Cholangiocarcinoma is a highly lethal carcinoma. Exosomes derived from cancer-associated fibroblasts (CAFs) serve key roles in the crosstalk between CAFs and cancer cells. Exploring the roles of CAF-derived exosomes and the mechanisms contribute to a better understanding of the development of cholangiocarcinoma.
Materials and MethodsCarcinoma and para-carcinoma tissues were collected from patients. Exosomes were isolated from CAFs and characterized by transmission electron microscopy, dynamic light scattering and western blot. Cholangiocarcinoma cells were cocultured with CAF-derived exosomes, and its proliferation, migration and invasion were evaluated with CCK-8, EdU incorporation and transwell assays, respectively. The interaction between a long non-coding RNA linc00152 and an RNA-binding protein hnRNPA2B1 was determined with RNA immunoprecipitation and RNA pull-down. The ubiquitination of hnRNPA2B1 was examined with western blot.
ResultsLinc00152 was highly expressed in cholangiocarcinoma tissues and cells, and its increased expression was associated with advanced tumor stage and poor prognosis. Linc00152 was highly enriched in CAFs and CAF-derived exosomes. CAF-derived exosomes promoted cholangiocarcinoma cell proliferation, migration, and invasion by delivering linc00152. Further analysis showed that hnRNPA2B1 recruited linc00152 and enhanced its loading into exosomes. The interaction between hnRNPA2B1 and linc00152 was identified, and linc00152 repressed the proteasome-dependent degradation of hnRNPA2B1 in cholangiocarcinoma cells. The oncogenic activities of linc00152 in cholangiocarcinoma cells were dependent on hnRNPA2B1 upregulation.
ConclusionsCAF-derived exosomes harboring linc00152 enhance malignancy in cholangiocarcinoma, identifying a novel role of exosomal linc00152 for intensifying the crosstalk between CAFs and cholangiocarcinoma cells.
Cholangiocarcinoma is a relatively rare but highly lethal cancer that originates from the bile ducts and classified as intrahepatic, perihilar, and distal cholangiocarcinoma according to its anatomical location [1]. As the second most common primary liver carcinoma, cholangiocarcinoma accounts for ∼15 % of all primary hepatic malignancy. It is particularly concerning that the incidence and mortality of cholangiocarcinoma are still increasing worldwide, especially in specific regions such as Thailand and China. The systemic treatment scenario involving adjuvant therapy and immunotherapy for cholangiocarcinoma is evolving [2–5]. Despite advances in the diagnosis and treatment for cholangiocarcinoma, the five-year survival rate is as low as 7–20 % with a high tumor recurrence rate [6,7]. Even when it is diagnosed at an early stage, patients only have a 20–30 % five-year survival due to its high invasion and chemotherapy resistance [8]. Therefore, further exploration of the development of cholangiocarcinoma is urgently needed for a better understanding of the pathogenesis and developing novel diagnostic and therapeutic approaches to improve patient prognosis.
Despite great advances in cancer biomarkers, the complex tumor microenvironment and the genetic instability of tumor cells make it difficult to apply molecular diagnosis and targeted therapy to clinical practice [9]. Cancer-associated fibroblasts (CAFs) are primary architects that shape the tumor microenvironment through various mechanisms such as secreting active factors, crosstalk with tumor cells, immune cell infiltration and extracellular matrix remodeling, driving cancer progression [10]. Exosomes are key mediators in CAF-cancer cell communication, and emerging evidence has demonstrated that CAF-derived exosomes reshape the tumor microenvironment and promote malignant behaviors via trafficking into cancer cells [11,12]. In intrahepatic cholangiocarcinoma, CAFs extensively interact with cancer cells to promote cancer growth [13], and exosomes derived from cholangiocarcinoma cells promote CAF activation [14]. Cholangiocarcinoma cell and tumor-associated macrophage-derived exosomes promote the progression of cholangiocarcinoma [15,16]. However, the biological activity of CAF-derived exosomes in shaping the tumor microenvironment, communicating with cancer cells, and regulating cancer progression in cholangiocarcinoma remains poorly understood.
Exosomes regulate cholangiocarcinoma progression via delivering cellular components such as lipids, proteins, and nuclear acids into cancer cells. Non-coding RNAs present in exosomes are emerging as key regulators in the pathogenesis and progression of cholangiocarcinoma. Exosomal lncRNA HCG18 enhances growth and metastasis in cholangiocarcinoma [13]. Exosomal miR-3124-5p facilitates the development of cholangiocarcinoma by targeting GDF11 [17]. Linc00152, located on chromosome 2p11.2, is a long non-coding RNA with a length of 828 bp. Linc00152 is upregulated in various cancers including lung, gastric and hepatocellular carcinomas and serves as a driver of cancer progression [18]. Only a previous preprint reported that linc00152 was highly expressed in cholangiocarcinoma, and its silencing inhibited the proliferation and migration of cholangiocarcinoma cells [19]. However, the roles of linc00152 and its action mechanisms in cholangiocarcinoma are largely unknown. We analyzed the TCGA and GEO database and found that linc00152 was aberrantly upregulated in cholangiocarcinoma. Moreover, a previous single-cell sequencing analysis of hilar cholangiocarcinoma cells divided tumor cells into ten subclusters. The subcluster ten accounted for 2.41 % with molecular markers COL1A1, COL1A2, ACTA2 and FN1, and is typical fibroblast cluster. The subcluster eight accounted for 4.99 % with molecular markers VWF, POSTN, COL4A1 and ACAM. Referring to the cluster analysis of intrahepatic cholangiocarcinoma fibroblasts by Zhang et al. [20], the cells in the subcluster eight resembled vascular fibroblasts. Both subcluster eight and subcluster ten showed high expression of linc00152, especially the subcluster ten, suggesting that linc00152 might exert functions in cholangiocarcinoma and cholangiocarcinoma-associated fibroblasts. A study identified that linc00152 was detected in exosomes derived from gastric cancer patient plasma [21]. In addition, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), a RNA-binding protein, facilitates RNA loading into exosomes via binding the EXO motif GGAG [22], and we identified potential interaction between linc00152 and hnRNPA2B1 through Starbase (a platform for systematically identifying the RNA-RNA and protein-RNA interaction networks, http://starbase.sysu.edu.cn/) [23] and RBPsuite (a tool for RNA-binding proteins site prediction, http://www.csbio.sjtu.edu.cn/bioinf/RBPsuite/) [24]. Thus, we hypothesized that CAF-derived exosomes might be involved in the regulation of cholangiocarcinoma progression via transporting linc00152 into cancer cells, and hnRNPA2B1 might control the packaging of linc00152 into exosomes.
In this study, we found that increased linc00152 expression was associated with cholangiocarcinoma progression and poor outcome of patients. hnRNPA2B1 facilitated the packaging of linc00152 into exosomes, and linc00152 suppressed hnRNPA2B1 degradation in cholangiocarcinoma cells. CAF-derived exosomal linc00152 promoted malignant phenotypes of cholangiocarcinoma cells by upregulating hnRNPA2B1, identifying a novel role of exosomal linc00152 in the crosstalk between CAFs and cholangiocarcinoma cells. Our study suggests potential diagnostic and therapeutic targets such as linc00152 for cholangiocarcinoma.
2Patients and Methods2.1Clinical specimens and ethical statementPatients (n = 24) were diagnosed with cholangiocarcinoma and divided into two groups according to tumor size (≤3, n = 11 and >3, n = 13), tumor cell differentiation (well-moderate, n = 12 and poor, n = 12), lymph node metastasis (yes, n = 12 and no, n = 12) or TNM staging (I-II, n = 15 and III-IV, n = 9). Cholangiocarcinoma and para-carcinoma tissues were collected from those patients. Patients provided written informed consent, and this study was approved by the Ethics Committee of our hospital.
2.2Isolation and culture of normal fibroblasts (NFs) and cancer-associated fibroblasts (CAFs)Cholangiocarcinoma and para-carcinoma tissues were cut into small pieces and digested in collagenase IV (500 μg/mL, Sigma, St. Louis, MO, USA) and DNase I (100 μg/mL, Sigma) solution for 2 h at 37 °C. The samples were filtered using a 70 μm-mesh, and cells were centrifuged and re-suspended in ACK buffer (Thermo Fisher Scientific, Waltham, MA, USA) for lysing red blood cells. Subsequently, cells were washed, and anti-human fibroblast microbeads from Miltenyi Biotec (Bielefeld, Germany) were applied to isolate CAFs and NFs from cholangiocarcinoma and para-carcinoma tissues, respectively. NFs and CAFs were maintained in DMEM F12 medium/10 % FBS (Thermo Fisher Scientific). The medium was removed, and fresh serum-free DMEM F12 medium (4 mL) was added for additional 24 h of cultivation. Then, the conditioned medium (CM) was harvested, filtered, and applied for subsequent assays.
2.3Isolation, characterization and internalization of NF and CAF-derived exosomesFor isolating NF and CAF-derived exosomes, NF and CAFs were cultured in serum-free DMEM F12 medium for 36 h, and the culture medium was collected. The debris and residual cells were removed through centrifugation, and exosomes were isolated from the supernatants with Total Exosome Isolation Reagent (Thermo Fisher Scientific) following the manual.
After isolation, exosomes were pelleted, fixed in 2.5 % glutaraldehyde solution and processed for sectioning, staining and imaging via transmission electron microscopy (TEM) as previously described [25]. The size distribution of isolated exosomes was determined with dynamic light scattering (DLS) using DynaPro NanoStar Dynamic Light Scattering Detector (Wyatt Technology, Santa Barbara, CA, USA) according to the manufacturer's suggestions. The abundance of positive and negative exosome biomarkers including CD63, TSG101 and GM130 was determined with western blot.
Exosomes were stained with PKH67 (Sigma) at 1 μM for 20 min and washed twice in PBS. Then, exosomes were co-cultured with HCCC-9810 cells for 12 h, and cells were fixed in 4 % paraformaldehyde and labeled with DAPI (Beyotime, Shanghai, China).
2.4Cell culture and transfectionHIBEC, CCLP-1, HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939 and RBE cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM/10 % FBS (Thermo Fisher Scientific). In some assays, HCCC-9810 and RBE cells were co-cultured with NF or CAF-derived exosomes at 100 μg/mL for 48 h. For genetic modification, linc00152 shRNA (KD linc00152), hnRNPA2B1 siRNA (si-hnRNPA2B1) and scramble controls (KD-NC and si-NC) were provided by RiboBio (Guangzhou, Guangdong, China). Linc00152 and hnRNPA2B1-coding sequences were cloned into pcDNA 3.1 vector (Thermo Fisher Scientific). HCCC-9810 and RBE cells and CAFs were transfected with indicated constructs using Lipofectamine 3000 following the manual. After 72 h, cells were harvested for subsequent analysis.
2.5Examination of cholangiocarcinoma cell proliferationAfter indicated treatment, cholangiocarcinoma cell proliferation was evaluated with CCK-8 and EdU incorporation assays. For CCK-8, the culture medium was removed, and 100 μL of fresh pre-warmed medium and 10 μL of CCK-8 reagent were mixed and added into each well. After 2 h of incubation, the absorbance at 450 nm was measured. The Click-iT™ EdU Imaging Kit (Thermo Fisher Scientific) was used for EdU incorporation analysis. Briefly, EdU (10 μM) was added into cells for incubation. After 2 h, cells were fixed and permeabilized. The Click-iT reaction cocktail was prepared, and EdU was detected using the Click-iT reaction cocktail following the manual. Cells were counter-stained with DAPI and imaged.
2.6Wound healing assayHCCC-9810 and RBE cells were co-cultured with exosomes or genetically modified, and their migratory capacity was evaluated with wound healing assay. Briefly, cells (5 × 105) per well were seeded in six-well plates and cultured into a monolayer. The wound was made on the monolayer with a 100 μL-pipette tip, and cells were incubated for additional 24 h. Wound closure was photographed and analyzed using the ImageJ software.
2.7Transwell invasion assayTranswell inserts (24 mm, Corning, Corning, NY, USA) were used to analyze cell invasion. HCCC-9810 and RBE cells (1 × 105) were suspended in serum-free medium and seeded in the Matrigel (Corning)-coated upper chamber, and DMEM/10 % FBS was added into the lower chamber. The transwell inserts were placed into six-well plates, and cells were incubated for 24 h. Cells that invaded into the lower chamber were washed, fixed, and labeled with crystal violet (0.5 %) for imaging and counting.
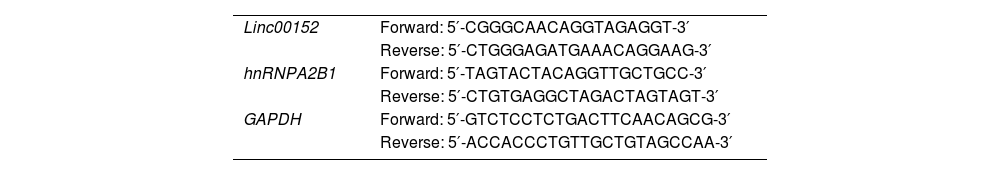
2.8RNA extraction and quantitative reverse transcription-PCR (qRT-PCR)RNA was extracted from NFs, CAFs, HIBEC, CCLP-1, HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939 and RBE cells, para-carcinoma and cholangiocarcinoma tissues using miRNeasy Tissue/Cells Advanced Micro Kit (Qiagen, Germantown, MD, USA) following the manual. Total Exosome RNA & Protein Isolation Kit (Thermo Fisher Scientific) was used to isolate RNA and protein from exosomes. RNA was quantified and reversely transcribed into cDNA with PrimeScript RT Reagent Kit (Takara, Kusatsu, Shiga, Japan). Linc00152 and hnRNPA2B1 were detected with quantitative PCR with SYBR Green Master Mix (Bio-rad, Hercules, CA, USA), and their expression was normalized to GAPDH and calculated using the 2−∆∆Ct method. Primers were shown in Table 1.
2.9Western blotProtein was extracted from cells and exosomes using Protein Extraction Kit (Beyotime) and Total Exosome RNA & Protein Isolation Kit (Thermo Fisher Scientific) following the manuals. Protein was quantified, and 30 μg of protein was electrophoresed and transferred to PVDF membrane. After block in 5 % BSA solution, membranes were incubated with a rabbit CD63 (1:2000, ab134045), TSG101 (1:1000, ab133586), GM130 (1:1000, ab52649), hnRNPA2B1 (1:500, ab31645) or β-actin (1:5000, ab8227) for 2 h. Then, membranes were washed and incubated in an HRP goat anti-rabbit IgG (1:10,000, ab6721) for 1 h. Bands were visualized after ECL (Beyotime) addition and analyzed using ImageJ software.
2.10RNA pull-down and immunoprecipitation (RIP) assaysCAFs, HCCC-9810 and RBE cells (5 × 106) were suspended and incubated in cold lysis buffer for 30 min on ice. Cell lysates were collected after centrifugation, and 10 μL of the lysates was used as the input control. For RNA pull-down, the biotin-conjugated linc00152 sense or antisense probe was added into cell lysates and mixed well, and samples were incubated with gentle rotation overnight. Next day, streptavidin magnetic beads (Beyotime) were added, and samples were incubated for additional 2 h with gentle rotation. The complexes attached on magnetic beads were eluted, and the abundance of hnRNPA2B1 was examined via western blot. For immunoprecipitation, Protein A/G magnetic beads (Abcam) were pre-coated with a rabbit hnRNPA2B1 antibody (5 μg, ab31645) or normal rabbit IgG (5 μg, ab37415). Beads were added into cell lysates, and samples were incubated overnight with gentle rotation. Subsequently, RNA was extracted from the complexes immunoprecipitated by the hnRNPA2B1 antibody and subjected to qRT-PCR analysis of linc00152.
2.11Statistical analysisData in our study was generated from at least three independent assays, analyzed using SPSS 17.0 and presented as mean ± SD. Data variance in two and more groups was analyzed by the Student's t-test and one-way analysis of variance (ANOVA), respectively. P value < 0.05 was statistically significant. The Kaplan–Meier curve was applied to analyzed the variance of survival rate of patients.
2.12Ethical statementWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee of Henan Provincial People's Hospital (APPROVAL NUMBER/ID: No.2021-144).
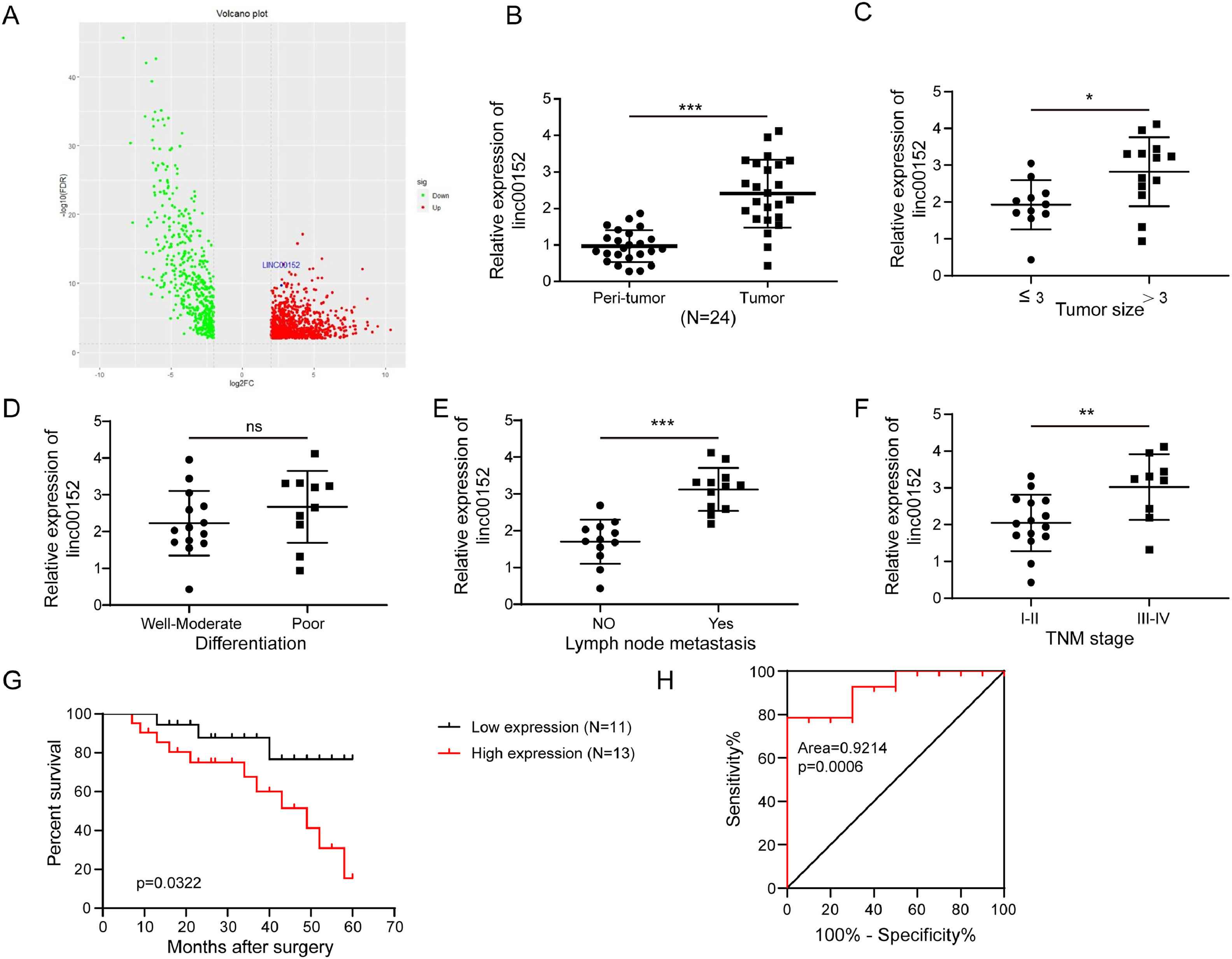
3Results3.1Highly expressed linc00152 was correlated to cholangiocarcinoma progressionTo explore the activity of linc00152 in cholangiocarcinoma, we downloaded The Cancer Genome Atlas Program (TCGA) data and analyzed linc00152 expression. Differential gene expression analysis showed increased linc00152 expression in cholangiocarcinoma (Fig. 1A). We collected cholangiocarcinoma and para-carcinoma tissues from cholangiocarcinoma patients and analyzed linc00152 expression. Compared to para-carcinoma tissues, cholangiocarcinoma tissues showed highly expressed linc00152 (Fig. 1B). Furthermore, we observed higher linc00152 expression in patients with larger tumor size (>3), lymph node metastasis or higher TNM stage (III-IV), but no significant change was observed in well-moderately and poorly differentiated tumors (Fig. 1C–F). Also, patients with higher linc00152 expression had lower survival (Fig. 1G). To analyze the diagnostic accuracy of linc00152, we measured the area under the receiver operating characteristic (ROC) curve (AUC). The AUC of linc00152 was 0.9214 (Fig. 1H), suggesting that linc00152 was a potential diagnostic biomarker for cholangiocarcinoma. These results suggested that increased expression of linc00152 was correlated to cholangiocarcinoma progression and indicated poor prognosis.
Increased linc00152 expression was correlated to cholangiocarcinoma progression. (A) Differential gene expression analysis of normal (n = 8) and cholangiocarcinoma (n = 33) tissues from TCGA. (B) qRT-PCR analysis of linc00152 in para-carcinoma and cholangiocarcinoma tissues collected from patients (n = 24). (C-F) Patients were divided into two groups based on tumor size (≤3, n = 11 and >3, n = 13), tumor cell differentiation (well-moderate, n = 12 and poor, n = 12), lymph node metastasis (yes, n = 12 and no, n = 12) or TNM staging (I-II, n = 15 and III-IV, n = 9), and the expression of linc00152 was examined with qRT-PCR. (G) The overall survival of patients with high or low expression of linc00152. (H) AUC was measured to analyze the diagnostic accuracy of linc00152 for cholangiocarcinoma. Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
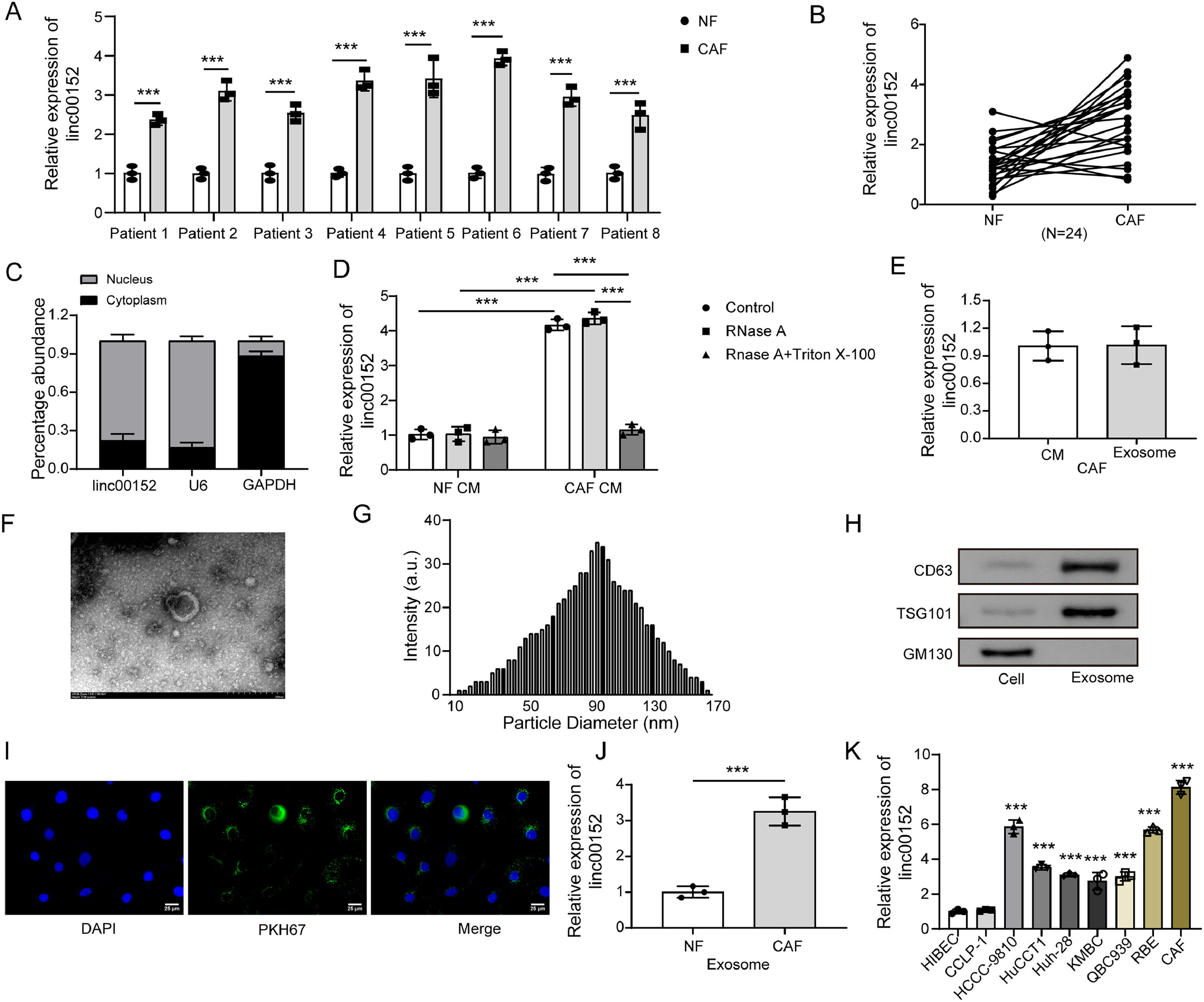
A previous study showed that linc00152 was highly expressed in CAFs from hepatocellular carcinoma (HCC), and linc00152 in CAFs regulated the proliferation and migration of HCC cells in vitro and HCC tumor growth in vivo [26]. To determine the roles of linc00152 in CAFs from cholangiocarcinoma, NFs and CAFs were isolated from patients, and CAFs contained more linc00152 compared to NFs (Fig. 2A and B). Through nucleoplasmic separation, we confirmed that linc00152 primarily localized in the nuclear fraction rather than the cytoplasmic fraction (Fig. 2C). We collected CM from NFs and CAFs and observed high abundance of linc00152 in CAF CM, and it was not affected by the treatment of RNase A alone but significantly reduced by RNase A in the presence of TritonX-100 (Fig. 2D), implying that linc00152 in the CAF CM was protected by structures with membranes. As CAFs contribute to cancer progression via releasing exosomes [22], we analyzed linc00152 expression in CAF CM and CAF-derived exosomes. As shown in Fig. 2E, same expression levels of linc00152 were observed. Subsequently, we examined the characteristics of CAF-derived exosomes. TEM showed typical nano-sized spherical vesicles (Fig. 2F), and CAF-derived exosomes showed normal size distribution (30–150 nm, Fig. 2G). CAF-derived exosomes were positive for exosome markers CD63 and TSG101, but negative for GM130, a non-exosome marker, and the exosomes could be internalized by HCCC-9810 cells (Fig. 2H and I). Furthermore, linc00152 was highly enriched in CAF-derived exosomes and cholangiocarcinoma cell lines including HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939 and RBE (Fig. 2J and K). Importantly, the highest expression was observed in CAFs (Fig. 2K). These observations suggested that linc00152 was primarily enriched in CAFs and CAF-derived exosomes.
Linc00152 was primarily enriched in CAFs and CAF-derived exosomes. (A) The expression of linc00152 in NFs and CAFs from randomly selected 8 patients using a table of random numbers [45–47] (n = 3). (B) The expression of linc00152 in NFs and CAFs from patients (n = 3). (C) Cytoplasmic and nuclear fractions were separated from CAFs, and the abundance of linc00152, U6 and GAPDH was analyzed (n = 3). (D) The abundance of linc00152 in NF CM and CAF CM treated with RNase A in the presence or absence of TritonX-100 was analyzed with qRT-PCR (n = 3). (E) qRT-PCR analysis of linc00152 in CAF CM and CAF-Exos (n = 3). (F) TEM examination of exosomes. (G) Exosomes were measured for their size distribution by dynamic light scattering. (H) The abundance of CD63, TSG101 and GM130 in CAFs and CAF-Exos was detected using western blot. (I) PKH67-labelled exosomes (Green) could be internalized by HCCC-9810 cells. (J) The abundance of linc00152 in NF-Exos and CAF-Exos was determined with qRT-PCR (n = 3). (K) qRT-PCR analysis of linc00152 in HIBEC, CCLP-1, HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939, RBE and CAFs (n = 3). Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
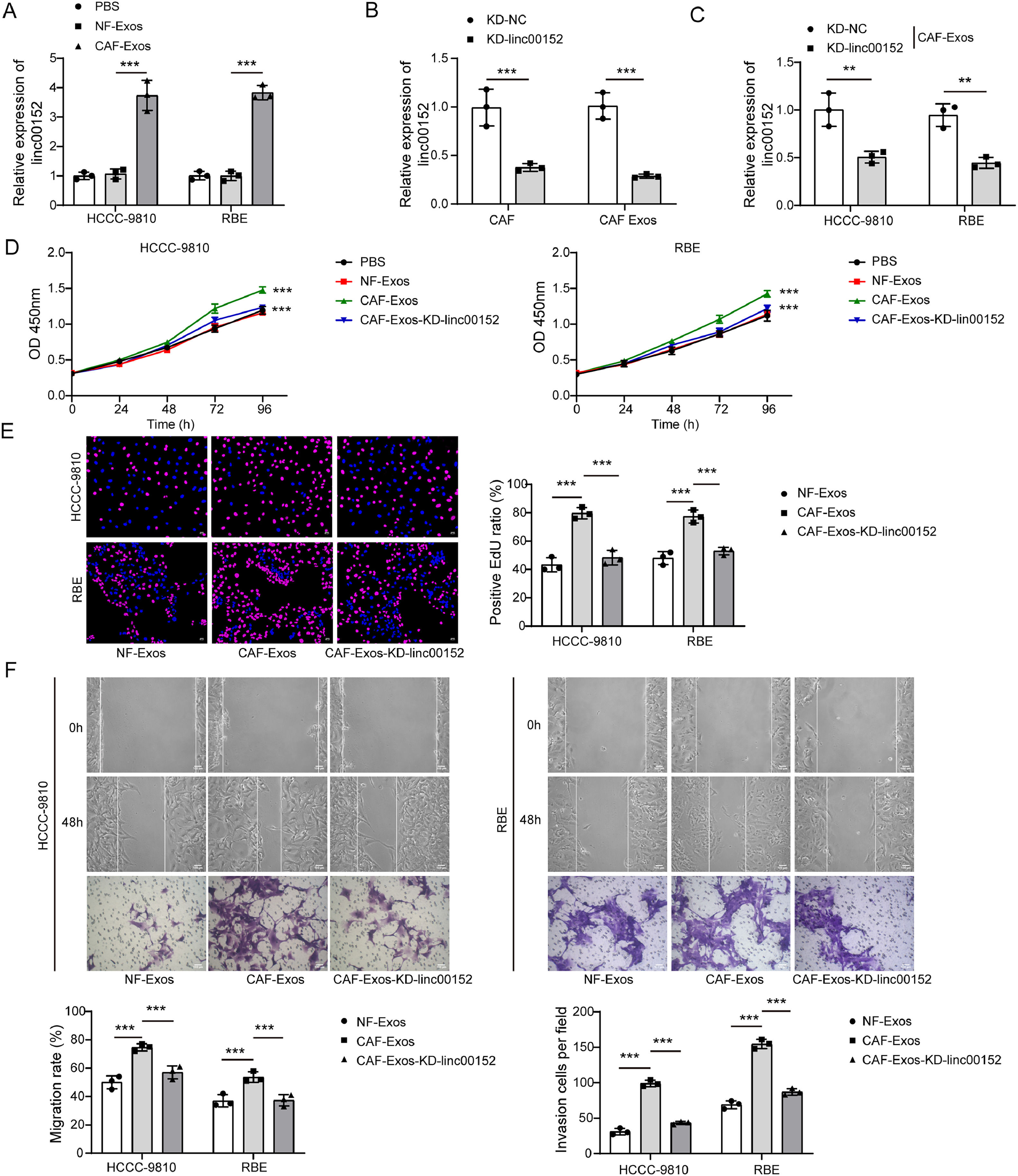
To examine whether CAF-derived exosomes regulate malignant phenotypes of cholangiocarcinoma cells via delivering linc00152, cholangiocarcinoma cells were co-cultured with NF or CAF-derived exosomes. Compared to PBS, NF-Exos did not affect linc00152 expression, but CAF-Exos significantly upregulated linc00152 in HCCC-9810 and RBE cells (Fig. 3A). To further demonstrate whether CAF-Exos increase linc00512 expression by delivering linc00512 into HCCC-9810 and RBE cells, we knocked down linc00152 in CAFs and isolated exosomes, and these exosomes had low linc00152 abundance (Fig. 3B). Then, these exosomes containing low linc00152 were cocultured with HCCC-9810 and RBE cells, and we found that linc00152 could not be significantly increased in HCCC-9810 and RBE cells (Fig. 3C). Thus, these data suggested that CAF-Exos increased linc00152 expression by delivering linc00152 into HCCC-9810 and RBE cells. Furthermore, compared to PBS and NF-Exos, CAF-Exos enhanced proliferation and EdU incorporation in HCCC-9810 and RBE cells, but these proliferation-promoting effects were abrogated by linc00152 knockdown (Fig. 3D and E). Besides, CAF-Exos promoted migrative and invasive capacities of HCCC-9810 and RBE cells, but CAF-Exos with linc00152 knockdown failed to affect migration and invasion (Fig. 3F). Collectively, CAF-derived exosomes facilitated cholangiocarcinoma cell proliferation, migration, and invasion via delivering linc00152.
CAF-derived exosomes promoted the proliferation, migration, and invasion of cholangiocarcinoma cells by delivering linc00152. (A) HCCC-9810 and RBE cells were cocultured with NF-Exos or CAF-Exos, and linc00152 expression in HCCC-9810 and RBE cells was examined by qRT-PCR (n = 3). PBS was used as a negative control. (B) Linc00152 was knocked down in CAFs, and linc00152 expression in CAFs and CAF-Exos was examined by qRT-PCR (n = 3). (C) HCCC-9810 and RBE cells were cocultured with CAF-Exos-KD-NC or CAF-Exos-KD-linc00152, and linc00152 expression in HCCC-9810 and RBE cells was examined by qRT-PCR (n = 3). HCCC-9810 and RBE cells were divided into PBS, NF-Exos, CAF-Exos and CAF-Exos-KD-linc00152 groups based on various treatments. (D and E) Cell proliferation was evaluated with CCK-8 and EdU incorporation assays (n = 3). (F) The migrative and invasive capacities of HCCC-9810 and RBE cells were analyzed with Transwell migration and invasion assays (n = 3). Data are shown as mean ± SD.**, P < 0.01; ***, P < 0.001.
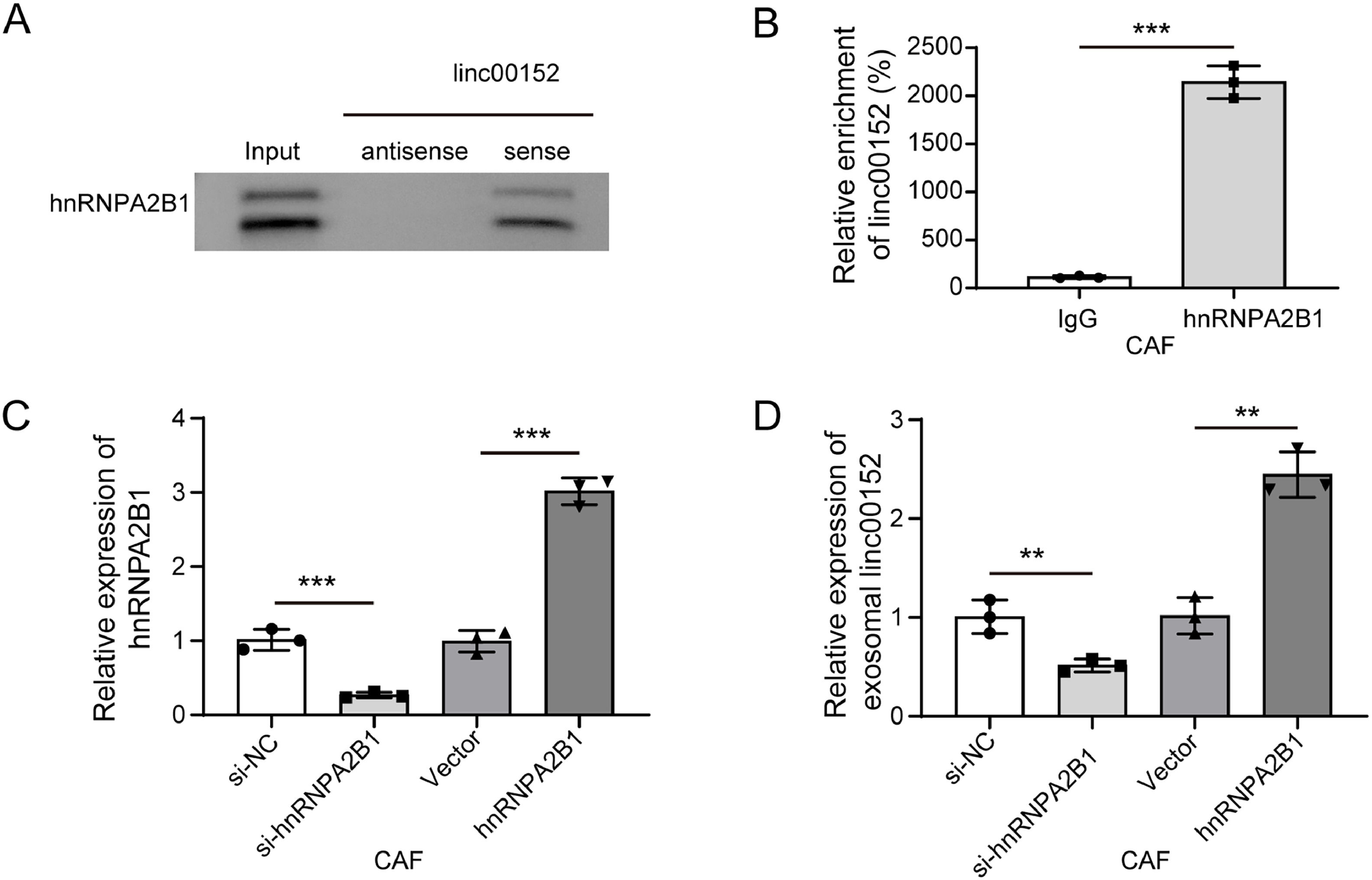
As the RNA-binding protein hnRNPA2B1 recruits RNAs with an EXO motif GGAG into exosomes [22], we investigated whether the packaging of linc00152 was dependent on hnRNPA2B1. A potential interaction between hnRNPA2B1 and linc00152 was identified through Starbase and RBPsuite. Moreover, hnRNPA2B1 was pulled down by the linc00152 sense probe, and no enrichment by the antisense probe was observed (Fig. 4A). RIP assays showed that linc00152 was efficiently enriched by an hnRNPA2B1 antibody (Fig. 4B). These data suggested an interaction between linc00152 and hnRNPA2B1. Subsequently, hnRNPA2B1 was overexpressed or knocked down in CAFs (Fig. 4C). Intriguingly, the abundance of exosomal linc00152 was reduced by hnRNPA2B1 knockdown but enhanced by hnRNPA2B1 overexpression (Fig. 4D). These data suggested that hnRNPA2B1 bound to linc00152 and might facilitate its loading into CAF-derived exosomes, which needs further investigation.
hnRNPA2B1 recruited linc00152 and facilitated its loading into CAF-derived exosomes. (A) The abundance of hnRNPA2B1 in the complexes pulled down by the linc00152 sense or anti-sense probe was examined by western blot. Total cell lysate was used as the input control. (B) The enrichment of linc00152 by hnRNPA2B1 was assessed with RIP-qPCR assays (n = 3). (C) qRT-PCR analysis of hnRNPA2B1 in CAFs transfected with si-hnRNPA2B1, si-NC, hnRNPA2B1 or vector (n = 3). (D) Exosomal linc00152 was analyzed with qRT-PCR (n = 3). Data are shown as mean ± SD. **, P < 0.01; ***, P < 0.001.
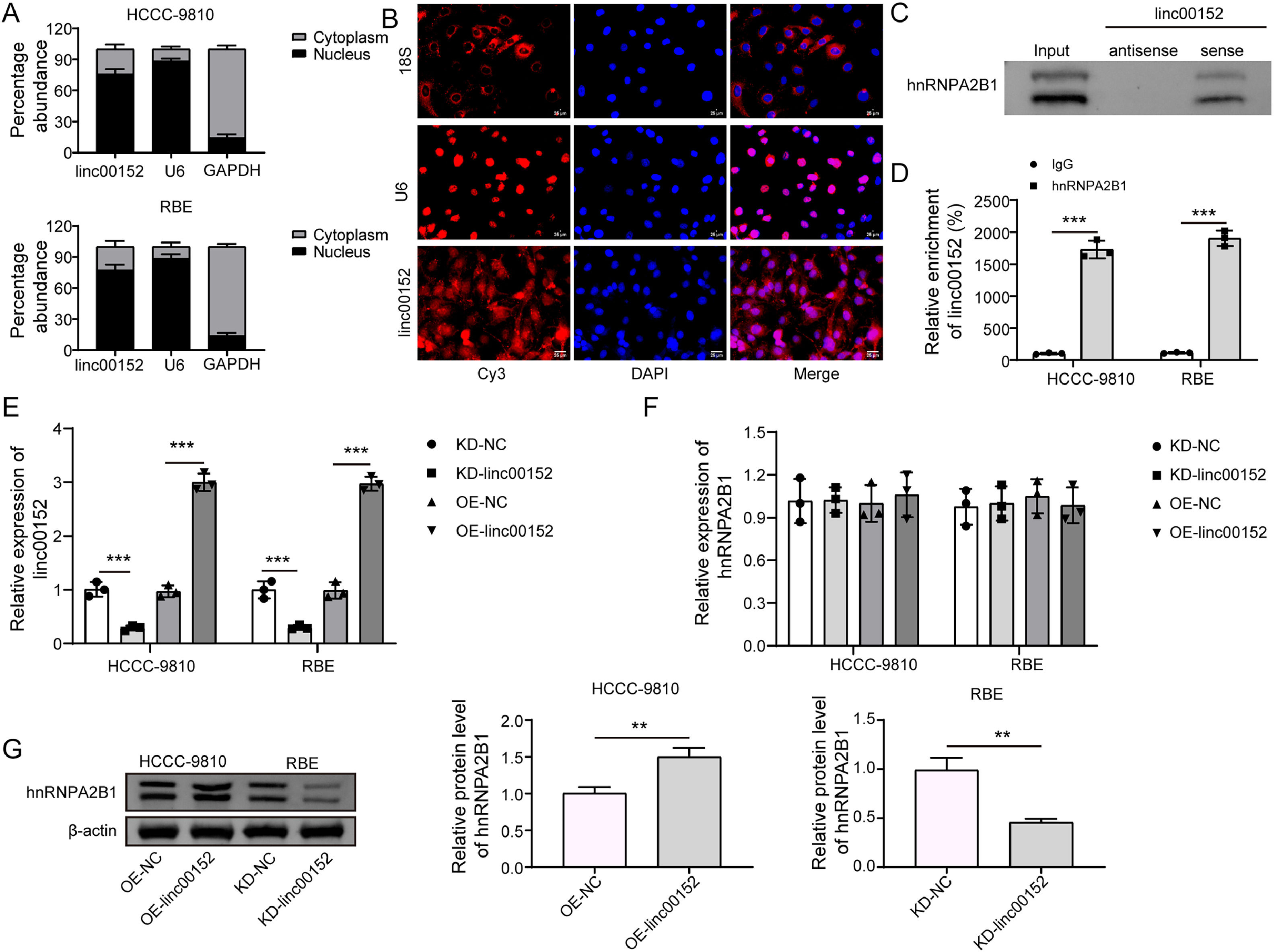
Furthermore, we found that linc00152 primarily localized in the nucleus although it was present in both the nucleus and cytoplasm of HCCC-9810 and RBE cells, which was confirmed by FISH assay (Fig. 5A and B). In HCCC-9810 and RBE cells, the linc00152 sense probe, not the antisense probe, pulled hnRNPA2B1 down, and linc00152 was enriched by an hnRNPA2B1 antibody (Fig. 5C and D), suggesting an interaction between linc00152 and hnRNPA2B1. Linc00152 was knocked down or overexpressed in HCCC-9810 and RBE cells, and we found that neither linc00152 knockdown nor its overexpression affected hnRNPA2B1 expression at the mRNA level (Fig. 5E and F). However, overexpression of linc00152 elevated the protein level of hnRNPA2B1, whereas linc00152 silencing reduced hnRNPA2B1 abundance in HCCC-9810 and RBE cells (Fig. 5G). Thus, linc00152 interacted with hnRNPA2B1 to promote hnRNPA2B1 expression at the protein level in cholangiocarcinoma cells.
Linc00152 interacted with hnRNPA2B1 to elevate its protein level in cholangiocarcinoma cells. (A) Cytoplasmic and nuclear fractions were separated from HCCC-9810 and RBE cells, and the abundance of linc00152, U6 and GAPDH was analyzed (n = 3). (B) FISH was applied to examine the subcellular location of linc00152. (C) The abundance of hnRNPA2B1 in the complexes pulled down by the linc00152 sense or anti-sense probe was examined by western blot. (D) The enrichment of linc00152 by hnRNPA2B1 was assessed with RIP-qPCR assays (n = 3). (E and F) Linc00152 was knocked down or overexpressed in HCCC-9810 and RBE cells, and the expression of linc00152 and hnRNPA2B1 was analyzed with qRT-PCR (n = 3). (G) Western blot analysis of hnRNPA2B1 in linc00152-overexpressing and linc00152-silencing cells. Data are shown as mean ± SD. **, P < 0.01; ***, P < 0.001.
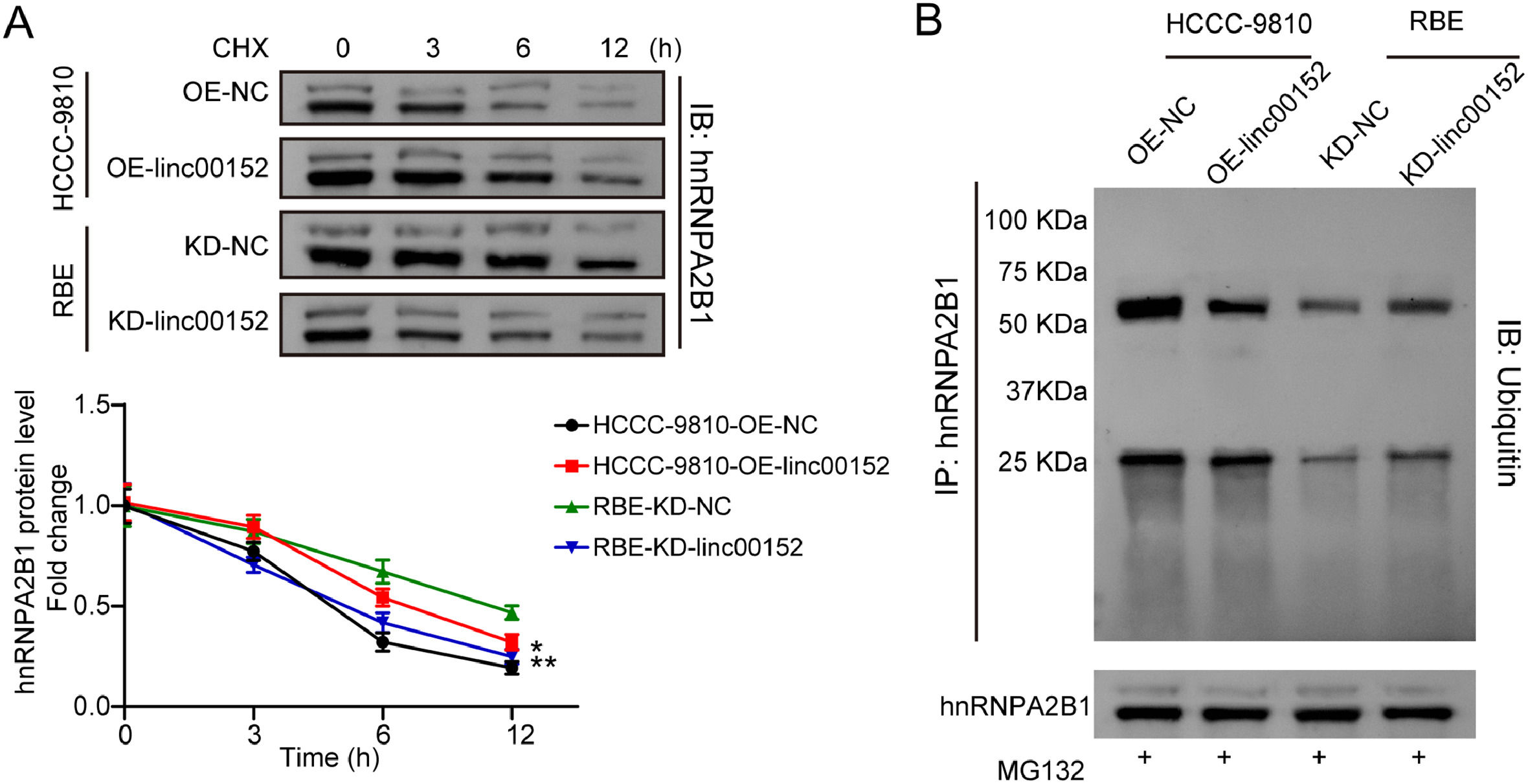
As linc00152 positively regulated hnRNPA2B1 protein expression but did not affect its mRNA level, we hypothesized that linc00152 might be involved in the regulation of hnRNPA2B1 protein degradation in cholangiocarcinoma cells. Linc00152 was overexpressed in HCCC-9810 cells and knocked down in RBE cells, and these cells were treated with CHX, a protein synthesis inhibitor. Overexpression of linc00152 inhibited hnRNPA2B1 degradation in HCCC-9810 cells, and knockdown of linc00152 accelerated its degradation in RBE cells (Fig. 6A). In addition, hnRNPA2B1 was immunoprecipitated from linc00152-overexpressing HCCC-9810 cells and linc00152-silencing RBE cells, and its ubiquitination was analyzed through western blot. The ubiquitination of hnRNPA2B1 was inhibited by linc00152 overexpression in HCCC-9810 cells but enhanced by linc00152 knockdown in RBE cells (Fig. 6B). Thus, linc00152 inhibited the proteasome-dependent degradation of hnRNPA2B1 in cholangiocarcinoma cells.
Linc00152 suppressed hnRNPA2B1 ubiquitination and degradation. (A) Linc00152-overexpressing HCCC-9810 and linc00152-silencing RBE cells were treated with CHX at 50 μg/mL for 0, 3, 6 and 12 h, and the abundance of hnRNPA2B1 protein was detected with western blot (n = 3). (B) hnRNPA2B1 was immunoprecipitated from linc00152-overexpressing and linc00152-silencing cell lysates, and its ubiquitination level was assessed with western blot. Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01.
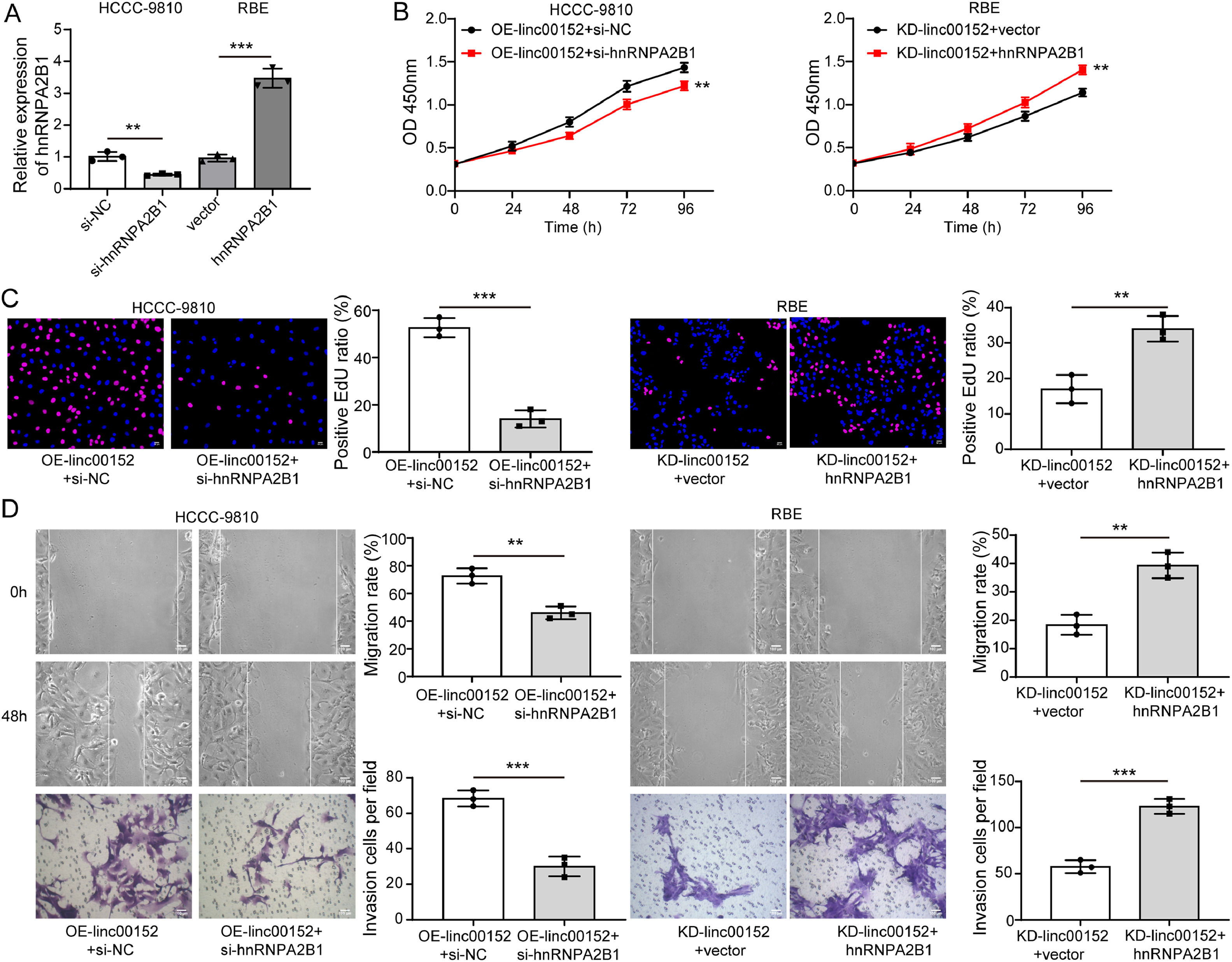
To further investigate whether linc00152 exerts biological activity in cholangiocarcinoma dependent on hnRNPA2B1, hnRNPA2B1 was knocked down in RBE cells and overexpressed in HCCC-9810 cells (Fig. 7A). Compared to KD-linc00152 + Vector, RBE cells in the KD-linc00152 + hnRNPA2B1 group showed enhanced proliferation, EdU incorporation, migration, and invasion (Fig. 7B–D). However, the proliferation, migration, and invasion of linc00152-overexpressing HCCC-9810 cells were inhibited by hnRNPA2B1 silencing (Fig. 7B–D). These findings indicated that linc00152 served its biological function in cholangiocarcinoma cells through hnRNPA2B1 upregulation.
Linc00152 served its biological function dependent on hnRNPA2B1 in cholangiocarcinoma cells. (A) qRT-PCR analysis of hnRNPA2B1 in RBE and HCCC-9810 cells with indicated transfection (n = 3). RBE cells were divided into KD-linc00152 + Vector and KD-linc00152 + hnRNPA2B1 groups, and HCCC-9810 cells were divided into OE-linc00152 + si-NC and OE-linc00152 + si-hnRNPA2B1 groups. (B and C) Cell proliferation was evaluated with CCK-8 and EdU incorporation assays (n = 3). (D) The migrative and invasive capacities of HCCC-9810 and RBE cells were analyzed with Transwell migration and invasion assays (n = 3). Data are shown as mean ± SD. **, P < 0.01; ***, P < 0.001.
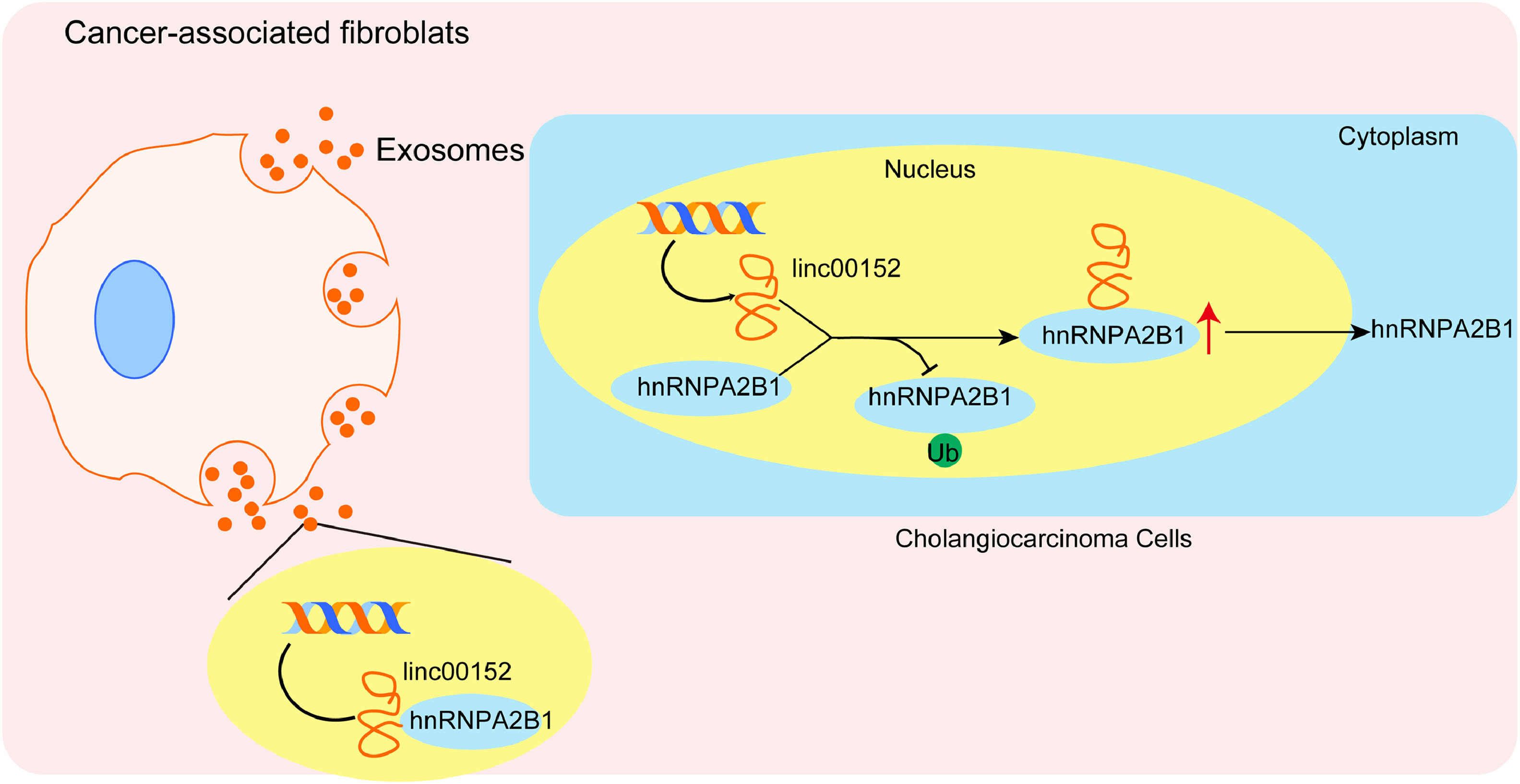
Cholangiocarcinoma is a rare cancer with an incidence of 0.3–6 per 100,000 inhabitants per year, but it has a high mortality rate of 1–6 per 1000, 100 inhabitants per year [13,27]. Unfortunately, the incidence and mortality rate of cholangiocarcinoma are on the rise globally. Surgery is the best chance for patients to survive from cholangiocarcinoma, but only 22 % of patents have resectable tumors. Even for those patients, the five-year survival rate is only 15–25 %. Most of patients are diagnosed with metastatic cholangiocarcinoma, palliative chemotherapy is the primary treatment, but the survival is as low as only 2 % [28]. Therefore, uncovering the pathogenesis of cholangiocarcinoma is quite important for identifying potential diagnostic and therapeutic targets. Here, we demonstrate that linc00152 is highly expressed in cholangiocarcinoma and associated with poor prognosis of patients. CAF-derived exosomes deliver linc00152 to promote the proliferation, migration, and invasion of cholangiocarcinoma cells via upregulating hnRNPA2B1. Our study reveals a mechanism by which linc00152 promotes cholangiocarcinoma progression and identifies a novel role of exosomal linc00152 in the crosstalk between CAFs and cholangiocarcinoma cells (Fig. 8).
Since microRNAs (miRNAs) are discovered, many non-coding RNAs such as miRNAs, long non-coding RNAs (lncRNAs) and circular RNAs have been identified to serve key roles as oncogenes, tumor suppressors or both in cancers [29]. LncRNAs are RNAs with a length of over 200 nucleotides lacking protein-coding capacity that have been linked to various cancers [30,31]. Growing studies have revealed that thousands of lncRNAs are dysregulated in cholangiocarcinoma tissues [32,33]. Zhu et al. reported that lncRNA TTN-AS1 facilitated cholangiocarcinoma progression by working as a sponge for miR-320a and downregulating neuropilin-1 [34]. LncRNA TUG1 was reported to enhance the proliferation and metastasis of cholangiocarcinoma cells via suppression of miR-29a [35]. Linc00152 is highly expressed in many cancers such as pancreatic cancer, hepatocellular cancer, breast cancer and thyroid cancer, and, in most cases, linc00152 exerts oncogenic activities in almost all aspects of the occurrence and progression of cancers [18]. In addition, a preprint reported increased linc00152 expression in cholangiocarcinoma [19]. In this study, we confirmed increased linc00152 expression in cholangiocarcinoma cells and tissues, and its high expression was associated with accelerated cancer progression and poor outcome of patients, supporting the notion that linc00152 functions as an oncogene in cholangiocarcinoma. Also, the AUC of 0.9214 indicated an increased diagnostic accuracy of linc00152 for cholangiocarcinoma. Our data suggests linc00152 as a potential diagnostic and therapeutic target for cholangiocarcinoma. However, to further evaluate its potential as a diagnostic biomarker for cholangiocarcinoma, more clinical specimens need to be collected for analyzing the levels of linc00152 and its association with cholangiocarcinoma diagnosis in our future studies. In addition, we cannot exclude the possibility that other RNAs may also exert a significant impact in cholangiocarcinoma. In our future studies, we will explore the potential impact of other RNAs that are significantly changed in cholangiocarcinoma.
CAFs are key players in shaping the tumor microenvironment via matrix remodeling, immune crosstalk, metabolic effects, and soluble secreted factors and vehicles, regulating cancer tumorigenesis, growth, and progression [13,36]. Exosomes transport biomolecules such as RNAs and proteins to neighboring cells to affect their behaviors [37]. CAFs are linked to intercellular communication with cancer cells via secreting exosomes that regulate cancer proliferation, invasion, angiogenesis, and metastasis [38]. Linc00152 was upregulated in fibroblasts isolated from HCC, and the CM collected from linc00152-silencing CAFs inhibited HCC cell proliferation and migration [26]. Linc00152 was likely present in exosomes from gastric and colorectal cancer patients [39,40]. All these observations raised the possibility that linc00152 might be transmitted by CAF-derived exosomes. Here, we firstly reported that linc00152 was enriched in CAFs and CAF-derived exosomes in cholangiocarcinoma, and CAF-derived exosomes promoted the malignant behaviors of cholangiocarcinoma cells by delivering linc00152, revealing a novel mechanism underlying the intercellular communication between CAFs and cholangiocarcinoma cells through exosomal linc00152. In addition, it will be interesting to investigate the expression of linc00152 in cholangiocarcinoma cell-derived exosomes and their roles in cancer progression in future studies.
Linc00152 has been well recognized as an oncogene as its high expression is significantly correlated to poor prognosis in many cancers. Linc00152 promotes cancer progression through various mechanisms including sponging miRNAs, epigenetically regulating gene expression and controlling protein abundance [18]. However, we reported a novel mechanism by which linc00152 was involved in cholangiocarcinoma. A novel direct interaction between linc00152 and hnRNPA2B1 was confirmed in cholangiocarcinoma cells. hnRNPA2B1, an RNA binding protein, contains two motifs for recognizing RNAs and serves vital roles in RNA transport, maturation, and metabolism and controlling the sorting of miRNAs to exosomes [41–43]. As expected, we found that hnRNPA2B1 might promote the loading of linc00152 into CAF-derived exosomes in cholangiocarcinoma. However, the enrichment of hnRNPA2BA in CAF-derived exosomes should be determined. As linc00152 destabilizes PTEN protein via enhancing its ubiquitination and degradation in breast cancer [44], we analyzed the ubiquitination and degradation of hnRNPA2B1. Strikingly, linc00152 inhibited the ubiquitination and degradation of hnRNPA2B1 in cholangiocarcinoma cells, thus upregulating hnRNPA2B1 to promote cancer malignancy. The contradicting effects conveyed by linc00152 on protein ubiquitination and degradation may be attributed to various genetic constellations and tumor microenvironment in different cancers, but it needs further investigation.
5ConclusionsIn conclusion, we are the first to report that CAF-derived exosomal linc00152 intensifies the crosstalk between cholangiocarcinoma cells and CAFs, thus functioning as an oncogene to promote the progression of cholangiocarcinoma by upregulating hnRNPA2B1. We provide evidence that CAF-derived exosomal linc00152 may be used as prognostic and diagnostic biomarkers, as well as a therapeutic target for cholangiocarcinoma. However, this study has several limitations: (1) the size of clinical samples is very limited, and we need to collect more patient samples to further investigate the impact of linc00152 on tumor progression and patient survival; (2) we only performed in vitro assays, and no animal models were involved, and we plan to use mouse tumor models to further evaluate the roles of exosomal linc00152 in the progression of cholangiocarcinoma; and (3) the detail mechanism underlying linc00152-mediated regulation of hnRNPA2B1 ubiquitination and degradation has not been further investigated, which is also a focus in our future study. Nevertheless, our study draws attention to the roles of CAF-derived exosomes in this highly fatal cancer and holds the potential of CAF-derived exosomal linc00152 for cholangiocarcinoma treatment. We still have several knowledge gaps, such as the impact of other RNAs that are enriched in CAF-derived exosomes, other source of linc00152, other targets rather than hnRNPA2B1 of CAF-derived exosomal linc00152, and downstream signaling pathways of hnRNPA2B1, and we will focus on these points in our future studies. Besides linc00152, we believe that the roles and molecular mechanisms of CAF-derived exosomes-mediated intracellular communication by delivering proteins and RNAs into cholangiocarcinoma cells will be further well elucidated, and more clinical efforts through CAF-derived exosomes will increasingly emerge in the next five years.
Authors' contributionsMin Hu conceived and designed the research. Min Hu and Yaxuan Niu performed the research and acquired the data. Jinlin Wang, Xiao Chen and Gang Li analyzed and interpreted the data. All authors were involved in drafting and revising the manuscript.
Availability of data and materialsAll data generated or analyzed are included in this article. Further inquiries can be directed to the corresponding author.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to give our sincere gratitude to the reviewers for their constructive comments.






![Linc00152 was primarily enriched in CAFs and CAF-derived exosomes. (A) The expression of linc00152 in NFs and CAFs from randomly selected 8 patients using a table of random numbers [45–47] (n = 3). (B) The expression of linc00152 in NFs and CAFs from patients (n = 3). (C) Cytoplasmic and nuclear fractions were separated from CAFs, and the abundance of linc00152, U6 and GAPDH was analyzed (n = 3). (D) The abundance of linc00152 in NF CM and CAF CM treated with RNase A in the presence or absence of TritonX-100 was analyzed with qRT-PCR (n = 3). (E) qRT-PCR analysis of linc00152 in CAF CM and CAF-Exos (n = 3). (F) TEM examination of exosomes. (G) Exosomes were measured for their size distribution by dynamic light scattering. (H) The abundance of CD63, TSG101 and GM130 in CAFs and CAF-Exos was detected using western blot. (I) PKH67-labelled exosomes (Green) could be internalized by HCCC-9810 cells. (J) The abundance of linc00152 in NF-Exos and CAF-Exos was determined with qRT-PCR (n = 3). (K) qRT-PCR analysis of linc00152 in HIBEC, CCLP-1, HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939, RBE and CAFs (n = 3). Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Linc00152 was primarily enriched in CAFs and CAF-derived exosomes. (A) The expression of linc00152 in NFs and CAFs from randomly selected 8 patients using a table of random numbers [45–47] (n = 3). (B) The expression of linc00152 in NFs and CAFs from patients (n = 3). (C) Cytoplasmic and nuclear fractions were separated from CAFs, and the abundance of linc00152, U6 and GAPDH was analyzed (n = 3). (D) The abundance of linc00152 in NF CM and CAF CM treated with RNase A in the presence or absence of TritonX-100 was analyzed with qRT-PCR (n = 3). (E) qRT-PCR analysis of linc00152 in CAF CM and CAF-Exos (n = 3). (F) TEM examination of exosomes. (G) Exosomes were measured for their size distribution by dynamic light scattering. (H) The abundance of CD63, TSG101 and GM130 in CAFs and CAF-Exos was detected using western blot. (I) PKH67-labelled exosomes (Green) could be internalized by HCCC-9810 cells. (J) The abundance of linc00152 in NF-Exos and CAF-Exos was determined with qRT-PCR (n = 3). (K) qRT-PCR analysis of linc00152 in HIBEC, CCLP-1, HCCC-9810, HuCCT1, Huh-28, KMBC, QBC939, RBE and CAFs (n = 3). Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001.](https://static.elsevier.es/multimedia/16652681/0000003000000001/v37_202504160609/S1665268124005283/v37_202504160609/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)










