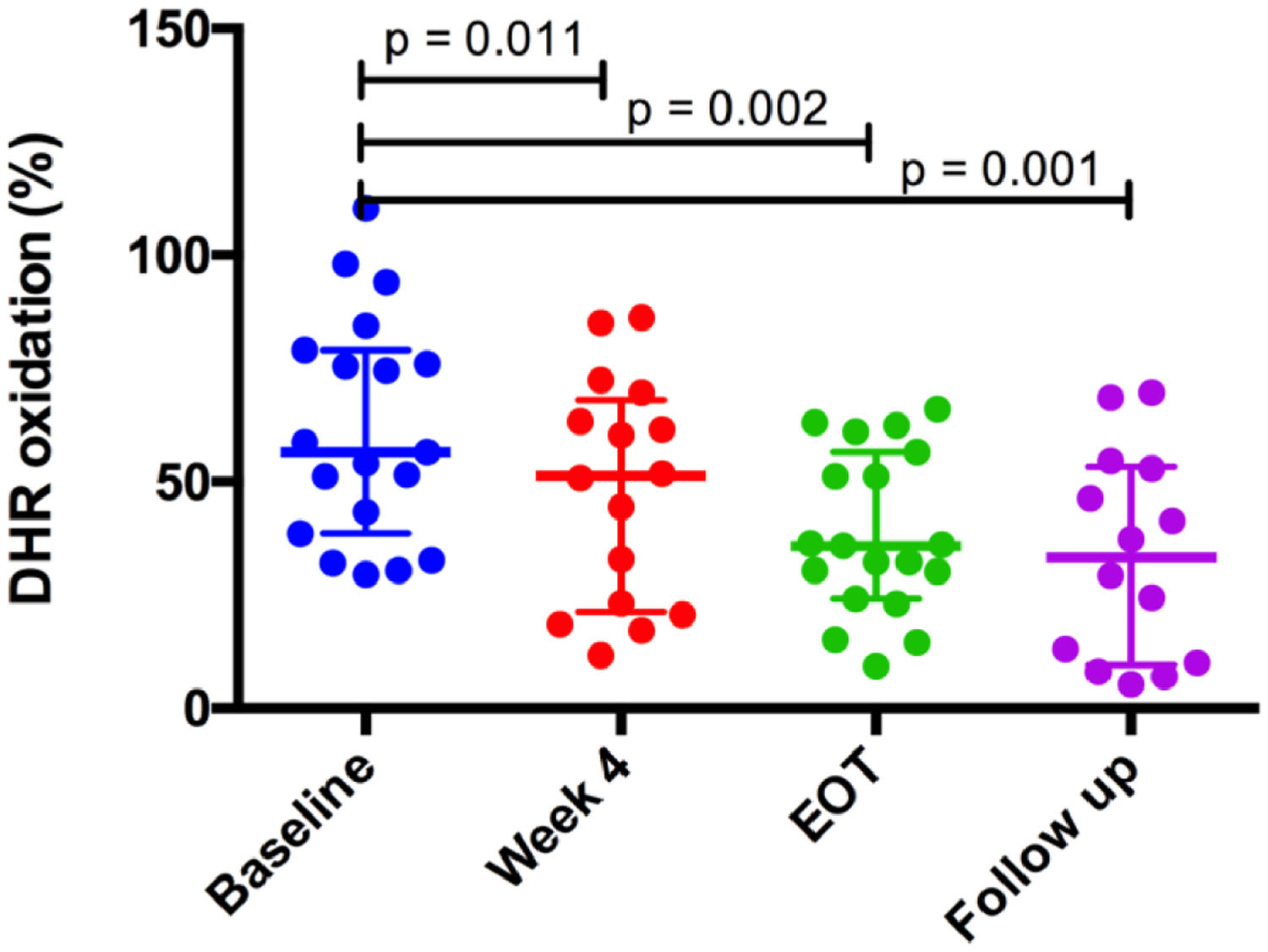
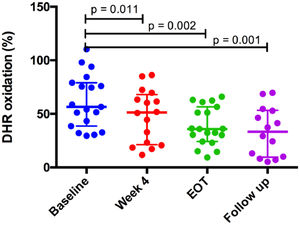
HCV infection is associated with an increased incidence of cardiovascular (CV) events. Mechanisms underlying this association remain unknown. In our study, twenty HCV patients (median age 60.5 years, 65% male and 80% with cirrhosis) were evaluated prior, during and after direct-acting antiviral treatment. Ninety percent of patients achieved sustained virological response (SVR). Significant changes were observed in LDL particle size index, measured by LDL-C/apoB ratio, which increased after treatment (p = 0.023). In addition, HDL antioxidant capacity improved gradually from 34.4% at baseline to 42.4% at 4 weeks (p = 0.011), 65.9% at end of treatment EOT (p = 0.002) and remained elevated at 12-week (p = 0.001) after EOT compared to baseline values. Our findings suggest that a shift to a less atherogenic lipid profile may be a possible mechanism associated with CV risk reduction in patients with HCV infection achieving SVR.
Chronic hepatitis C virus (HCV) infection is one of the most common causes of chronic liver disease, cirrhosis, hepatocarcinoma and a leading indication for liver transplantation worldwide [1]. During its replication, HCV uses and modulates lipid-related pathways in the host inducing profound changes in lipid metabolism [2]. Rapid changes in lipid homeostasis and insulin resistance have been demonstrated in chronic HCV patients treated with IFN-free antivirals [3].
Despite the presence of relatively low levels of circulating total cholesterol and LDL-C, HCV infection has been linked to an increased incidence of cardiovascular (CV) adverse events [4–6]. Consistently, a reduction in CV risk has been associated with viral clearance and eradication upon antiviral treatment [7].
In recent years, HDL functionality has emerged as an important factor linked to relevant CV outcomes [8,9]. In addition, apolipoprotein levels and their ratios to cholesterol levels –as indicators of lipoprotein particle size- have been proposed as surrogate predictors of CV events [10,11]. In our study, the changes in serum lipid levels, apolipoproteins and their ratios, and HDL functionality were evaluated in patients with chronic HCV infection undergoing treatment with direct-acting antivirals agents (DAAs).
2Material and methods2.1Study designThis was a single-center prospective observational study. Consecutive patients with chronic HCV infection being treated with DAA were included. Blood samples were obtained prior to the start of DAA, 4 weeks within treatment, at the end of treatment (EOT, 12 or 24 weeks), and 12 weeks after treatment completion.
Demographic, laboratory data and virological variables were evaluated at different stages of treatment. Standard lipid profile, apolipoprotein A-I and B, ApoB/ApoA-I ratio and LDL/ApoB ratio were calculated, and the antioxidant HDL function was determined using a validated dihidrorodhamine (DHR)-based fluorescent assay [12]. Whole plasma was used for antioxidant HDL function determination as previously described [12]. Informed consent was obtained prior to enrollment and the study was approved by the Ethical Review Board of the Facultad de Medicina, Pontificia Universidad Católica de Chile (study protocol 14-019).
For the statistical analysis, descriptive statistics (i.e. medians, IQR, frequencies with CI) were first performed to assess the characteristics of the study sample. Analyses were aimed to determine variations in lipid serological variables during and after DAA treatment. Paired-sample analyses were conducted, using Fisher's Exact Test for categorical variables and Mann–Whitney's or Student's T tests for quantitative data, according to normality tests. One-way ANOVA for paired samples with correction for multiple group comparisons was performed to assess the changes in the clinical, laboratory and virological variables during and after treatment. Stata 13.0 ® and Prism 6 Graphpad ® software were used for statistical analyses.
3ResultsTwenty patients were assessed during our study. Their median age was 60.5 years (IQR 56–70), 65% (13/20) were male, 80% (16/20) had cirrhosis, and most were classified as Child-Pugh class A (81.5%). HCV genotype 1b was responsible for 100% of infections, and 45% (9/20) had a previously failed antiviral treatment with IFN-based regimens. One patient had a CV event (MI, stroke) before entering the study, and no CV event was seen in patients included during the study period. None of the patients were using statins at enrollment or during the study period. The patient's baseline characteristics are depicted in Supplementary Table 1. Treatment regimens included asunaprevir + daclatasvir (ASV-DCV) for 24 weeks, sofosbuvir + daclatasvir (SOF-DCV) for 12 weeks and sofosbuvir + ledipasvir (SOF/LDV) for 12 weeks. Upon DAA treatment 90% (18/20) of the infected patients achieved SVR12 and HCV load was undetectable at week 4 of treatment, with a rapid decrease in ALT serum levels as expected.
Increases in total cholesterol (134.1 mg/dl to 157.5 mg/dl, p = 0.0122) and LDL-C (67.0 mg/dl to 83.8 mg/dl, p = 0.0136) levels were observed in patients achieving SVR12 when baseline values were compared with the week 12 of the follow-up period. No changes in triglycerides, HDL-C, apolipoprotein A-I and B levels and apo B/A-I ratio were found during treatment or in the follow-up period. However, the LDL-C/apo B ratio, which estimates LDL particle size, increased after treatment (0.93 to 1.28, p = 0.023). Changes in the lipid panel values at each point are detailed in supplementary Fig. 1.
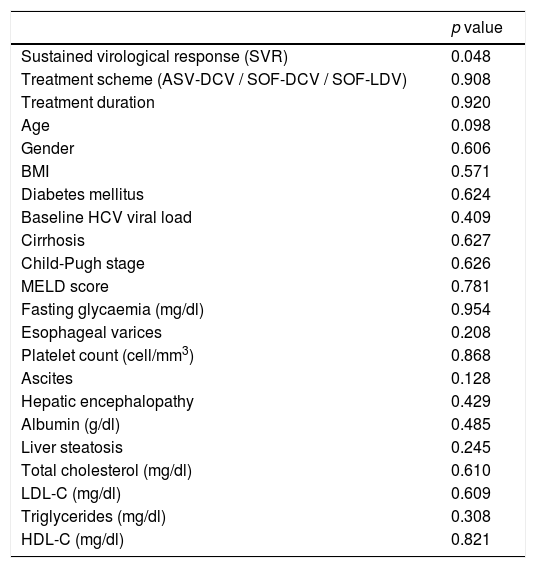
HDL antioxidant capacity improved gradually from 34.4% at baseline to 42.4% at week 4 of treatment (p = 0.011), 65.9% at the end of treatment (p = 0.002) and remained stable at the 12-week follow-up period (62.2%, p = 0.001) after the end of treatment, compared to baseline values (Fig. 1). HDL antioxidant capacity did not improve in the two patients (10% of the sample) who did not achieve SVR12. Further analysis indicated that SVR was the only variable associated with improvement of HDL function (p = 0.048) (Table 1).
Variables associated with improvement in HDL antioxidant capacity in HCV patients after DAA treatment.
| p value | |
|---|---|
| Sustained virological response (SVR) | 0.048 |
| Treatment scheme (ASV-DCV / SOF-DCV / SOF-LDV) | 0.908 |
| Treatment duration | 0.920 |
| Age | 0.098 |
| Gender | 0.606 |
| BMI | 0.571 |
| Diabetes mellitus | 0.624 |
| Baseline HCV viral load | 0.409 |
| Cirrhosis | 0.627 |
| Child-Pugh stage | 0.626 |
| MELD score | 0.781 |
| Fasting glycaemia (mg/dl) | 0.954 |
| Esophageal varices | 0.208 |
| Platelet count (cell/mm3) | 0.868 |
| Ascites | 0.128 |
| Hepatic encephalopathy | 0.429 |
| Albumin (g/dl) | 0.485 |
| Liver steatosis | 0.245 |
| Total cholesterol (mg/dl) | 0.610 |
| LDL-C (mg/dl) | 0.609 |
| Triglycerides (mg/dl) | 0.308 |
| HDL-C (mg/dl) | 0.821 |
In our study in patients with chronic HCV infection, SVR resulted in an increase in total and LDL cholesterol levels. This observation is in accordance with previous reports regarding serum lipids changes after HCV eradication [3]. Even though there was an increase in cholesterol levels, both before and after treatment total and LDL cholesterol remained below the reported averages for the Chilean population [13]. In addition, our results link HCV elimination after DAA therapy with changes in LDL particle size and HDL antioxidant capacity.
It could be argued that the increase in cholesterol among treated patients, which included mainly cirrhotic individuals (80%), is due to a recovery in liver function. However, even though attractive, this possibility seems unlikely. Firstly, most of our patients had compensated liver disease (Child-Pugh A) with baseline preserved synthetic liver function (median albumin level 4 g/dL). Noteworthy, lipids decrease in patients after HCV infection, a phenomenon that is independent of liver fibrosis [14]. Furthermore, the changes of LDL after treatment are not associated with advanced liver disease [15]. We and others have shown that increases in total and LDL cholesterol are very rapid after starting antiviral therapy (4 weeks) (supplementary Fig. 1) [15], suggesting that viral clearance is the most plausible explanation for the changes in lipid values rather than a normalization in liver function, that could typically take months or even years.
The increase in LDL-C particle size, estimated by LDL cholesterol/ApoB ratio, indicates a shift to a less atherogenic lipoprotein particle profile in patients achieving SVR. The LDL-C/ApoB ratio has been questioned as a reliable marker to determine LDL particle size [16]. However, and consistent with our findings, early changes in LDL particle size were recently described in patients achieving SVR with DAA treatment [3] using a direct method to measure LDL size. The increase in LDL-C/ApoB ratio observed in treated HCV patients indicates a shift from atherogenesis-prone small dense LDL particles to larger and less atherogenic low-density lipoproteins. This change in LDL particles after HCV eradication may imply a reduced CV risk in these patients compared to their previous infectious state.
HDL functionality has been identified as a major determinant of relevant CV outcomes [8,9]. Several functions have been attributed to HDL particles, one of them being its antioxidant capacity preventing LDL-oxidation, an essential step in the formation of macrophage-derived foam cells and atherogenesis [17]. Results presented herein show that the HDL antioxidant capacity improved significantly in patients that achieve SVR but remained impaired in patients without SVR. Improvement in HDL antioxidant capacity associated with HCV eradication with DAA is a novel finding. The fact that HDL function improved upon HCV clearance implicates a potential causative role of HCV replication in the impairment of functional HDL properties. Previous work reported decreased blood levels of paraoxonase-1 (PON-1), a key determinant of HDL antioxidant activity, in HCV patients [18]. Then, normalization of PON-1 may be involved in the restoration of HDL function after HCV therapy. Further studies should address possible mechanisms that explain this reversible HDL dysfunctionality associated with HCV chronic infection. Oxidative stress plays a pathogenic role in HCV pathogenesis and complications [19], and it may also be relevant for increased CV risk in HCV patients. Thus, reduction in antioxidant HDL activity due to HCV replication may be a key mechanism underlying oxidative damage and increased CV risk associated with HCV infection.
The evidence linking HCV and CV risk continues to growth in recent years. A large database recent study indicated that treatment with direct-acting antiviral and IFN-based therapy for HCV infection was associated with a significantly reduced risk of cardiovascular disease events [20]. Complementarily, another study showed that HCV eradication by DAA resulted in improved carotid atherosclerosis [21]. Although the number of studies linking HCV infection and CV risk continues to grow, most of these reports comes from epidemiological data and database research, reassuring the association's importance. Not many experimental studies have explored the nature of the HCV-CV risk link. Thus, our study provides a novel possible mechanism to explain this association using validated biochemical methods and conjugate HCV infection with a well-described CV determinant as HDL functionality.
One of the limitations of our study is a limited sample size. However, every patient had samples analyzed in four different periods, with determinations in triplicate and the changes in lipid metabolism were entirely consistent with prior studies. Our work is a proof-of-concept study linking chronic HCV infection to HDL functionality. As such, we believe that our results will prompt the research for more data regarding the molecular basis of the association between HCV chronic infection and HDL functionality. On the other hand, we used a previously validated cell-free assay to assess HDL functionality. This assay was recently used to evaluate HDL functionality in patients with chronic heart failure and linked to relevant CV outcomes [22].The more prominent studies evaluating HDL functionality and CV risk have used cell-based HDL functionality assays, however those methods are more expensive and not widely available. The findings of our study using a biochemical approach to assess HDL functionality will probably allow the inclusion of a larger number of patients and samples in future studies.
5ConclusionOverall, the results from this study suggest that HCV replication induces an abnormal LDL-C particle size and HDL dysfunctionality and show that viral clearance results in a significant increase in LDL particle size and improvement in HDL functionality. We propose that reduced LDL-particle size and HDL dysfunctionality are possible mechanisms underlying CV risk associated with HCV infection. These findings also provide additional support for the universal treatment of patients with chronic HCV infection.
Financial supportThis study was funded by grants FONDECYT #1130357 and #1191389 to AS, #1150311 to FB and #1150399 to AR from CONICYT, Chile.
Conflict of interestJNone.
None.