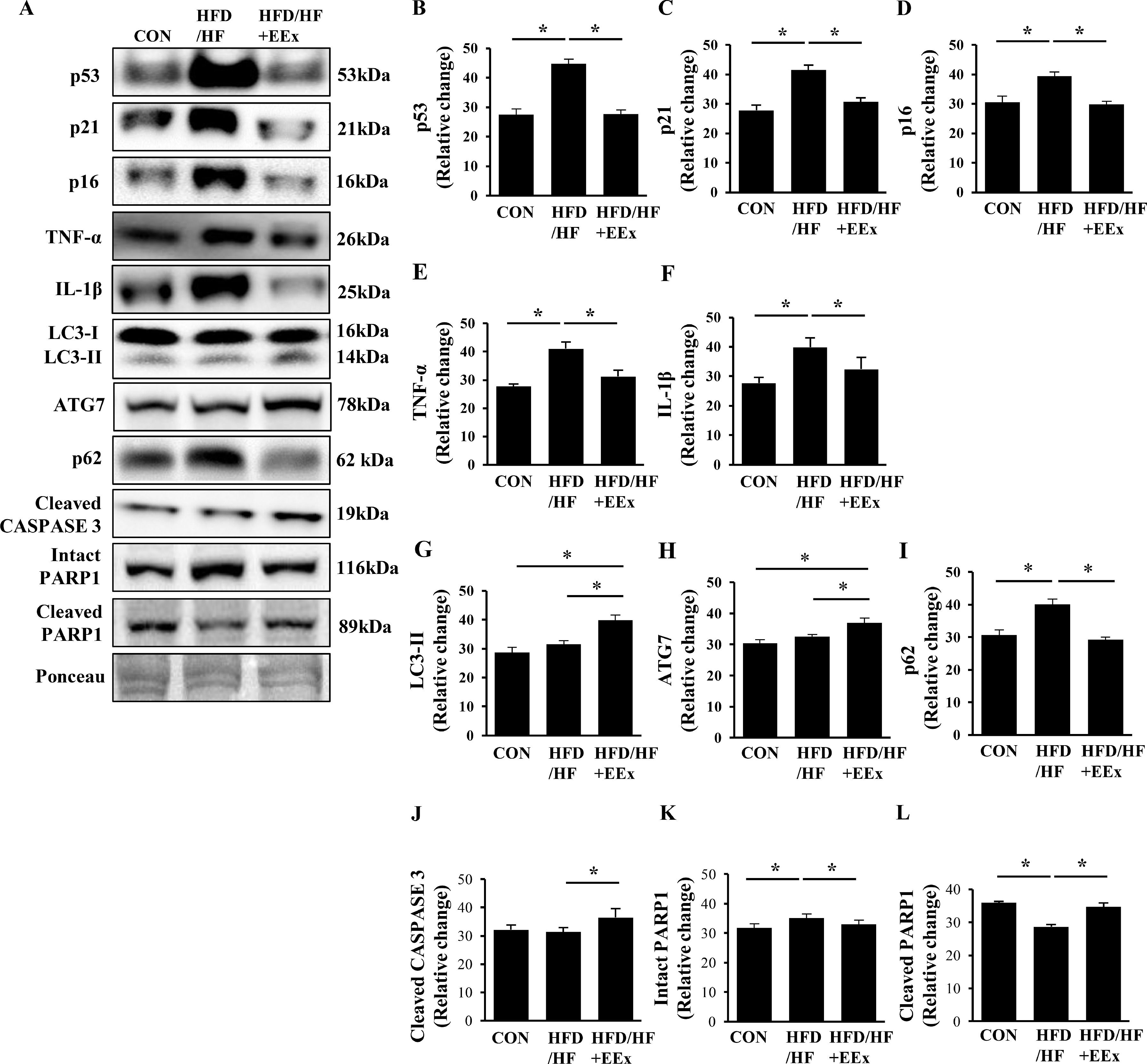
NAFLD is known to increase senescence, characterized by a cell cycle arrest (preventing apoptosis) and inflammation. To explore if EEx appeases NAFLD-induced senescence, we assessed senescence-related proteins such as tumor-suppressing proteins (p53) and cyclin-dependent kinase inhibitors (p21 and p16). Our results showed that HFD/HF upregulated p53, p21, and p16; conversely, EEx completely prevented the HFD/HF-induced upregulation of those proteins (Fig. 6A-D). The release of inflammatory cytokines along with a cell cycle arrest is a primary hallmark of senescence. Our data showed that HFD/HF diet markedly increased pro-inflammatory cytokines TNF-α and IL-1β; contrarily, EEx prohibited the upregulation of those cytokines (Fig. 6A, E, and F).
Assessment of hepatic senescence, autophagy, and apoptosis. A. Representative Western blot images of senescence (p53, p21, and p16), pro-inflammatory cytokines (TNF-α and IL-1β), autophagy (LC3-II, ATG7, and p62), and apoptosis markers (cleaved Caspase 3, intact PARP1, cleaved PARP1) in the liver. Ponceau staining was used as a loading control. B-G. Quantification of the senescence, autophagy, and apoptosis markers. Data show that EEx prevents HFD/HF diet-induced senescence and improves autophagy and cell turnover (n=7-8 per group). An asterisk (*) indicates significant differences at p <0.05. p53: tumor suppressor protein 53, p21: cyclin-dependent kinase inhibitor 1, p16: cyclin-dependent kinase inhibitor 2A, TNF-α: tumor necrosis factor- α, IL-1β: interleukin-1 β, LC3: microtubule-associated protein 1A/1B-light chain 3, ATG7: autophagy related protein 7, p62: ubiquitin-binding protein 62, CASPASE 3: cysteine-dependent aspartate-directed protease 3, PARP1: Poly (ADP-ribose) polymerase 1.
Since defective autophagy (e.g., accumulation of p62 with or without LC3-II upregulation) is associated with the pathogenesis of NAFLD and EEx promotes autophagy in the liver, we examined if EEx-induced autophagy restoration against HFD/HF diet contributes to ameliorating NAFLD. HFD/HF diet resulted in no changes in LC3-II and ATG7, but EEx elevated those autophagy related proteins compared to the CON and HFD/HF groups (Fig. 6A, G and H). More importantly, while HFD/HF diet led to accumulate p62 levels, EEx completely reversed its accumulation (Fig. 6A and I). Next, we examined apoptosis. EEx increased the active (cleaved) form of CASPASE 3 levels, but its levels were not altered in the HFD/HF group. (Fig. 6A and J). Cleaved PARP1 levels were lower in the HFD/HF group, compared to both CON and HFD/HF + EEx groups (Fig. 6A, and K).
4DiscussionThe present study provides four prime mechanisms demonstrating how EEx targets multilateral interactions of cellular signaling and modulates various metabolic paradigms to prevent HFD/HF diet-induced NAFLD in young adult female mice. First, EEx-induced counteraction against lipogenesis and improvement in mitochondrial biogenesis is linked to the reduced size of lipid droplets in the liver. Second, EEx reprograms metabolic processes to deter gluconeogenesis. Third, EEx-mediated activation of the pentose-phosphate pathway is associated with improving the antioxidative defense system. Fourth, EEx prevented hepatic senescence by enhancing autophagy and cell turnover.
The disequilibrium of fat metabolism (e.g., reduced lipolysis and increased lipogenesis) within the hepatocytes contribute to NAFLD in both male and female mice [5, 6, 34]. Our study supports this notion because enhanced lipolysis by EEx concurred with reduced lipid droplet size in the liver of HFD/HF-fed female mice. However, we cannot attest to this finding as a sole mechanism responsible for the reduced lipid droplet size in response to EEx because HFD/HF treatment per se in our study and another study promoted lipolysis [4], prompting us to postulate that modulation of lipogenesis would contribute to EEx-induced reduction in the size of the lipid droplets.
Lipogenic enzymes ACLY and DGAT1 appear to play a pivotal role in the pathogenesis of hepatic steatosis since downregulation of ACLY and DGAT1 via adenovirus-mediated RNA interference and knockout, respectively, in the liver inhibited hepatic lipogenesis and prevented hepatic steatosis [19, 35]. Our data also support their finding because EEx-mediated ACLY and DGAT1 downregulation was associated with a significant decline in the size of lipid droplets. This finding is important in that our results for the first time suggest that EEx-induced lipogenic protein suppression may be a crucial mechanism associated with attenuating NAFLD in female mice.
Mitochondrial impairment and deficient mitochondrial biosynthesis are linked to the development of NAFLD in male mice [36, 37]. In contrast, we observed that HFD/HF diet elevated most of OXPHOS (e.g., CI, CIII, and CV) and CPT-1A, indicating that HFD/HF per se facilitates fatty acid oxidation via mitochondrial biogenesis in female mice. Consistent with our observation, two human studies (female dominant) using isotope tracers have demonstrated that the rate of mitochondrial fatty oxidation (e.g., beta-oxidation, Krebs cycle, ETC) significantly increases in NAFLD patients [38, 39]. These findings suggest that NAFLD in female subjects may enhance mitochondrial biogenesis as an adaptive response. Besides HFD/HF-induced mitochondrial biogenesis, EEx synergistically promoted more mitochondrial biogenesis (e.g., increased all five mitochondrial OXPHOS: CI-CV) in conjunction with CPT-1A. Collectively, it should be noted that HFD/HF-mediated mismatched mitochondrial biogenesis (e.g., upregulation of CI, CIII, CV, but downregulation of CII without a change in CIV) seems to signify potential mitochondrial impairment and contribute to NAFLD. For example, downregulation of CII is associated with NAFLD [40]. Also, since CIV, a rate-limiting enzyme in the ETC, determines the rate of oxidative phosphorylation [41], a partial increase in OXPHOS activities in the absence of a proportional increase in CIV may pose a backlog of electrons along with the ETC, leading to superoxide production. This maladaptive mitochondrial response may be the potential pathogenesis of NAFLD. On the contrary, restoration of CII and upregulation of CIV by EEx suggests that EEx-mediated improvement in mitochondrial function in parallel with enhanced lipolysis and FA transportation capacity may underlie the attenuation of NAFLD in female mice.
Excessive fatty acid oxidation promotes hyperglycemia in the human fatty liver by promoting gluconeogenesis [38, 39]. Since we reported in our previous study that EEx improved HFD/HF diet-induced hyperglycemia [24], we examined gluconeogenesis pathway in the present study, and found that EEx prevented HFD/HF diet-induced upregulation of prime gluconeogenic enzymes (e.g., PCK1, GAPDH, and G6PC) and increased GLUT2 levels. This finding suggests that EEx-induced gluconeogenesis suppression may be a novel blood glucose regulatory mechanism that attenuates NAFLD-induced hyperglycemia.
NADPH can be utilized for fatty acid synthesis, antioxidant production (e.g., glutathione reduction) and as an electron donor to NADPHOx that generates a potent free radical superoxide anion. The PPP is a prime process by which NADPH is produced through enzymes glucose-6-phosphate dehydrogenase (G6PD) and 6-phospho-gluconolactone dehydrogenase (PGD). A recent study has reported that increased PPP activity is associated with lipogenesis in the obese rat liver [42]. Also, an increase in a liver specific NADPHOx2 is linked to oxidative injuries in human NAFLD patients [43] whereas NADPHOx2 deficient mice ameliorate NAFLD [44]. Our data support the possibility of maladaptive PPP activities in the pathogenesis of NAFLD because HFD/HF diet-induced PPT activities (e.g., increased G6PD and PGD levels) were associated with increased NADPHOx2 levels, reduced GPx, and inflammation. Conversely, although increasing the PPT activities, EEx intervention completely reversed those oxidative stress, indicating that NADPH produced from the enhanced PPP in response to EEx may be used to facilitate an antioxidant defense capacity. This potential protective mechanism of EEx against NAFLD-mediated oxidative stress in female mice is novel. As such, it remains unknown whether this mechanism is sex-specific or not; thus, further gender studies are warranted.
Recent studies have reported that senescence is exhibited in NAFLD of male mice and rats [45, 46]. Our results also showed that the HFD/HF diet triggered hepatic senescence in female mice, suggesting that premature senescence exhibited in NAFLD is not a gender-specific phenomenon. As opposed to the HFD/HF diet, EEx entirely prevented senescence phenotypes (e.g., senescence biomarkers and anti-apoptosis). This finding suggests that EEx-induced restoration of normal cell turnover (e.g., apoptosis) may exert a potential role in safeguarding the liver against NAFLD. However, Fealy et al. have reported a contradictory result that EEx instead reduces apoptosis [47]. The reason for this discrepant result may be explained by the fact that aside from the difference in the model system (mice vs. humans), gender (female vs. mixed), and exercise duration (long-term for our study vs. acute for their study) could generate the conflicting observation. It appears probable that acute EEx may initially suppress apoptosis; then, chronic EEx may proceed to apoptosis as senescent cell numbers grow to remove dysfunctional cells at a level equivalent to those in normal diet mice. Further studies should consider this attention to identify acute and chronic mechanisms of EEx-induced protection against NAFLD on a gender basis.
Proper management of autophagy plays a crucial role in cell protection against NAFLD, as the absence of autophagy leads to the accumulation of TG in hepatocytes [29]. Autophagy is assessed by measuring microtubule-associated protein B-light chain 3 II (LC3-II: active form of LC3), autophagy rate-limiting protein ATG7 [29], and p62 as an autophagy flux indicator [48]. A recent clinical study has shown that p62 accumulation (i.e., autophagic flux dysfunction) contributes to NAFLD [49]. We observed similar results showing p62 accumulation in HFD/HF-treated mice, supporting the notion that impaired autophagic flux may be associated with NAFLD development. EEx has been shown to enhance hepatic autophagy in male mice [30]. In the present study, we found that EEx reversed HFD/HF-induced autophagic dysfunction (e.g., abolished p62 accumulation and increases in LC3-II and ATG7 levels) in female mice. This finding suggests that EEx-induced autophagy improvement may delay the progression to NAFLD, possibly independent of gender since EEx-induced autophagy has been reported in both genders. It is important to note that because autophagy levels were assessed 24 hours after the last session of EEx, we affirm that EEx stimulates basal autophagy.
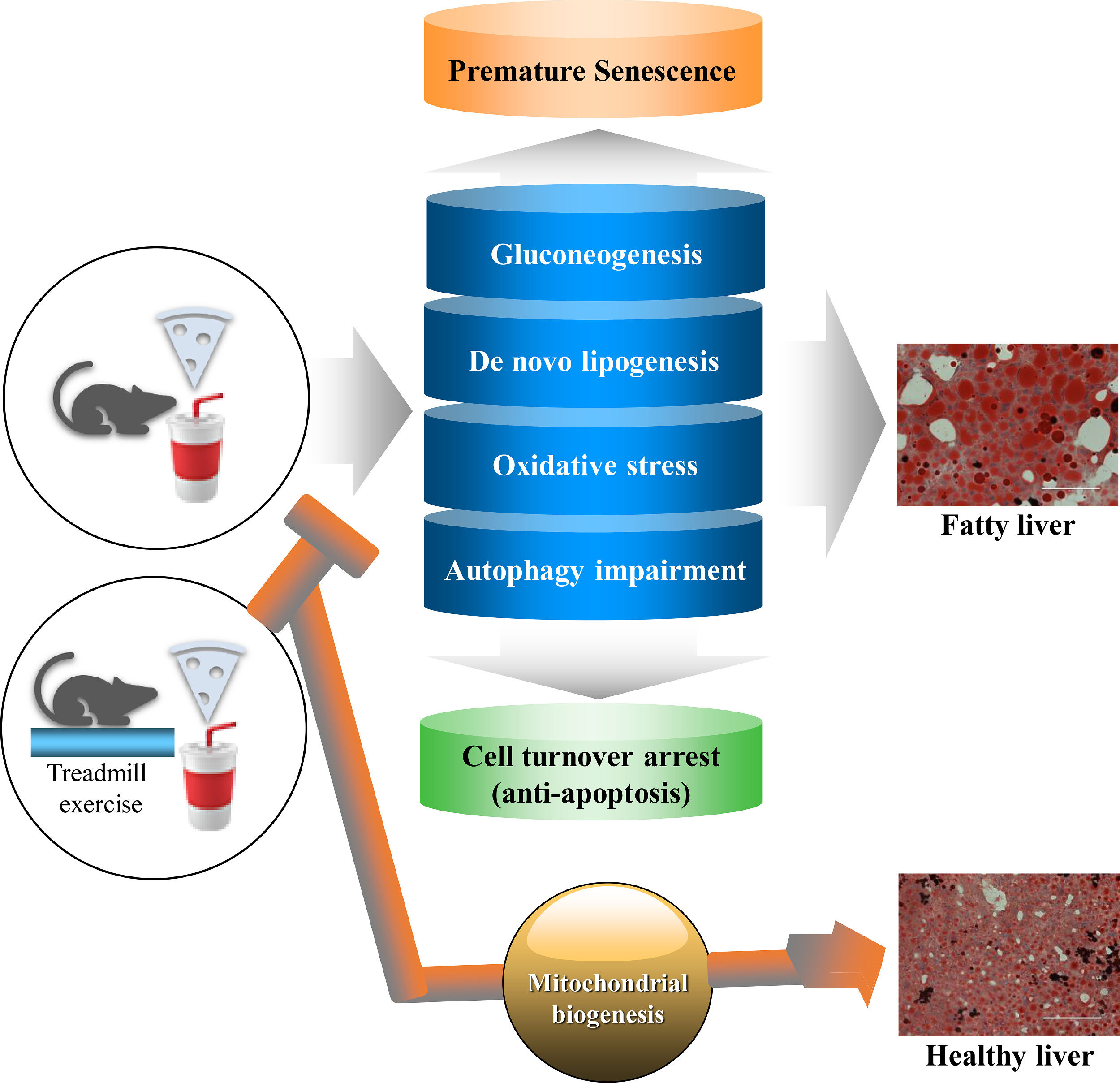
5ConclusionAs illustrated in Fig. 7, our study using a female mouse model of NAFLD shows that the EEx-induced metabolic reprogram (e.g., suppressed gluconeogenesis and de novo lipogenesis), potentiation of the pentose phosphate pathway toward establishing an antioxidant capacity, and restoration of autophagy against HFD/HF concur with prevention of premature senescence and apoptosis arrest. Thus, the present study suggests that EEx-induced hepatic protection against NAFLD may require comprehensive cellular interactions that help maintain normal hepatic function and morphology
Summary of a potential protective mechanisms of EEx against HFD/HF-induced NAFLD. EEx-induced mitigation of gluconeogenesis, de novo lipogenesis, oxidative stress, and autophagy impairment results in preventing premature senescence and improving cell turnover via apoptosis, leading to liver protection against NAFLD. Our study suggests that regular EEx may restore normal liver function by reversing the canonical paradigms of HFD/HF-induced NAFLD.
This project was funded by awards from the University of West Florida Kugelman Honors Program (Joshua J. Cook), Office of Undergraduate Research (Joshua J. Cook), and Usha Kundu Research Endowment (Youngil Lee).
Declaration of interestNone.
Author contributionsJC, MW, BS, LC, JS, ST, YK, SK, and YL participated in the design of the study; JC, MW, BS, and YL conducted animal handling, biochemical assays, and data collection; JC, BS, and YL contributed to data analysis and interpretation of results. All authors contributed to the manuscript writing. All authors have read and approved the final version of the manuscript and agreed with the order of presentation of the authors. Youngil Lee is the corresponding author in charing of the integrity of the work as a whole.