Fontan-associated liver disease is a hepatic disorder arising from hemodynamic changes and systemic venous congestion following Fontan surgery. The histological changes produced in the liver are similar but not equivalent to those seen in other forms of cardiac liver disease. While the natural history of this form of liver disease is not well established, over time many Fontan patients develop portal hypertension-related complications such as ascites, variceal hemorrhage or encephalopathy. Fontan survivors also show an increased risk of hepatocellular carcinoma. Early diagnosis of advanced liver disease is mandatory for the prevention and treatment of complications such as hepatocellular carcinoma, esophageal varices and malnutrition. This review updates current knowledge of the pathophysiology and management of Fontan-associated liver disease including new diagnostic methods and treatments.
Fontan surgery includes several techniques that divert systemic venous return to the pulmonary arterial system, usually without an intervening ventricle. This form of surgery is the palliative procedure of choice for many patients with a single functional ventricle. Fontan procedure survivors almost invariably experience long-term complications involving the heart, lungs, kidneys, brain, gut and liver. Fontan-associated liver disease (FALD) comprises a wide spectrum of structural and functional alterations of the liver caused by hemodynamic disturbances following Fontan surgery. As in all forms of chronic liver disease, FALD progresses through several stages before reaching a final stage, when the main complications of portal hypertension and hepatocellular carcinoma occur. Although liver damage in Fontan patients is universal, it likely starts before surgery and its progression differs in each patient. The extent of this damage mainly depends on heart disease progression, local complications in the Fontan circulation and acute cardiopulmonary events such as cardiac arrhythmias or pulmonary thromboembolism. As an ever increasing number of Fontan patients reach adulthood, the expertise of the hepatologist is essential. This review examines the pathophysiology and management of FALD and addresses issues related to heart and liver transplantation.
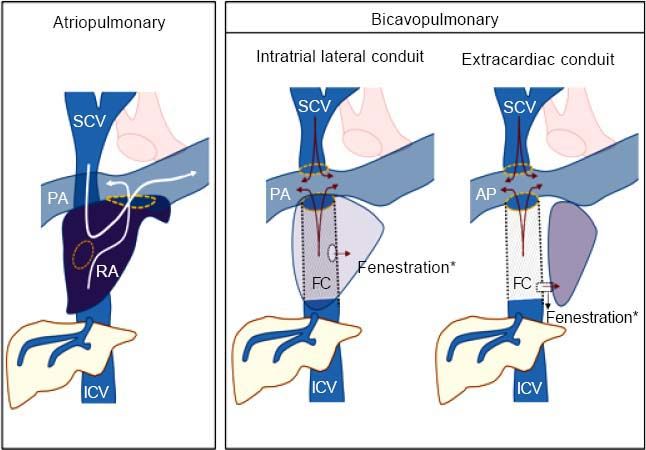
Fontan Surgery: Definition and TypesThe Fontan procedure is used in patients who have a single functioning ventricle due to a complex congenital heart disease and when biventricular repair is not feasible. In these patients, intracardiac mixing of venous and arterial blood leads to severe hypoxemia. The Fontan procedure separates the pulmonary and systemic circulation and allows systemic venous return to the pulmonary arteries, avoiding the right ventricle. In simple terms, the Fontan technique creates a connection between systemic venous return from both the inferior and superior venae cavae and the pulmonary arteries, which will passively transmit blood to the single ventricular chamber. Surgery serves to preserve adequate oxygenation of the blood, maintaining cardiac output slightly below normal and ultimately creating new circulatory dynamics.1
There are two major variants of the technique: atriopulmonary and bi-cavopulmonary (Figure 1). The original Fontan procedure (atriopulmonary anastomosis) converts the atrium into a canal that connects blood from both cava veins to the pulmonary artery. An anastomosis is created between the atrium and the pulmonary artery and both the tricuspid valve and atrial septal defect are closed. Although initially it was thought that a hypertrophied right atrium would serve as a functional pump, this was later found to increase the risk of tachyarrhythmia and atrial thrombi.2,3 The more recent bi-cavopulmonary procedure is carried out in two stages: in the first, the superior vena cava is connected to the pulmonary arteries (bidirectional Glenn procedure); and in the second, or Fontan completion stage, the inferior vena cava is also connected through an artificial conduit to the pulmonary arteries, thus completing the system.
The Fontan technique was first described in 1968, though it was not until the 1980s that it was widely adopted.4 The procedure is indeed one of the major advances in Pediatric Cardiology of our time as it guarantees survival rates of around 80% at 20 years and should be viewed as a great success considering the severity of the underlying anatomical cardiac defects.5,6 However, follow up over decades of the growing Fontan population has revealed the emergence of multiple and varied consequences on several organ systems. These include arrhythmias and single ventricle dysfunction, aortic valve disease, exercise intolerance, poor somatic growth, delayed puberty, plastic bronchitis, thromboembolic events, kidney disease due to glomerular and tubular injury, delayed neurocognitive development, peripheral venous insufficiency, protein-losing enteropathy and liver disease.7,8
The term used to describe the multisystemic clinical consequences of the Fontan circulation is “Fontan Failure” (Table 1). This failure may be the final outcome of elevated pulmonary resistance, pulmonary thrombi, narrowing and scarring of the Fontan pathway or pulmonary arteries, arrhythmias and failure of the systemic ventricle. Such alterations can damage target organs through two different mechanisms:9–12
- •
Venous passive congestion. Chronic elevation of systemic venous pressure promotes congestion in the splanchnic venous circulation (lacking self-regulating flow capacity) and reduces lymphatic return through the thoracic duct.
- •
Arterial ischemia. Reduced cardiac output from the functioning single ventricle, secondary to both diastolic and systolic dysfunction, causes ischemia in target organs.
Systemic consequences of the “failure” of the Fontan circulation.
| Organ/system | Complication | Mechanism | Clinical Findings |
|---|---|---|---|
| Lungs | Veno-venous/atrial shunts Plastic bronchitis Chylothorax Thromboembolism Pulmonary hypertension | Gradient-dependent passive circulation ↓ Lymphatic return ↓ Lymphatic return Hypercoagulability Vascular hyperreactivity | Cyanosis, dyspnea, hypoxia, exercise intolerance |
| Kidneys | Proteinuria Kidney injury (acute/chronic) | Hyperfiltration due to venous hypertension Ischemia due to ↓ CO | Edema, ascites Dyspnea, oliguria |
| Bowel | Protein-losing enteropathy | ↓ Lymphatic return Splanchnic venous congestion Systemic inflammation Hormonal activation | Malnutrition, edema, ascites, diarrhea |
| Liver | Chronic liver disease | Liver congestion Ischemia due to ↓ CO | Ascites, varices, encephalopathy, hepatocarcinoma |
| Brain | Cerebrovascular disease | Cardioembolic Ischemia due to ↓ CO | Decreased executive skills |
| Heart | Bradi and Tachyarrhythmias Ventricular dysfunction | Atrial and ventricular remodeling Activation of neurohormonal systems | Hemodynamic instability Dyspnea, exercise intolerance |
| Vascular system | Varicosities | Venous hypertension ↓ Lymphatic return | Edema, varicose veins |
CO: Cardiac output.
The pathogenesis of liver fibrosis in the Fontan population is not well-documented. Fibrogenesis is thought to be driven by a non-inflammatory mechanism, as the inflammatory infiltrate in biopsy and autopsy samples is minimal or absent.13,14
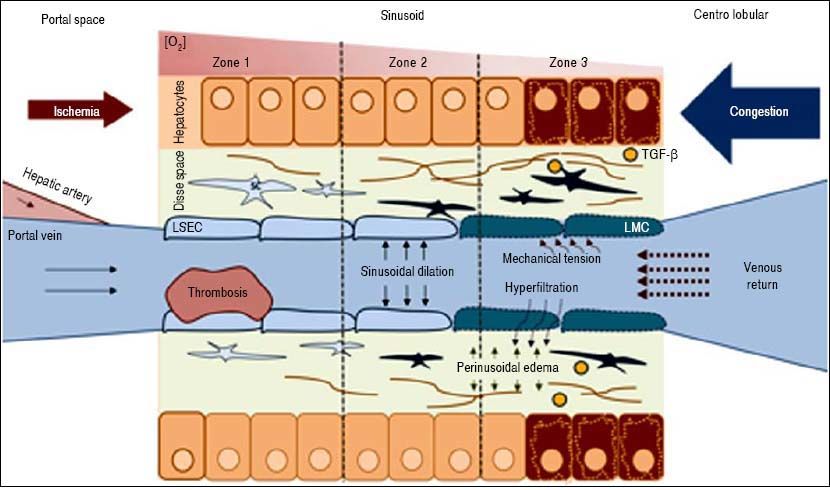
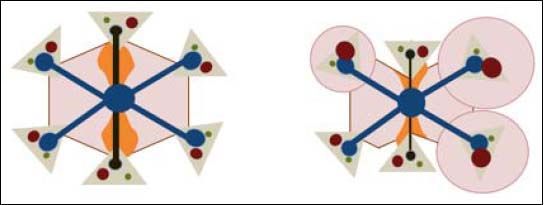
The key point in the pathophysiology of FALD is a disturbance in the liver’s vascular supply and drainage (Figure 2). Elevated systemic venous pressure leads to inefficient blood drainage of the liver, determining a state of chronic passive congestion. This state promotes sinusoidal dilation and blood hyperfiltration causing perisinusoidal edema and hypoxia in centrilobular hepatocytes.15 In addition, mechanical tension also likely plays an important role by inducing a phenotypic change in the endothelial cell and enabling the activation of hepatic stellate cells and fibroblasts. As in other forms of liver fibrosis, TGF-β seems to be the central profibrogenic molecule driving the process.16 Finally, in a murine model, Simonetto, et al. recently observed that sinusoidal thrombosis and mechanical strain secondary to blood stasis were the main fibrogenic promoters. These authors also confirmed minimal inflammatory activity, supporting the hypothesis that inflammation is not a common feature of cardiac liver disease.17 Wan-less, et al. have also argued that intrahepatic thrombosis is the main cause of cardiac cirrhosis.18 In line with data suggesting that Fontan patients show an inherent prothrom-botic state,17 the hypothesis of intrahepatic thrombosis becomes more important and opens the way for future therapeutic strategies such as anticoagulation (Figure 3).
Pathophysiology of FALD. LSEC: Liver Sinusoidal endothelial cell. SC: Stellate cell. TGF-β. Transforming growth factor beta. Systemic venous hypertension secondary to FS results in a decrease in venous drainage, which increases pressure and dilates the sinusoid. There will be phenomena of hyperfiltration towards the space of Disse and the mechanical stress will induce a LSEC phenotypic change. The secretion of some molecules will activate autocrine SC, promoting fibrogenesis. Hypoxia and perisinusoidal fibrosis will eventually lead to hepatocyte parenchymal necrosis, more evident in zone 3 (near the centrolobular vein).
Finally, it should be highlighted that Fontan patients feature additional risk factors for chronic liver disease which are unrelated to this unique vascular system. These include a higher prevalence of hepatitis C virus infection or the use of hepatotoxic antiarrythmia drugs (eg, amiodar-one).19,20
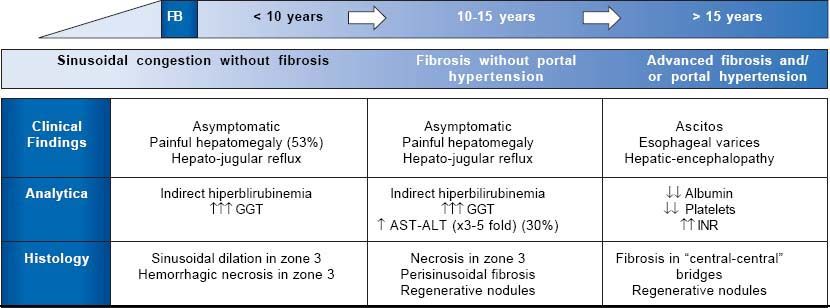
Natural History of Fontan-Associated Liver DiseaseSymptomatic FALD is frequently the first manifestation of Fontan failure and may coincide with other systemic manifestations such as protein-losing enteropathy or a progressive decline in functional capacity. However, liver damage begins before symptoms appear. Although its natural history is still poorly understood, 3 main stages have been described (Figure 4):
- •
Liver congestion and sinusoidal dilation. This stage starts even before Fontan surgery and continues into the following few years.21,22 Although many patients are asymptomatic, 53% develop painful hepatomegaly and/ or hepatojugular reflux. Analytically, this phase is characterized by mild indirect hyperbilirubinemia and increased GGT, in relation to perisinusoidal edema. Biopsy usually reveals sinusoidal dilation and hepato-cellular necrosis in zone 3 of the lobule.23
- •
Fibrosis without portal hypertension. Around 5-10 years after surgery, patients show perisinusoidal fibrosis, regenerative nodules and hepatocellular necrosis. In this stage, necrosis can be aggravated if low cardiac output further decreases, so slight elevations in AST, ALT and LDH are frequent. At this stage fibrosis is potentially reversible if the patient undergoes heart transplantation.24
- •
Advanced fibrosis with portal hypertension. This is the last stage of liver disease in general. Laboratory abnormalities are hypoalbuminemia, prolonged coagulation time and a low platelet count. At this stage, there is an increased, though not well quantified, risk of developing hepatocarcinoma and portal hypertension-related complications such as ascites, bleeding from esophagogastric varices or hepatic encephalopathy.25
In any of these stages, a clinical picture of its own entity may occur: ischemic hepatitis, characterized by markedly elevated AST, ALT and LDH in the context of an acute low cardiac output. This situation is usually reversible once hemodynamic stability has been reestablished.
As we are not dealing with primary liver disease, the chronology of FALD is difficult to establish and its progression depends on the cardiological and hemodynamic situation. Table 2 lists the variables associated with an increased risk of liver damage.26–28 However, the main risk factor for Fontan liver disease is the time since surgery. This means that compared to a post-Fontan time under 5 years, the odds of having a hepatic complications for post-Fontan durations of 11-15 years or 16-20 years are 4.4-fold (confidence interval [CI]: 1.1-17.2) or 9.0-fold (CI: 2.2-36.2), respectively.29
Risk factors for liver damage in FS patients.
| Related to the hemodynamic situation |
| ↓ Cardiac output. |
| ↑ Pulmonary capillary pressure. |
| ↑ Central venous pressure. |
| Related to the surgery |
| Pulmonary atresia as surgery precipitant. |
| Classical technique (atriopulmonary variant). |
| Fontan conduit stenosis / thrombosis. |
| Time since surgery. |
| Related to cardiological events |
| Cardiac arrhythmias. |
| Systolic ventricular dysfunction. |
| Others |
| Hepatotrope virus infection. |
| Exposure to hepatotoxic drugs (e.g. amiodarone). |
Liver damage in Fontan patients is universal. However, as not all patients develop liver-related complications, invasive and non-invasive diagnostic methods are needed to identify who may require close and targeted monitoring.30
Serological testsA basic blood panel, including liver function test, blood cell count and basic coagulation parameters may be sufficient as an initial assessment of liver disease in Fontan patients. A platelet count < 150,000/µL is the main indicator of hypersplenism, which is notoriously associated with portal hypertension. Serum albumin is a classic marker of liver dysfunction and, although protein-losing enteropathy can facilitate its loss, low albumin levels usually indicate some extent of liver damage. Multiple simple methods that combine analytical and radiological findings have been recently developed. Although designed to diagnose and stratify patients with liver fibrosis other than Fontan patients, many of these methods have been used in the latter patients (Table 3). However, these methods have not been validated against the gold standard, so we lack well-defined cut-off values. In a cohort of 204 patients (26% with hepatic decompensation), Baek, et al. compared several serological tests and identified Forns index as the best predictor of advanced liver damage with an area under the ROC curve of 0.786.29 In patients with cirrhosis, MELD score is the main prognostic tool. However, as many Fontan patients are under anticoagulation therapy, MELD is artificially increased. To overcome this limitation, a new score MELD-XI has been designed.31 In Fontan patients, this new score has shown good correlation with the extent of liver fibrosis but is unable to define a cut-off for FALD.32
Serological methods for diagnosis FALD y FS patients.
| Serological test | Parameters | Evidence |
|---|---|---|
| APRI | AST, platelets | It has shown correlation with radiological findings. |
| AST/ALT | AST, ALT | It has shown correlation with radiological findings. |
| FIB-4 | Age, AST, ALT, platelets | It has shown correlation with radiological findings. |
| Forns | Age, GGT, cholesterol, platelets | It has shown correlation with radiological findings. |
| ELFTM score | TIMP-1, PIIINP, hyaluronic acid | Not useful. |
| Plaquetas | Platelets count | It has shown correlation with radiological findings. |
| MELD-XI | Bilirrubin, Creatinine | Correlated with histological findings. Non cutt-off points with adequate sensitivity and specificity. |
| FibroSure® | ALT, A2-macroglobulin, apolipoprotein A-1, bilirubin, GGT, haptoglobin, AST, glucose, cholesterol, triglycerides. | No correlation with histological findings. |
ALT: alanine transaminase. APRI: aspartate transaminase to platelet ratio index. AST: aspartate aminotransferase. FIB-4: fibrosis index based on four factors. GGT: gamma-glutamyl transpeptidase. MELD-XI: model for end stage liver disease-XI. PIIINP: N-terminal propeptide of procollagen type III. TIMP-1: Tissue inhibitor-1 of metalloproteinase.
Doppler ultrasonography is today the most useful radiological tool for the assessment of liver disease. By identifying a few simple signs detailed in table 4, a diagnosis of advanced liver disease can be made. The most specific ultrasound finding for this purpose is the irregular surface of the parenchyma as determined by a high frequency trans-ducer.33,34 Inverted portal flow offers a specificity of 100% for identifying portal hypertension.35 The most frequent echographic findings in Fontan patients are heterogeneous echogenicity, a nodular surface and small-sized hyperechoic nodules.36 In a controlled study in 106 individuals, Kutty, et al. found a higher resistance and pulsatility index in the celiac trunk and mesenteric artery with a significant decrease in portal velocity in their Fontan patients.37 While the loss of the three-phase Doppler pattern in the hepatic veins is universal following bi-cavopulmonary surgery (as there is no atrial beat), observation of a monophasic pattern indicates advanced liver injury.26
Suggestive sonographic findings of advanced liver damage and portal hypertension in FALD.
| Mode B |
| Nodular liver surface. |
| Heterogenicity of hepatic parenchyma. |
| Intrahepatic venous-venous fistulas. |
| Diameter of the portal vein > 12 mm. |
| Splenomegaly (Area > 50 cm2). |
| Doppler |
| Decreased portal velocity (< 16 cm/s). |
| Inverted portal flow Resistance index > 0.71 and pulsatility index > 1.3 of principal hepatic artery. |
| Single-phase or biphasic flow in the hepatic veins. |
Elastography is the main non-invasive tool for the assessment of liver fibrosis in the majority of liver diseases.38 There are two ways to measure liver stiffness: transient elastography (Fibroscan®), which is easier and widespread in Europe, and sonoelastography, which needs to be conducted and interpreted by an expert operator and is more popular in the US. Transient elastography serves to stratify patients into four fibrosis stages and has been validated for use in many liver diseases. This method thus avoids liver biopsy in a large number of situations. Its major drawback is its rate of false positives including hepatic congestion.39 Consequently, in Fontan patients elastography may overestimate liver stiffness. Fontan surgery induces an immediately increased Liver stiffness (LS) due only to hepatic congestion.40 It has been well described that over time, signs of Fontan failure will appear. LS values will then increase, sometimes exceeding 15 kPa, and fibrosis progression may be to blame.41,42 A recent study in which liver fibrosis was assessed through Shearwave sonoelastography has shown good correlation between liver stiffness and histology.37 Although cut-offs still remain to be established, elastography is an accurate noninvasive method of measuring fibrosis.
Magnetic resonance imagingThe usefulness of MRI in FALD patients stems from its accuracy in detecting and characterizing liver nodules.43 Further, since cardiac resonance is now one of the main tools used in the follow up of the Fontan population, simultaneous dynamic hepatic resonance reduces costs and the number of medical visits. MRI-elastography is a novel and useful technique for the estimation of hepatic fibrosis, but its high cost and scarce availability limits its use in clinical practice. In Fontan patients, MRI-elastography has shown positive correlation between estimated stiffness and APRI index, MELD, pressures in the Fontan conduit and even histological damage.44
Hepatic hemodynamicsIn patients with chronic liver disease, hepatic venous pressure gradient (HVPG) is the best prognostic factor and has also been proposed as a key tool for the differential diagnosis of ascites in patients with FALD. Measuring HVPG is a quick, simple and minimally invasive procedure that does not require sedation.45 The procedure consists of jugular puncture to insert a balloon catheter through the Fontan connection into one of the hepatic veins. Hepatic pressure readings facilitate the differential diagnosis of portal hypertension such that high hepatic pressures (free and wedged) and a normal HVPG suggest a post-hepatic origin, and this is the most frequent finding after Fontan surgery.27 In patients with advanced parenchymal damage, HVPG may exceed 6 mmHg, or even 10 mmHg, which indicates a high risk of decompensation in most liver diseases.46 Nevertheless, there are several situations in which HVPG may be underestimated such as vascular fistulas between the hepatic veins themselves and the portal branches. In our experience, the development of such communications is very frequent in Fontan patients, which makes the HVPG reading difficult to interpret. We strongly recommend that HVPG measurements in these patients are carried out by expert staff and interpreted with caution.
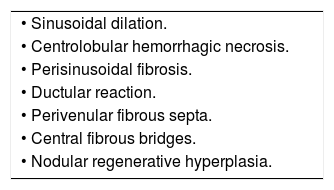
Liver biopsyLiver biopsy is the gold standard diagnostic procedure and, pending the proper validation of non-invasive diagnostic methods, is still necessary to determine the extent of liver damage. Typical histological features of FALD are provided in table 5. Sinusoidal dilation is present in 90-97% of Fontan patients and is the earliest parenchymal change. Typically, it is more pronounced than in other causes of cardiogenic hepatopathy. The distribution of fibrosis in the early stages is typically perisinusoidal (in the space of Disse); this is not the case in other forms of cardiac-derived liver disease. Extensive bridges of centrolobular fibrosis associated with regenerative nodules are a late finding in patients with advanced liver disease. Periportal inflammation is usually minimal or absent, allowing the differential diagnosis with other liver disease etiologies.47 Some authors recommend a liver biopsy in all patients 10 years after Fontan surgery.25 In a cohort of 67 patients, this strategy revealed that all patients developed hepatic fibrosis and that its extent increased with time. However, the authors found no correlation between the degree of fibrosis and clinically relevant events, hemodynamic or analytical parameters,48 thus questioning its utility in clinical practice. Hepatic biopsy is currently recommended when the etiology of liver disease is not clear and in candidates for heart and/or liver transplantation.37,39,49
Hepatic Complications of Fontan SurgeryAs in other forms of hepatic cirrhosis, in advanced FALD stages esophageal varices, ascites, hepatic encephalopathy, hepatocarcinoma and splenomegaly with thrombocytopenia may occur. The characteristics and natural history of these complications in FALD remain largely unknown, probably because no attention had been paid to FALD until the last five years.
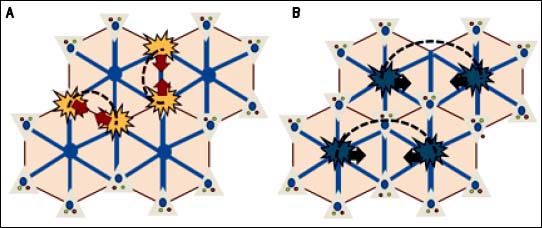
Hepatic nodules and hepatocellular carcinomaAs in Budd-Chiari syndrome and other vascular diseases of the liver, the development of large regeneration nodules in the hepatic parenchyma is a frequent finding in FALD.15,50 These nodules are usually multiple, hypervascular, hyperechoic, < 3 cm and located in the outer margins of the liver. Histologically, they usually correspond to nodules of regeneration, focal nodular hyperplasia or adenomas.28,36,51,52 Although the pathophysiology behind benign liver nodules in patients with FALD is unknown, their peripheral location and radiological behavior indicate a vascular origin (Figure 5).53 In recent years, some cases and small case series of hepatocarcinoma have been reported in young Fontan patients.53,66 These are often patients with advanced liver disease and a post-Fontan duration longer than 5-10 years. Malignant nodules are usually hypervascular, show washout and alpha-fetoprotein is typically elevated. However, these features are not pathognomonic and their diagnostic accuracy in FALD has not been addressed, making the differential diagnosis of nodules a main clinical challenge. Hence, until more data becomes available, the diagnosis of hepatocarcinoma in patients with FALD always requires histological confirmation.44
Regenerative nodular hyperplasia in FALD. A. Hepatic congestion and reduced cardiac output cause a decrease in portal blood flow predominantly in periphery of the liver, resulting in focal ischemia. B. Extinction of the parenchyma secondary to ischemia triggers an arteria vasodilatory response that stimulates the proliferation of healthy hepatocytes resulting in nodular regenerative hyperplasia (B).
Screening every 4-12 months with radiological techniques has proven to be cost-effective and to increase survival in patients with cirrosis. Nandwana, et al. retrospectively analyzed a cohort of 145 Fontan patients subjected to periodical liver imaging: in the initial assessment one case of hepatocarcinoma was present and four incident cases were detected during a median follow up of 3.05 years.67 Most experts recommend periodic screening, although the optimal radiologic technique and interval are unknown.25,51 Treatment of hepatocarcinoma should follow clinical practice guidelines used in other forms of cirrhosis.68
Esophageal varicesFollowing Fontan surgery, the prevalence of esophageal varices has been estimated at between 2 and 43%.25,30,69 In the VAST study on a retrospective cohort of 73 Fontan patients, the presence of varices along with other manifestations of portal hypertension was associated with an increased risk of death, heart transplantation and hepatocarcinoma.70 Case reports exist of variceal bleeding, some with a fatal outcome, highlighting the need for screening and proper prevention performed in a systematized manner when other signs of portal hypertension are present.25 In other forms of cirrhosis, the cornerstone of variceal bleeding prophylaxis is non-cardioselective beta-blockers. However, the effects of this form of prophylaxis have not yet been addressed in FALD.71 Unlike in all other forms of cirrhosis, the portal hypertension model in patients with FALD is characteristically hypodynamic such that the efficacy of these drugs is debatable. Further, if we also consider that betablockers may have deleterious effects on the Fontan circulation, as prophylaxis, it is advisable to consider rubber band ligation. An acute episode of gastrointestinal bleeding should be managed with vasoactive drugs (somatostatin, terlipressin or octreotide) and endoscopic therapy (band ligation). In cases of bleeding refractory to standard treatment, a transjugular intrahepatic portosystemic shunt (TIPS) has been shown to improve survival in other types of hepatopathy.71 Notwithstanding, the resultant increase in cardiac preload may precipitate pulmonary hypertension and cardiac failure. To the best of our knowledge, there is only one reported case of a 42-year-old man with cirrhosis after Fontan surgery in whom variceal hemorrhage was controlled by TIPS. Hence, its use should be restricted to highly selected cases not responding to conventional treatment when cardiac function is within the normal range or minimally impaired.72
AscitesAscites is a late manifestation of cirrhosis and is associated with reduced survival and a poor quality of life.73 It is the most frequent sign of clinical hepatic decompensation and its prevalence in the Fontan population ranges from 2 to 17%.51 In chronic liver disease with intrahepatic portal hypertension, ascites appears when HVPG ≥ 10 mmHg, which also has prognostic capacity.46 However, in the Fontan patient, HVPG is usually normal and ascites may appear in the absence of cirrhosis. This means its value as a prognostic marker and its pathophysiological mechanisms differ from those related to other forms of liver dis-ease.28,74 There are several causes of ascites in Fontan patients (Table 6).74
Differential diagnosis of ascites in Fontan patients.
| • Systemic venous hypertension due to failure of the Fontan circulation (arrhythmias, thrombosis/stenosis of Fontan conduit, ventricular dysfunction, pulmonary hypertension). |
| • Post-hepatic portal hypertension. |
| • Sinusoidal portal hypertension (advanced liver fibrosis). |
| • Hypoalbuminemia/Hypoproteinemia secondary to proteinlosing enteropathy. |
Ascites is usually manageable by optimizing cardiac function, and nutrition along with the use of loop diuretics and anti-aldosterone drugs since the renin-angiotensin aldosterone system is hyperactivated in portal hypertension and heart failure.46,75 Large-volume paracentesis could be a rescue treatment, although it is rarely required. TIPS does not seem a suitable option in Fontan anatomy and no case reports of this exist.
Hepatic encephalopathyIt is estimated that 30-40% of patients with liver cirrhosis will present this condition at some point,76 although in FALD this event is poorly documented. Only 3 cases of hepatic encephalopathy following Fontan surgery exist in the literatura.25,30,69 However, it seems likely that its real incidence and prevalence are underestimated because of the retrospective nature of most studies and the possibility that hepatic encephalopathy was not considered in the differential diagnosis.
Liver transplantationIn the twentieth century, cirrhosis was conceived as an irreversible disease. However, current knowledge arising from studies on viral and alcoholic cirrhosis indicate that fibrosis is effectively reversible.76,77 In cardiac cirrhosis, experimental models and small case series point to the idea that if cardiac function is restored, liver disease may also improve and even fully normalize.73,78,79 Considering that the severity of heart disease is directly linked to liver damage and that liver damage is practically universal in Fontan patients, the key question arises as to which subgroups of patients will require an isolated heart transplant or a double cardiohepatic transplant.
To shed light on this question, we have data available from two studies which are nevertheless retrospective, have a small sample size, and suffer from methodological flaws. Simpson, et al. analyzed 20 abdominal CT scans of Fontan patients before heart transplantation: 7 had morphological changes compatible with liver cirrhosis, 5 had liver changes not meeting cirrhosis criteria and the remaining were normal. Cirrhotic patients were older (17.6 vs. 9.6 years, p = 0.002) and showed a longer time since surgery (180 vs. 50 months, p < 0.05); without significant differences in laboratory parameters. One-year survival was similar between cirrhotic and non-cirrhotic patients (86% vs. 77%, p = 0.681). In the group of transplanted cirrhotic patients, two patients died, none of them of a hepatic cause.58 The absence of biopsy and lack of a detailed description of cirrhosis stage in some subjects limit the validity of this research. In 2016, D’Souza, et al. described 7 Fontan patients who received a double transplant. The criterion for liver transplantation was the presence of bridge fibrosis or cirrhosis in a pre-transplantation biopsy. Three patients had portal hypertension and ascites, while the other four had no complications related to FALD. All patients survived a median follow up of 4.6 years.39,47 From our perspective, this approach is too aggressive since liver damage in patients with compensated disease will likely be insufficient to justify liver transplantation; especially considering the current shortage of organs and the potential improvement of liver function after heart transplantation. In addition, no study has shown that compensated liver disease is a perioperative risk factor or a long-term poor prognostic marker after cardiac transplantation in FALD. At present, there are no consensus guidelines regarding indications for double transplantation. The institutions with more experience in this field recommend an individual analysis of each case by a multidisciplinary committee.
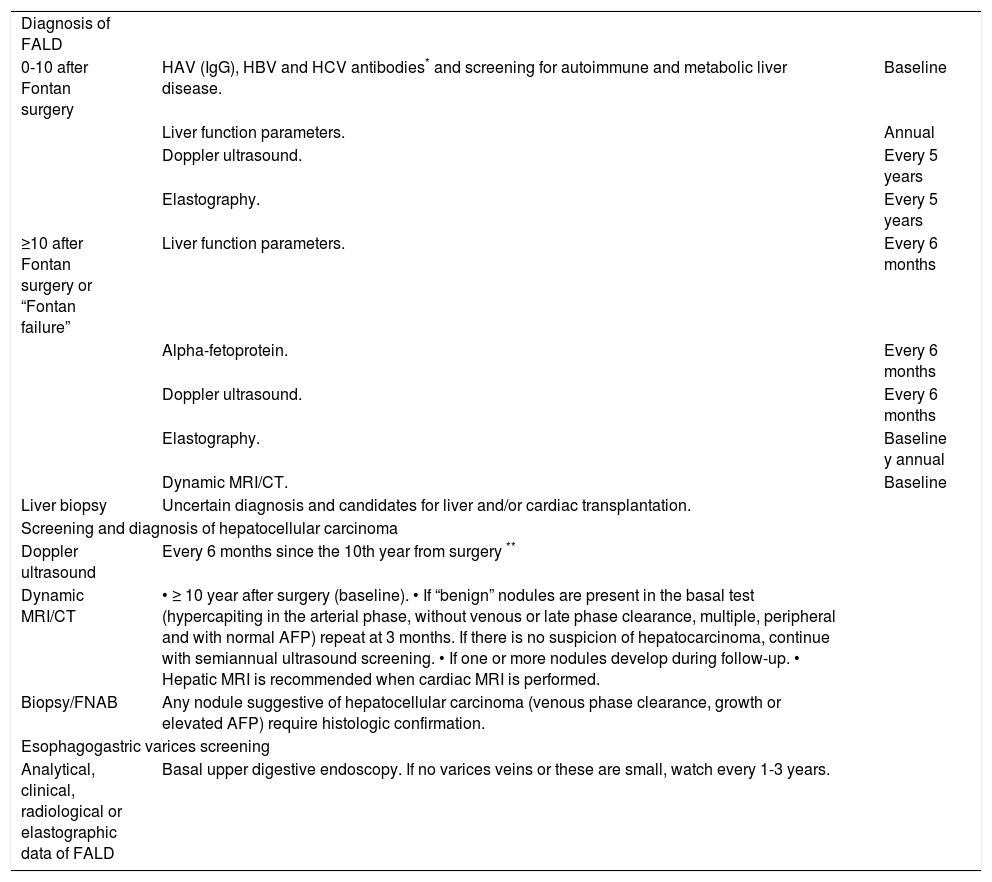
ConclusionsLiver damage following Fontan surgery is universal. The main risk factors are time since surgery and poor cardiac function. Although the natural history of FALD is not well established, patients operated on more than 10 years ago should be closely followed to check for the onset of portal hypertension-related complications or hepatocellular carcinoma. Its peculiar pathophysiology and clinical behavior make FALD a unique entity of liver disease that requires an individualized management program designed by a multidisciplinary committee (Table 7). It is essential to create multicenter research groups to gather scientific evidence on which to base the optimal follow up and treatment of the growing adult Fontan population.
Follow-up recommendations related liver disease after Fontan surgery at Hospital Universitario Ramón y Cajal.
| Diagnosis of FALD | ||
| 0-10 after Fontan surgery | HAV (IgG), HBV and HCV antibodies* and screening for autoimmune and metabolic liver disease. | Baseline |
| Liver function parameters. | Annual | |
| Doppler ultrasound. | Every 5 years | |
| Elastography. | Every 5 years | |
| ≥10 after Fontan surgery or “Fontan failure” | Liver function parameters. | Every 6 months |
| Alpha-fetoprotein. | Every 6 months | |
| Doppler ultrasound. | Every 6 months | |
| Elastography. | Baseline y annual | |
| Dynamic MRI/CT. | Baseline | |
| Liver biopsy | Uncertain diagnosis and candidates for liver and/or cardiac transplantation. | |
| Screening and diagnosis of hepatocellular carcinoma | ||
| Doppler ultrasound | Every 6 months since the 10th year from surgery ** | |
| Dynamic MRI/CT | • ≥ 10 year after surgery (baseline). • If “benign” nodules are present in the basal test (hypercapiting in the arterial phase, without venous or late phase clearance, multiple, peripheral and with normal AFP) repeat at 3 months. If there is no suspicion of hepatocarcinoma, continue with semiannual ultrasound screening. • If one or more nodules develop during follow-up. • Hepatic MRI is recommended when cardiac MRI is performed. | |
| Biopsy/FNAB | Any nodule suggestive of hepatocellular carcinoma (venous phase clearance, growth or elevated AFP) require histologic confirmation. | |
| Esophagogastric varices screening | ||
| Analytical, clinical, radiological or elastographic data of FALD | Basal upper digestive endoscopy. If no varices veins or these are small, watch every 1-3 years. | |
Perform HAV, HCV and HBV ELISA (HBsAg, HBcAb and HBsAb) in all patients with Fontan surgery. If not immunized, vaccination against HAV and HBV should be indicated and its efficacy tested with new serologies. 10 years after effective vaccination against HBV, levels of HBsAg should be determined and a new dose should be indicated if levels are <100 IU/L.
It will be advanced in those patients with “Fontan failure”, Fontan’s duct thrombosis or transitional elastography ≥ 15 KPa. AFP: Alpha-fetoprotein. CT: Computed tomography. HAV: Hepatitis A virus. HBsAg: Hepatitis B surface antigen. HBcAb: Hepatitis B core antibody. HBsAb: Hepatiti B surface antibody. HBV: Hepatitis B virus. HVC: Hepatitis C virus. MRI: Magnetic resonance imaging.
- •
ALT: alanine transaminase.
- •
APRI: AST to platelet Ratio Index.
- •
AST: aspartate aminotransferase.
- •
CT: computed tomography.
- •
FALD: Fontan-associated liver disease.
- •
GGT: gamma-glutamyl transpeptidase.
- •
HVPG: hepatic vein pressure gradient.
- •
LDH: lactate dehydrogenase.
- •
LS: liver stiffness.
- •
MELD: model for end stage liver disease.
- •
MR: nuclear magnetic resonance.
- •
TGF- : transforming growth factor beta.
- •
TIPS: transjugular intrahepatic portosystemic shunt.
This work was financed by grants from the Spanish Ministry of Health, Instituto de Salud Carlos III (no. PI14/00876 and PI051871, CIBERehd) awarded to Agustín Albillos. Ciberhed is funded by the Instituto de Salud Carlos III.
Conflicts of InterestAll authors have nothing to disclosure.
ACKNOWLEDGEMENTWe thank the Department of Pediatric Cardiology of the Hospital Universitario Ramón y Cajal, in particular María Jesús del Cerro and Elvira Garrido-Lestache, for their enthusiastic dedication to Fontan-associated liver disease.