Steatotic liver disease (SLD), previously known as Nonalcoholic fatty liver disease (NAFLD), is a chronic liver disease closely associated with metabolic conditions [1–3]. It is the leading cause of liver disease globally and a growing public health problem [4]. The actual prevalence is probably underestimated due to insufficient attention to metabolic disorders in patients with hepatic steatosis [5]. In addition, SLD is a risk factor for liver-related events, cardiovascular diseases, and extrahepatic manifestations [2,6–9]. Recently, a multi-society Delphi conference proposed a new nomenclature of metabolic dysfunction-associated steatotic liver disease (MASLD) [10]. In China, MASLD has become the most prevalent liver disorder due to dramatic lifestyle changes over the last 20 years [11–13].
However, the shift from NAFLD to an inclusion-based definition of MASLD has yet to be extensively studied. The impetus for this change was the proposition put forth in the year 2020 to reclassify NAFLD as metabolic-associated fatty liver disease (MAFLD) [14]. The criteria for MAFLD with metabolic abnormality did not exclude individuals with significant alcohol intake or chronic viral hepatitis. Some studies have suggested that new nomenclature, which includes metabolic disorders, is more suitable for fatty liver disease [15,16]. However, those studies were hampered by no histologic data and limited sample size, and most importantly, coexisted confounders of other chronic liver diseases [17].
MAFLD and MASLD involve not only the shift of name but also the appropriate definition of terminology. The modified Delphi process was led by the American Association for the Study of Liver Disease (AASLD), the European Association for the Study of the Liver (EASL), and the Association Latinoamericana para el Estudio del Higado (ALEH). They provide a comprehensive understanding of the various etiological factors contributing to steatotic liver disease while encompassing all its subcategories: “cryptogenic SLD,” “pure” MASLD, “MetALD,” and “MASLD+ALD.” However, it is essential to note that the metabolic criteria can potentially impact the identification of patients in both epidemiological and clinical research. There is a degree of uncertainty around the terminology in stratifying patients of this umbrella terminology, SLD [18].
Furthermore, one issue with the proposal is whether it affects diagnostic algorithms and medication development [19]. Before asserting the generalizability of data on NAFLD from previous decades to the new nomenclature, it is imperative to thoroughly examine the amount to which definitions of subgroups influence different study populations. Nonetheless, there is limited knowledge regarding the attributes of groups that align with the former criteria of NAFLD yet are presently omitted from the recently introduced MASLD definition.
Although a liver biopsy is not obligatory for diagnosing MASLD, providing histopathological features will accurately assess fibrosis progression, which is a significant risk factor for the onset of hepatocellular carcinoma and mortality [20]. Moreover, it would greatly help to evaluate the significant difference between the novel MASLD criteria and the prior NAFLD.
Thus, to assess the impact of the new SLD definition on fatty liver cohort stratification and risk factors for significant fibrosis, this study aimed further to evaluate the MASLD criteria in a biopsy-proven cohort and to characterise patients who were currently undiagnosed by MASLD criteria.
2Methods2.1Study designThis is a single-centre, cross-sectional study. Subjects who underwent liver biopsy between January 2009 and December 2022 at Beijing Ditan Hospital, Capital Medical University, Beijing, China, were retrospectively included.
2.2Demographic variables and laboratory parametersPrior to undertaking the liver biopsy, demographic variables were obtained as follows: age, sex, body mass index (BMI), blood pressure, history of type 2 diabetes mellitus (DM) or prediabetes (Fasting serum glucose≥ 5.6 mmol/L or 2 h post-load glucose levels≥ 7.8 mmol/L or HbA1c≥ 5.7 % or specific treatment for DM), hyperlipidemia, hypertension (blood pressure ≥130/85 mmHg or antihypertensive treatment), and history of steatogenic medication (i.e., corticosteroids). Liver stiffness measurement (LSM), haematological data, and baseline biochemical data were retrieved at the time of biopsy within 48 hours, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), platelet count (PLT), haemoglobin A1c (HbA1c), fasting glucose, triglyceride (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), plasma high-sensitivity C-reactive protein level and insulin resistance score.
2.3Histological evaluationLiver biopsies were obtained by percutaneous liver biopsy under real-time transabdominal ultrasound guidance. All specimens were stained with hematoxylin-eosin and Masson trichrome stains. An adequate biopsy specimen was defined as having a length of no less than 10 mm and no less than six portal tracts. The presence of ballooning and NAFLD activity score (NAS) [21], which is based on a standardised grading system of steatosis (on a scale of 0-3), lobular inflammation (on a scale of 0–3), and ballooning (on a scale of 0–2), were reevaluated by three experienced histopathologists who were blinded to the clinical data. A final consensus had to be reached. If the evaluation by three pathologists was discordant, the patients were excluded as “missing data”. Metabolic dysfunction associated steatohepatitis (MASH) was defined as NAS ≥5. Fibrosis stages 1a, 1b, and 1c were considered stage 1 for analysis. Significant and advanced fibrosis was defined as fibrosis stages 2–4 and 3, 4, respectively.
2.4Diagnostic criteria and definition of groupsHepatic steatosis of various etiologies proved by liver biopsy was diagnosed as steatotic liver disease (SLD). NAFLD was defined as SLD in the absence of any secondary causes of steatosis, consisting of steatogenic drug use (i.e., corticosteroids, amiodarone, and methotrexate) and excessive alcohol consumption (>20 g/d in females or >30 g in males). MASLD is diagnosed according to the criteria above in conjunction with at least one of five cardiometabolic risk factors (CMRF): (1) BMI≥ 24 kg/m2 OR WC> 90 cm (M) 80 cm (F), defined as BMI subgroup; (2) Fasting serum glucose ≥ 5.6 mmol/L OR 2-hour post-load glucose levels ≥7.8 mmol/L OR HbA1c ≥5.7 % OR diagnosis of DM OR treatment for DM, defined as DM subgroup; (3) Blood pressure ≥130/85 mmHg OR specific antihypertension treatment, defined as HT subgroup; (4) Plasma triglycerides ≥1.70 mmol/L OR lipid-lowering treatment, defined as TG subgroup; (5) Plasma HDL ≤1.0 (M) and ≤1.3 mmol/L (F) OR lipid-lowering treatment, defined as HDL subgroup. Patients with hepatic steatosis who do not meet CMRF are diagnosed as cryptogenic SLD [10]. Patients were grouped according to the NAS: no MASH (NAS0-4) and MASH (NAS≥5).
2.5Statistical analysisData for continuous variables were expressed as means± standard deviations, median with interquartile range, or numbers (percentages) where applicable. Data for categorical variables were expressed as frequency and percentage. Statistically significant differences between groups at a 2-tailed P < 0.05 were evaluated by Student's t-test or the nonparametric Mann-Whitney U or Chi-square test. Multivariate logistic regression was used to assess risk factors of significant fibrosis, and 95 % confidence intervals (CIs) were calculated. All analyses were conducted using SPSS 25.0 (IBM Corp., Armonk, NY, USA) and R version (4.1.2 for Windows).
2.6Ethical statementThe study protocol conformed to the guidelines of the 1975 Declaration of Helsinki (6th revision,2008) and was approved by the Ethics Committee of Beijing Ditan Hospital (DTEC-KT2023-006-01). Informed consent for liver biopsy was obtained from all subjects before the procedure, and clinical data used to analyse were anonymous.
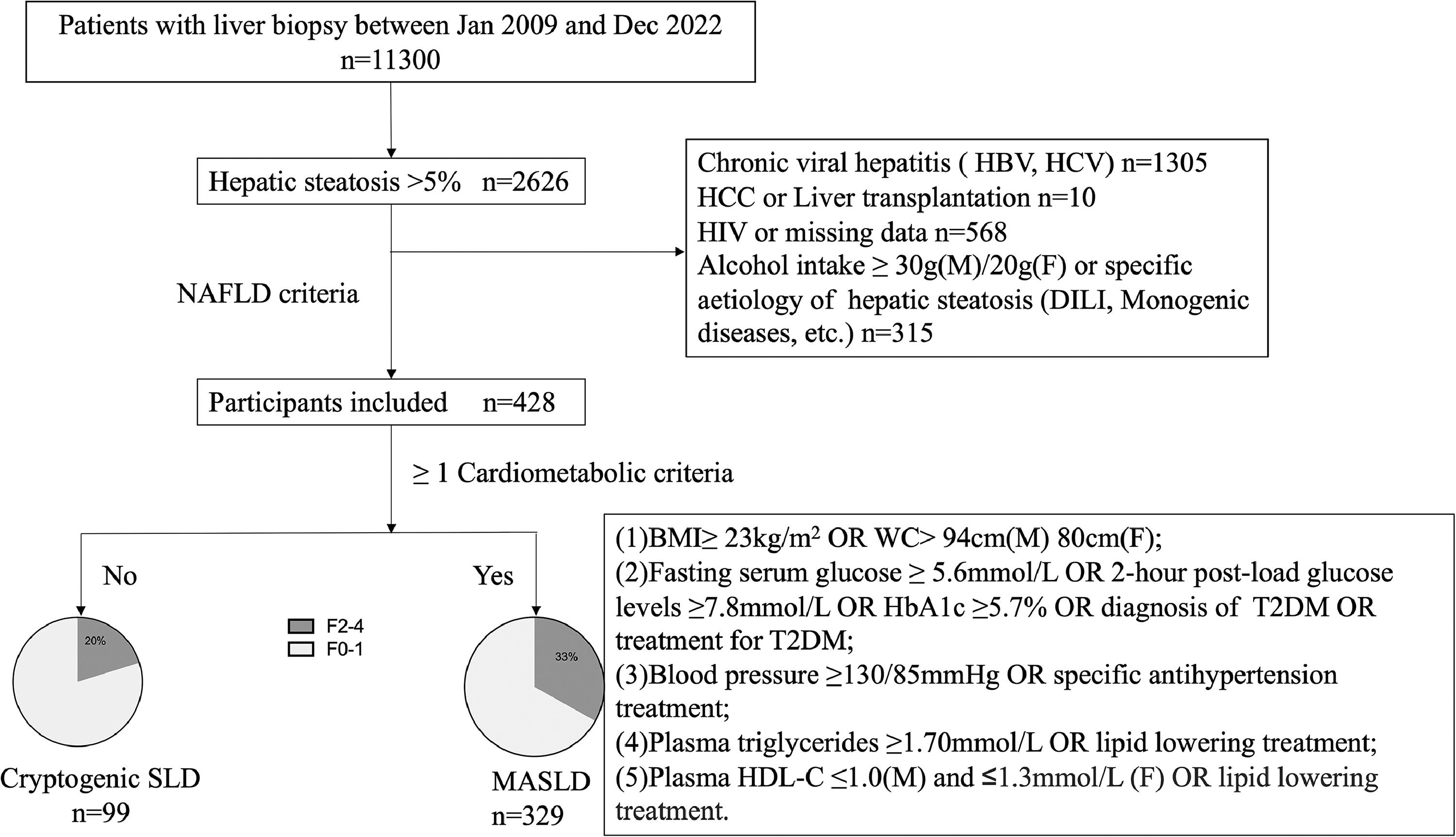
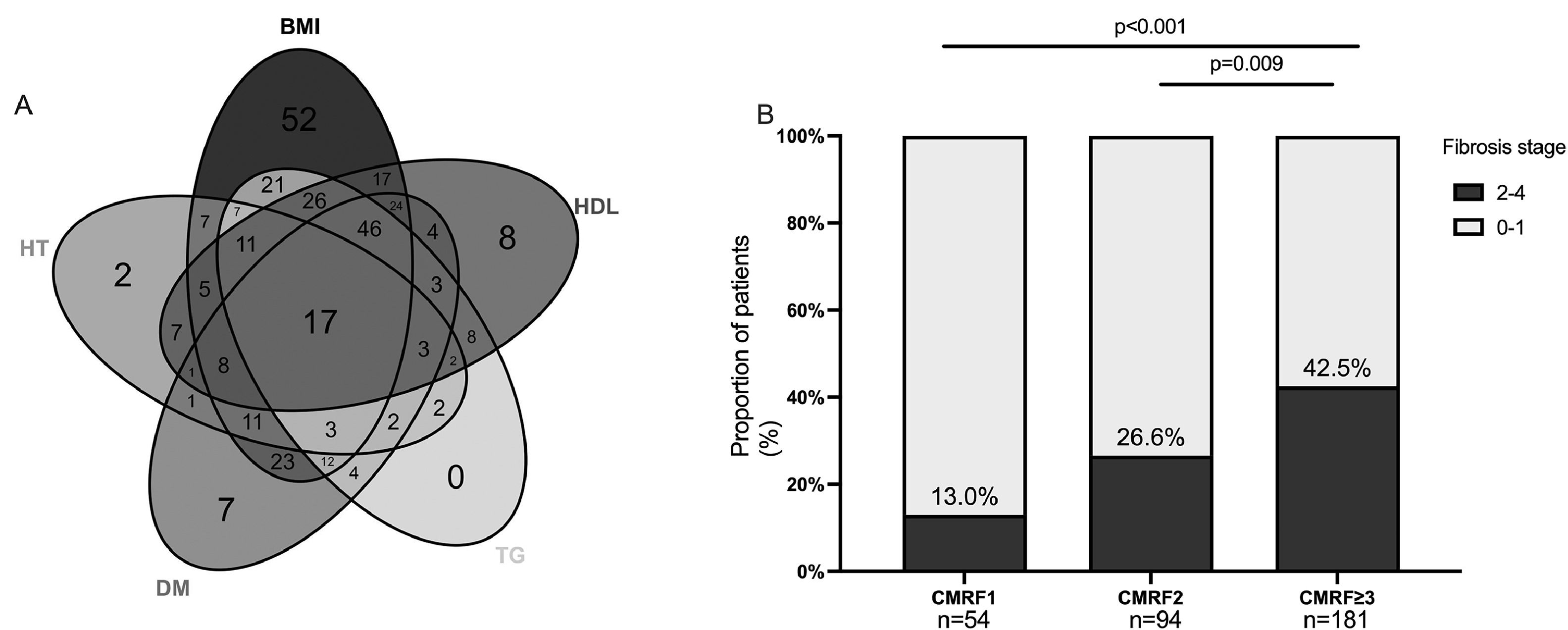
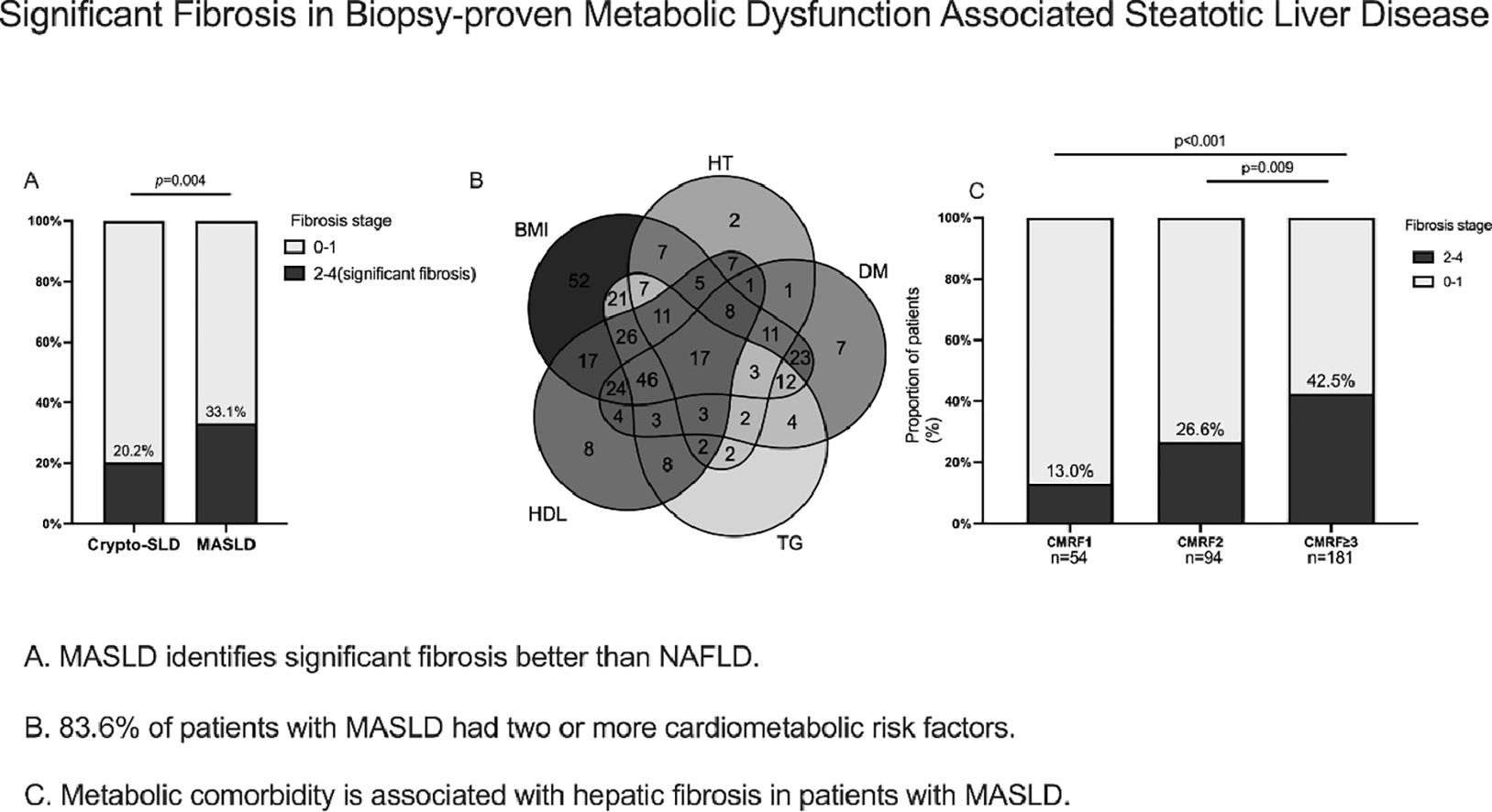
3Results3.1Patient demographics, characteristics, and laboratory dataBetween Jan 2009 and Dec 2022, 2626 patients who underwent liver biopsy were proved to have hepatic steatosis; among them, 2198 were excluded, leaving 428 patients who met NAFLD criteria for analysis (Fig. 1). The study included predominantly males (59.3 %), with a mean age of 41 ± 0.7 years. Reliable LSMs were available in 70.3 % (n = 301) of participants. Metabolic comorbidities were common, resulting in a 76.9 % prevalence of MASLD (n = 329). Patients who did not fulfil the CMRF criteria were divided into the Cryptogenic-SLD group (n = 99). In the MASLD group, the most common CMRF criteria were overweight (BMI subgroup, n = 275, 83.6 %), followed by the HDL subgroup (n = 190, 57.8 %), DM subgroup (n = 169, 51.3 %), TG subgroup (n = 167, 50.8 %) and HT subgroup (n = 89, 27.1 %). A total of 181 (42.3 %) patients were diagnosed to have more than three CMRFs. The three most co-existing CMRFs were overweight, high TG, and HDL (n = 100).
Study design. BMI, body mass index; DILI, drug-induced liver injury; F, female; HBV, hepatitis B virus; HbA1C, Glycosylated hemoglobin type A1C; HCV, hepatitis c virus; HCC, hepatocellular carcinoma; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; M, male; MASLD, metabolic associated steatotic liver disease; SLD, steatotic liver diseases; T2DM, type 2 diabetes mellitus; WC, waist circumference.
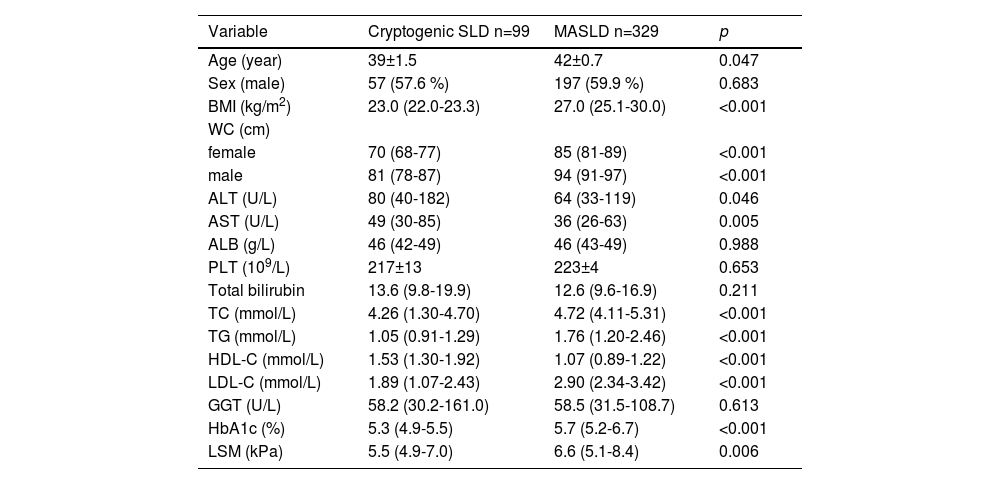
The first set of questions aimed to determine factors linked with a diagnosis of MASLD. Baseline characteristics are shown in Table 1, stratified by the presence or absence of CMRF. Patients with MASLD were three years older (42 vs. 39, p = 0.047) than Cryptogenic-SLD. Characteristics such as sex, ALB, PLT, GGT, and total bilirubin were not statistically different in the two groups.
Clinical characteristics of MASLD and cryptogenic SLD.
ALT, alanine aminotransferase; AST aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transferase; HbA1c, Glycosylated hemoglobin type A1C;LDL-C, low-density lipoprotein cholesterol; LSM, Liver stiffness measurement; PLT, platelet; MASLD, metabolic dysfunction-associated steatotic liver disease; SLD steatotic liver diseases; TC, total cholesterol.
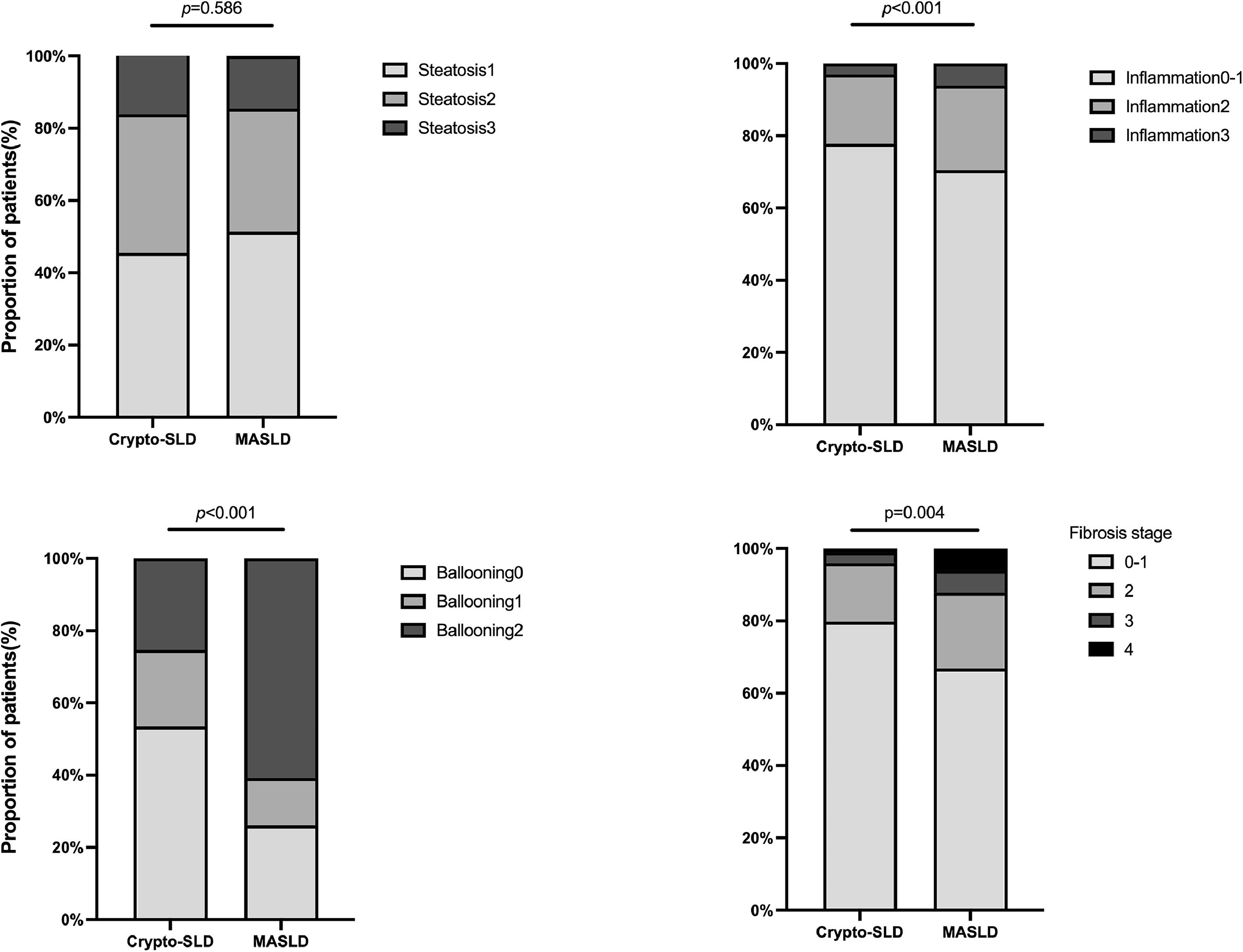
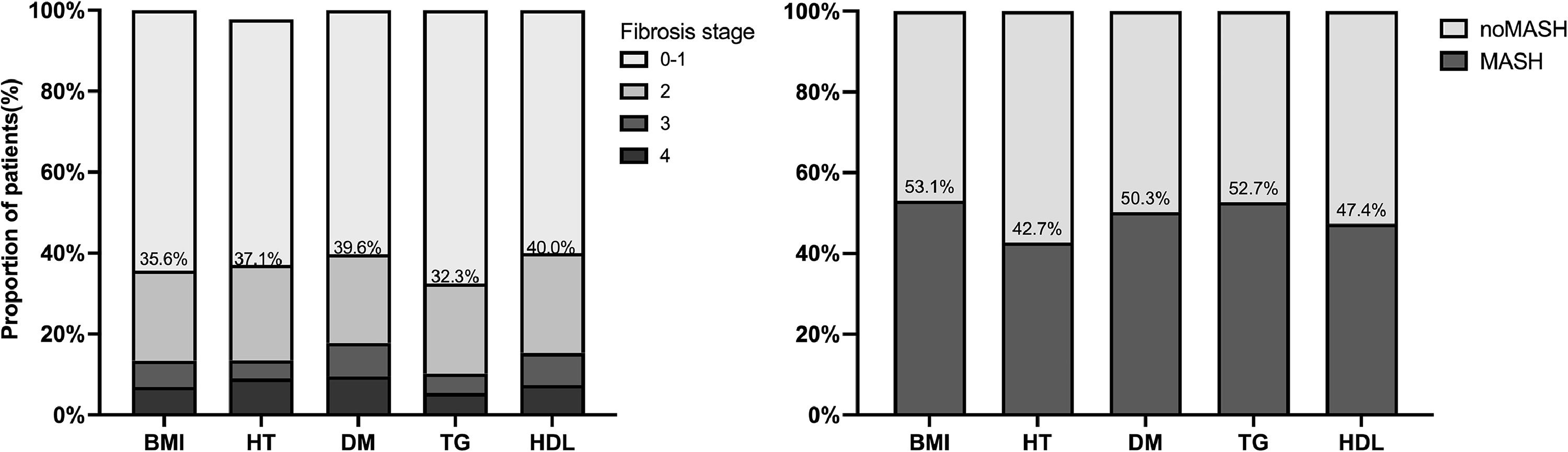
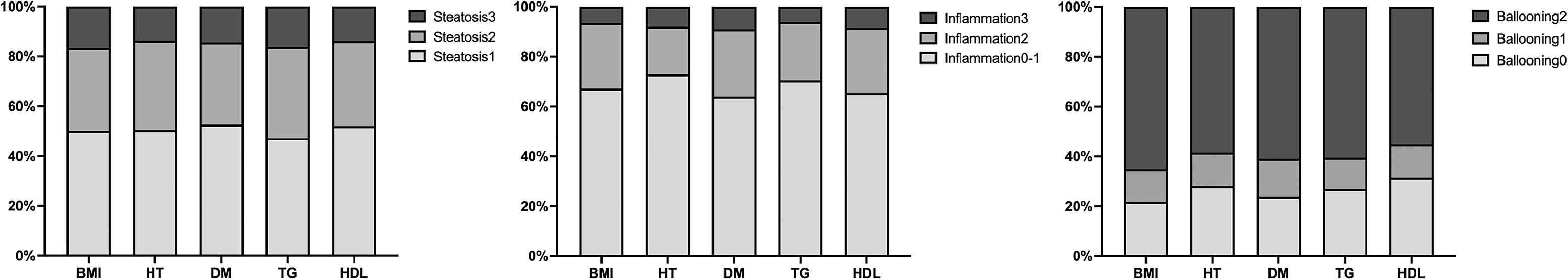
We further investigated the burden of steatosis, inflammation, and fibrosis in patients with MASLD. Histologically, those with steatosis grades 3 were 16.2 % in the Cryptogenic-SLD group, similar to 14.6 % in the MASLD group (p = 0.586). The grades of lobular inflammation and ballooning were significantly higher in the MASLD group than in the Cryptogenic-SLD group (Supplementary Table S1, Fig. 2). Cryptogenic-SLD had a median NAS score of 3, while MASLD had a median NAS score of 4 (p = 0.001). Of the 329 patients in the MASLD group, 60 (18.2 %) had NAS 1-2, 113 (34.3 %) had NAS 3-4, and 156 (47.4 %) had NAS 5-8. Thus, the proportion of patients with MASH was significantly higher in MASLD than that of Cryptogenic-SLD (47.4 % vs. 22.1 %, p < 0.001). In all 428 patients with NAFLD (MASLD + Cryptogenic SLD), significant fibrosis occurred in 30 %. A higher proportion of significant fibrosis in the MASLD group was observed (Figs. 1 and 2) compared to the Crypto-SLD group (33.1 % v.s 20.2 %, p = 0.014). Furthermore, the sensitivity for MASLD to detect significant fibrosis and MASH were 84.5 % and 87.6 %, respectively. Patients with MASLD were categorised by CMRF criteria (Fig. 3), including overweight (BMI group), diabetes or prediabetes (DM group), hypertension (HT group), high triglycerides (TG group), and low high-density lipoprotein cholesterol (HDL group). Fig. 4, Supplementary Table S2, and Fig. 5 show the distribution of patients according to the NAS scores in five subgroups of MASLD.
3.4Metabolic comorbidity is associated with fibrosis in patients with MASLDTo clarify the impact of metabolic disorders on hepatic fibrosis, patients with MASLD were further stratified by the numbers of CMRF: CMRF1 (n=54), CMRF2 (n=94), CMRF≥3 (n=181). The proportion of males was higher in the CMRF1 group (74.1 %) and CMRF2 group (66.0 %) compared to the CMRF≥3 group (52.5 %, p = 0.006). In the CMRF≥3 group, the median age was 53 for females and 36 for males. There is a trend for older patients diagnosing more metabolic disorders (31.5 vs. 43 vs. 47 years old in CMRF1, CMRF2, and CMRF≥3 group, respectively, p < 0.001). No difference existed between the three groups regarding ALT and AST (p = 0.745,0.535, respectively). While the level of total cholesterol was significantly lower in patients with CMRF1 compared with CMRF 2 and CMRF≥3 (4.56 v.s 4.66 v.s 4.86, p = 0.010).
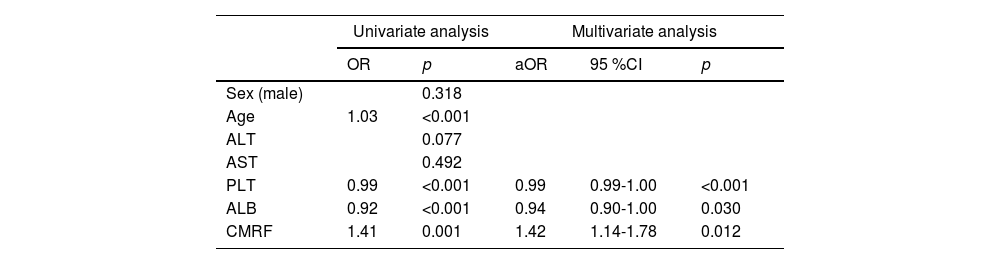
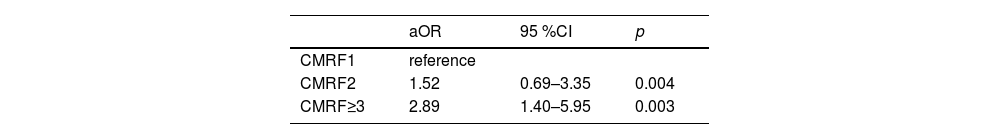
Further analysis of the data revealed the role of the numbers of CMRFs in fibrosis progression. The prevalence of significant fibrosis increased from 13 % and 26.6 % for one and two criteria present to 42.5 % for meeting three or more CMRF criteria (Fig. 3). The next section of the survey concerned clinical variables and laboratory parameters associated with significant fibrosis. As can be seen in Table 2, ALB (aOR:0.94,95 %CI:0.90-1.00;p = 0.030), lower levels of PLT (aOR:0.99, 95 %CI:0.99-1.00; p < 0.001), and more metabolic comorbidities (aOR:1.42, 95%CI:1.14-1.78; p = 0.012) were independent risk factors of significant fibrosis. As shown in Table 3, the coexistence of three or more CMRFs was associated with a nearly 3-fold increase in significant liver fibrosis than patients with one CMRF. Notably, among 17 patients with all five CMRFs, one had fibrosis stage F3, and cirrhosis (F4) could be diagnosed in 4 patients.
Distribution of fibrosis stages and proportion of MASH among five subgroups. Patients with MASLD were divided according to the five admission criteria: overweight (BMI group), hypertension (HT group), type 2 diabetes (T2DM group) and two hyperlipidemia risk factors (TG and HDL group).
Factors associated with significant fibrosis in MASLD: univariate analysis and multivariate analysis.
aOR, adjusted odds ratio; ALT, alanine aminotransferase; AST aspartate aminotransferase; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; CMRF, cardiometabolic risk factor; PLT, platelet; MASLD, metabolic dysfunction-associated steatotic liver disease.
To assess the impact of steatosis and inflammation of hepatic fibrosis, binary logistic regression was conducted. There was no statistically significant correlation between steatosis grade and fibrosis stage (p = 0.495). Further analysis showed a significant correlation between NAS score and fibrosis stage (p < 0.001).
4DiscussionThis cross-sectional, liver biopsy-based study aimed to better the new nomenclature MASLD and confirm that it is better than NAFLD in identifying significant fibrosis.
Several reports have shown that epigenetic factors and other elements, such as obesity, lipodystrophy, and insulin resistance, may contribute to the development and advancement of MASLD independently or in conjunction with genetic factors [22,23]. Hagström H et al reported that the most common CMRF was a BMI ≥25 kg/m2 (88.5%), which is in accordance with our study [24]. Previous studies have demonstrated that obesity leads to the development of (88.5 %), which is in accordance with our study of metabolic syndrome and comorbidities, including SLD [25–27], and lean patients with SLD were significantly metabolically healthier than obese patients [28,29]. What stands out in Fig. 2 and Table 1 is the higher rate of significant fibrosis in MASLD than in Cryptogenic SLD in which all “lean SLD” were included with a median BMI of 22 kg/m2. International Diabetes Federation has recommended cutoffs of 90 cm in men or 80 cm in women to define central obesity in Chinese people [30]. According to the Chinese BMI classification, overweight was defined as 24 to <28 kg/m2, and obesity as ≥28 kg/m2[31]. It is believed that the cutoff we use might be more suitable for defining central obesity in the Chinese population. This study supports evidence from previous observations in the long-term prognosis in NAFLD with obesity [32,33]. Obesity has become an increasingly common condition, and those who have been diagnosed with MASLD, such as these obese people, were given more attention by clinicians. Consistent with the literature, in our study, obese or overweight is the most common CMRF in MASLD patients. Considering the absence of authorized pharmacological therapies for MASLD, it is a natural approach to focus on addressing obesity as a potential strategy for its therapy.
A higher fibrosis stage was observed among patients with MASLD who had more cardiometabolic risk factors. The NAS score is predominantly higher in MASLD than in cryptogenic SLD. The possible explanation is that in MASLD patients, the body's storage capacity in the subcutaneous adipose tissue is surpassed [34–36]. Accordingly, the metabolic disorder may be the primary culprit for hepatic fibrosis progression instead of steatosis. This would be the reason for the inflammation, and MASLD may progress into MASH over time, resulting in fibrosis [37]. In general, NAFLD is more frequently recognized in men than women [38]. In our study, there were more women than men (52.5 % vs. 47.5 %) in the CMRF ≥ 3 group and the median age was significantly different (53 years vs. 36 years). However, sex was not an independent risk factor for significant fibrosis in our study. Previous reports show a trend in increasing NAFLD prevalence among postmenopausal women, and this sex difference is reduced [39–42]. In a research of 1266 patients in the US nonalcoholic steatohepatitis (NASH) network, 64 % were female, and those with NASH were more likely to be female [43]. Several studies reported postmenopausal women showed a significant association with an increased risk of diabetes, hypertriglyceridemia, and central obesity, which are important criteria for diagnosing MASLD [44,45]. Although the prevalence of metabolic syndrome is slightly higher in men, it reverses after 50 years [46]. These data might explain our result that in patients with more metabolic discordant, the majority were women with a median age of 53 years. Whether sex is independently associated with hepatic fibrosis in different phases of sex hormones is currently unknown. Conflict data might be explained by different interests between men and women in participating in studies. Features of hepatic injury and inflammation in response to metabolic stress appear to be diverse and this area requires further research.
The most striking result to emerge from the data is that more than 40 % of patients with CMRF≥3 were reported to have significant fibrosis, and it is statistically significant when compared to the patients with CMRF =2 (Table 3). Besides, the majority of patients diagnosed with MASLD are those who exhibit three or more cardiometabolic disorders. What emerges from the results reported here is that patients with three or more metabolic disorders should exercise caution. All five metabolic comorbidities were present in 17 patients (5 %) in our study, and the prevalence of advanced fibrosis can be 23.5 % in these patients. Despite the limited number of patients within this specific cohort, it is crucial to acknowledge the rising prevalence of fatty liver disease, as the severity of fibrosis in these individuals poses a significant concern. Consequently, there is a pressing need to ensure timely access to appropriate treatment interventions. This observation suggests that healthcare practitioners should diligently focus on this matter from a clinical perspective. In light of the significant burden posed by MASLD, it is imperative for future policy and research endeavours to prioritize the identification and resolution of existing knowledge gaps.
Some limitations in our study included a retrospective study design, data from a single centre, selection bias for biopsy, and shortage of LSM in some patients. Given the limited sample size, it is not possible to perform a separate analysis of subgroups according to different CMRFs. Considerably more work will need to be done to confirm the stratification of patients with different subgroups of CMRFs and evaluate the impact of these risk factors during long term follow-up.
5ConclusionsIn conclusion, this study found that there was a substantial burden of liver fibrosis in patients with MASLD, with a high prevalence of cardiovascular risk factors. The results of this observational study support the name shift. MASLD identifies more significant fibrosis than NAFLD. These findings also provided evidence for stratifying liver fibrosis in SLD patients, especially in individuals with three or more cardiometabolic risk factors.
Author contributionsShan Hong: Conceptualization, Investigation, Data curation, Writing – original draft. Lei Sun: Resources, Data curation, Writing – review & editing. Yiwei Hao: Formal analysis, Validation. Ping Li: Yuling Zhou: Data curation. Xiuxia Liang: Data curation. Julong Hu: Data curation. Hongshan Wei: Conceptualization.
This paper was funded by grants from the National Natural Science Foundation of China [Grant No. 82170541), the Capital Foundation for Clinical Characteristic Applied Research Projects [Grant No. Z181100001718084].