The coronavirus disease 2019 (COVID-19) pandemic has been a challenge globally. In severe acute respiratory syndrome (SARS) epidemic 60% of patients had hepatic injury, due to phylogenetic similarities of the viruses it is assumed that COVID-19 is associated with acute liver injury. In this meta-analysis, we aim to study the occurrence and association of liver injury, comorbid liver disease and elevated liver enzymes in COVID-19 confirmed hospitalizations with outcomes.
Materials and methodsData from observational studies describing comorbid chronic liver disease, acute liver injury, elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels and outcomes of COVID-19 hospitalized patients from December 1, 2019, to June 30, 2020 was extracted following PRISMA guidelines. Adverse outcomes were defined as admission to intensive care unit (ICU), oxygen saturation <90%, invasive mechanical ventilation (IMV), severe disease and in-hospital mortality. Odds ratio (OR) and 95% confidence interval (95% CI) were obtained.
Results24 studies with 12,882 confirmed COVID-19 patients were included. Overall prevalence of CM-CLD was 2.6%, COVID-19-ALI was 26.5%, elevated AST was 41.1% and elevated ALT was 29.1%. CM-CLD had no significant association with poor outcomes (pooled OR: 0.96; 95% CI: 0.71–1.29; p=0.78). COVID-19-ALI (1.68;1.04–2.70; p=0.03), elevated AST (2.98; 2.35–3.77; p<0.00001) and elevated ALT (1.85;1.49–2.29; p<0.00001) were significantly associated with higher odds of poor outcomes.
ConclusionOur meta-analysis suggests that acute liver injury and elevated liver enzymes were significantly associated with COVID-19 severity. Future studies should evaluate changing levels of biomarkers amongst liver disease patients to predict poor outcomes of COVID-19 and causes of liver injury during COVID-19 infection.
The WHO declared coronavirus disease 2019 (COVID-19) as a global pandemic on March 11, 2020 [1]. With approximately 5 million cases and over 160,220 deaths, the USA remains the worst affected country as of August 8, 2020 [2]. Following this, India (4.3M), Brazil (4.1M), and Russia (1M), are among the other countries that are greatly affected. Total cases worldwide are around 27 million, and thus it remains an emergency of international concern [3].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) mainly affects the respiratory system but as we are gaining more insight about this novel disease, many published studies have also provided evidence of its organotropism and multisystem organ inflammation nature [4,5]. Multiple studies have observed association of elevated liver enzymes in patients with COVID-19 infection assuming that it can cause liver damage either via direct hepatotoxic injury with viral infection, or drug toxicity, or immune mediated response. In the past, it has been reported that 60% of patients developed liver damage due to the SARS epidemic [6]. Since SARS-CoV-2 belongs to the same coronavirus family, we assume it may cause liver injury. The biliary epithelium has expression of angiotensin-converting enzyme (ACE-2) receptor, which is also the binding site of SARS-CoV-2 [7]. The ACE2 receptor expression in hepatocytes has been shown to be upregulated in animals’ models of liver injury but hepatocytes have lower expression in humans [8,9].
Current literature has many published clinical studies focusing on implications of hepatic involvement in COVID-19. However, most of them are diverse because of variation in definition of liver injury, different clinical presentations and severity of the disease in individual studies. Additionally, there is no strong evidence showing association of outcomes of COVID-19 in patients with pre-existing chronic liver disease or liver injury. One study in China reported that of patients who had preexisting hepatitis B infection, 32.1% of them got severe COVID 19 infection in comparison to 15.7% of patients who had no hepatitis B [10]. Hence, we aim to systematically study the occurrence of liver injury, comorbid liver disease and elevated liver enzymes in COVID-19 confirmed hospitalizations and also identify their association with outcomes.
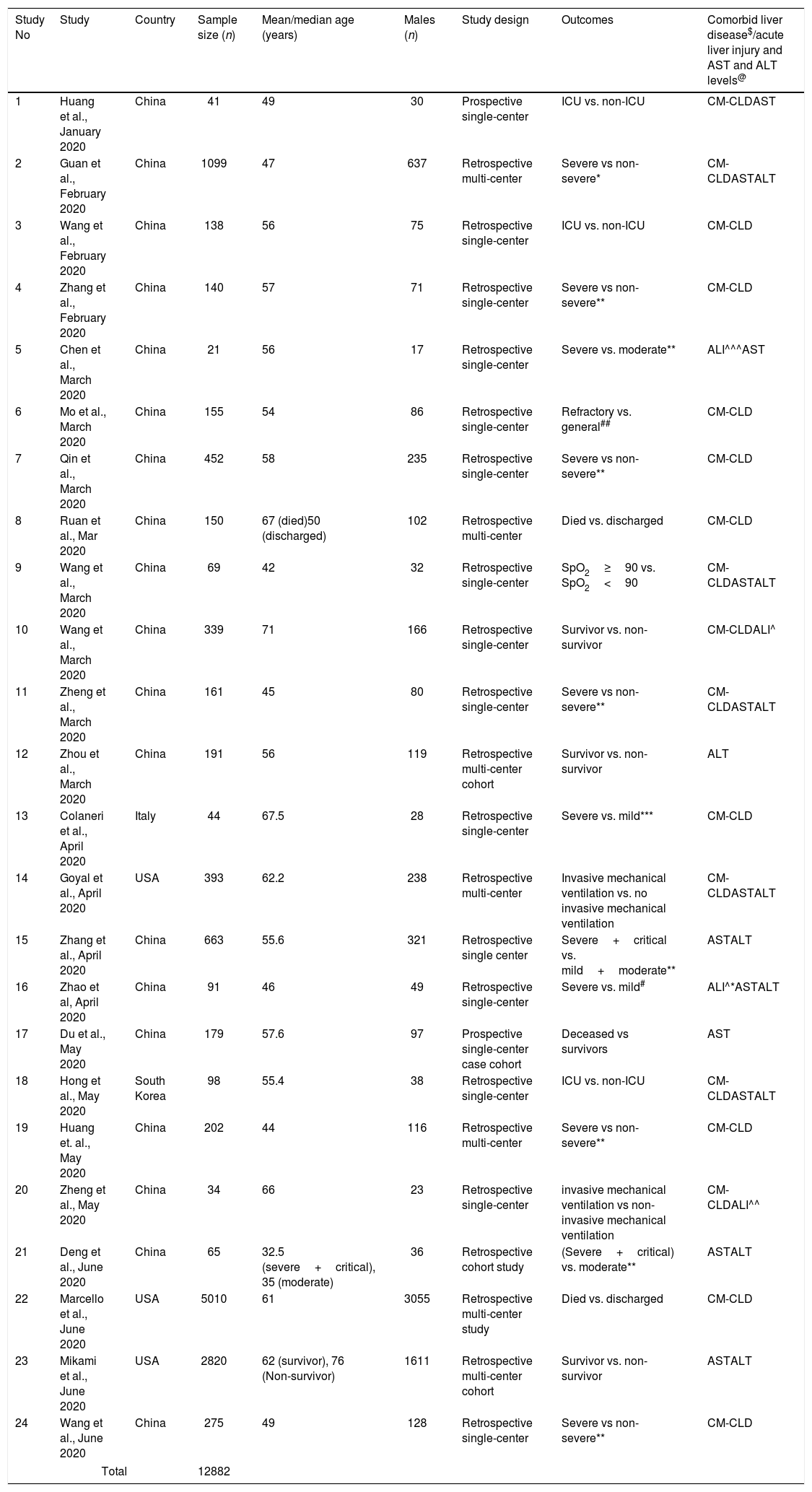
2Methods2.1EndpointThe aim of the study is to evaluate the role of the comorbid chronic liver disease (CM-CLD), elevated liver enzymes and COVID-19 associated acute liver injury (COVID-19 ALI) in predicting the outcomes in confirmed COVID-19 hospitalized patients. COVID-19 confirmation was evaluated by combined findings of RT-PCR, serology, symptoms, and MRI chest in majority of those studies. Poor outcomes were defined by intensive care unit (ICU) admission, oxygen saturation <90%, invasive mechanical ventilation (IMV) utilization, severe disease, and in-hospital mortality. Study-specific poor outcomes and definitions of CM-CLD, COVID-19 ALI and cut-off levels of liver enzymes in each individual study are mentioned in Table 1.
Study characteristics, outcomes and Liver disease.
| Study No | Study | Country | Sample size (n) | Mean/median age (years) | Males (n) | Study design | Outcomes | Comorbid liver disease$/acute liver injury and AST and ALT levels@ |
|---|---|---|---|---|---|---|---|---|
| 1 | Huang et al., January 2020 | China | 41 | 49 | 30 | Prospective single-center | ICU vs. non-ICU | CM-CLDAST |
| 2 | Guan et al., February 2020 | China | 1099 | 47 | 637 | Retrospective multi-center | Severe vs non-severe* | CM-CLDASTALT |
| 3 | Wang et al., February 2020 | China | 138 | 56 | 75 | Retrospective single-center | ICU vs. non-ICU | CM-CLD |
| 4 | Zhang et al., February 2020 | China | 140 | 57 | 71 | Retrospective single-center | Severe vs non-severe** | CM-CLD |
| 5 | Chen et al., March 2020 | China | 21 | 56 | 17 | Retrospective single-center | Severe vs. moderate** | ALI^^^AST |
| 6 | Mo et al., March 2020 | China | 155 | 54 | 86 | Retrospective single-center | Refractory vs. general## | CM-CLD |
| 7 | Qin et al., March 2020 | China | 452 | 58 | 235 | Retrospective single-center | Severe vs non-severe** | CM-CLD |
| 8 | Ruan et al., Mar 2020 | China | 150 | 67 (died)50 (discharged) | 102 | Retrospective multi-center | Died vs. discharged | CM-CLD |
| 9 | Wang et al., March 2020 | China | 69 | 42 | 32 | Retrospective single-center | SpO2≥90 vs. SpO2<90 | CM-CLDASTALT |
| 10 | Wang et al., March 2020 | China | 339 | 71 | 166 | Retrospective single-center | Survivor vs. non-survivor | CM-CLDALI^ |
| 11 | Zheng et al., March 2020 | China | 161 | 45 | 80 | Retrospective single-center | Severe vs non-severe** | CM-CLDASTALT |
| 12 | Zhou et al., March 2020 | China | 191 | 56 | 119 | Retrospective multi-center cohort | Survivor vs. non-survivor | ALT |
| 13 | Colaneri et al., April 2020 | Italy | 44 | 67.5 | 28 | Retrospective single-center | Severe vs. mild*** | CM-CLD |
| 14 | Goyal et al., April 2020 | USA | 393 | 62.2 | 238 | Retrospective multi-center | Invasive mechanical ventilation vs. no invasive mechanical ventilation | CM-CLDASTALT |
| 15 | Zhang et al., April 2020 | China | 663 | 55.6 | 321 | Retrospective single center | Severe+critical vs. mild+moderate** | ASTALT |
| 16 | Zhao et al, April 2020 | China | 91 | 46 | 49 | Retrospective single-center | Severe vs. mild# | ALI^*ASTALT |
| 17 | Du et al., May 2020 | China | 179 | 57.6 | 97 | Prospective single-center case cohort | Deceased vs survivors | AST |
| 18 | Hong et al., May 2020 | South Korea | 98 | 55.4 | 38 | Retrospective single-center | ICU vs. non-ICU | CM-CLDASTALT |
| 19 | Huang et. al., May 2020 | China | 202 | 44 | 116 | Retrospective multi-center | Severe vs non-severe** | CM-CLD |
| 20 | Zheng et al., May 2020 | China | 34 | 66 | 23 | Retrospective single-center | invasive mechanical ventilation vs non-invasive mechanical ventilation | CM-CLDALI^^ |
| 21 | Deng et al., June 2020 | China | 65 | 32.5 (severe+critical), 35 (moderate) | 36 | Retrospective cohort study | (Severe+critical) vs. moderate** | ASTALT |
| 22 | Marcello et al., June 2020 | USA | 5010 | 61 | 3055 | Retrospective multi-center study | Died vs. discharged | CM-CLD |
| 23 | Mikami et al., June 2020 | USA | 2820 | 62 (survivor), 76 (Non-survivor) | 1611 | Retrospective multi-center cohort | Survivor vs. non-survivor | ASTALT |
| 24 | Wang et al., June 2020 | China | 275 | 49 | 128 | Retrospective single-center | Severe vs non-severe** | CM-CLD |
| Total | 12882 | |||||||
World Health Organization and the National Health Commission of China interim guidelines defined disease severity and improvement as follows: Mild cases: The mild clinical symptoms and no pneumonia in imaging. Moderate cases: symptoms like fever and respiratory tract symptoms, etc., and pneumonia can be seen in imaging. Severe cases: Meeting any of the following – respiratory distress, respiratory rate≥30breaths/min; SpO2≤93% at rest; and PaO2/FIO2≤300. Patients with >50% lesion progression within 24–48h. Critical/extremely severe cases: if they have one of the following: respiratory failure requiring mechanical ventilation, shock, and other organ failure requiring ICU treatment.
Patients were included in the mild disease group if they did not need high-flow oxygen support and in the severe disease group if they were provided with high-flow oxygen support.
General COVID-19 included following criteria:(i) obvious alleviation of respiratory symptoms (e.g. cough, chest distress and breath shortness) after treatment; (ii) maintenance of normal body temperature for ≥3 days without the use of corticosteroid or antipyretics; (iii) improvement in radiological abnormalities on chest CT or X-ray after treatment; (iv) a hospital stays of ≤10 days. Otherwise, it was classified as refractory COVID-19.
All the studies mentioned chronic liver disease as a comorbidity.
^ Liver enzyme abnormalities.
^^ Acute liver injury defined as an increase in alanine aminotransferase (ALT) over two times the upper limit of the range (ULN) or an increase in conjugated bilirubin or a combined increase in aspartate aminotransferase (AST), alkaline phosphatase and total bilirubin provided that one of them was above two times ULN.
^^^ Acute liver injury was defined as Jaundice with a total bilirubin level of 3mg/dl or higher and an acute increase in ALT of at least 5 times the upper limit of the normal range and/or an increase alkaline phosphatase of at least twice the upper limit of the normal range.
^* Liver injury was judged ALT and AST levels.
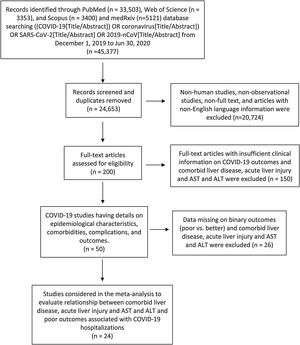
A systematic search was conducted on published studies using PRISMA guidelines [11] and followed MOOSE checklist [12] from December 1, 2019 to June 30, 2020. We searched PubMed, Web of Science, Scopus, and medRxiv for observational studies that described comorbidities, complications and laboratory findings of COVID-19 patients following keyword/MESH terms: ((COVID-19[Title/Abstract]) OR coronavirus [Title/Abstract]) OR SARS-CoV-2[Title/Abstract] OR 2019-nCoV [Title/Abstract]. Studies were included in this meta-analysis if they had comorbid liver disease, elevated liver enzymes, acute liver injury and outcomes of COVID-19 hospitalized patients. Literature other than observational studies, non-English literature, non-full text, and animal studies were excluded. Flow diagram of the literature search and study selection process is described in Fig. 1.
2.3Study selectionAbstracts were reviewed, and articles were retrieved and reviewed for availability of data on comorbid liver disease, elevated liver enzymes, acute liver injury and outcomes of COVID-19 patients. Studies which gave details on outcomes were selected for quantitative analysis. PM and DM independently screened all identified studies and assessed full-texts to decide eligibility. Any disagreement was resolved through discussion with another reviewer UP.
2.4Data collectionFrom the included studies, we extracted the following variables including comorbid liver disease, elevated AST and ALT levels, acute liver injury and outcomes. Details on binary outcomes like ICU vs. non-ICU admission, severe vs non-severe disease, IMV vs no-IMV use, oxygen saturation <90% vs >90%, in-hospital mortality vs discharged alive and survivors were collected using prespecified data collection forms by two authors (PM and DM) with a common consensus of author (UP) upon disagreement. We have presented the study characteristics like the first author's last name, publication month and year, country of origin, sample size, mean or median age, males, outcomes and definitions of comorbid liver disease and acute liver injury and cut offs for elevated AST and ALT levels assessed in that individual study in Table 1 (references for the studies are in Supplemental file 1.1).
2.5Statistical analysisData analysis was performed using Review Manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). If the study has more than one outcome comparison then we have used data from the most severe outcome in the analysis to minimize the overall selection bias of our study.
The Maentel–Haenszel formula was used to calculate dichotomous variables to obtain odds ratios (ORs) along with its 95% confidence intervals to describe the association of comorbid liver disease, elevated liver enzymes, acute liver injury and outcomes of COVID-19 patients in each study. Random-effect models were used regardless of heterogeneity to estimate the combined effect and its precision, to give a more conservative estimate of the ORs and 95% CI. The I2 statistic was used to assess statistical heterogeneity and I2>50% was considered significant heterogeneity. The p<0.05 was considered significant. Publication bias was assessed visually using funnel plots and the Newcastle-Ottawa Scale (NOS). Newcastle-Ottawa Scale (NOS) [13] was used to assess the quality and bias in the included studies, which rates selection, comparability and outcome (Supplemental file 1.2). All studies were assessed to be of high quality.
The pooled OR and 95% CI are represented in the form of forest plots. Each square on the chart area represents individual study and the area of each square is equivalent to the weight of the study, which is the inverse of the study variance. The diamond represents the pooled OR and the width corresponds to the 95% CI.
3ResultsReview of the databases identified 45,377 articles on July 1, 2020, out of which 200 full text articles were assessed for eligibility after removing duplicated articles, non-human studies, non-observational studies, and articles with non-English language by July 7. During the second round, 150 articles with insufficient clinical information on COVID-19 outcomes and comorbid liver disease, elevated liver enzymes, acute liver injury were excluded and 50 articles on comorbidity, complications and outcomes were extracted for final evaluation by July 15. So, after detailed assessment and considering strict inclusion and exclusion criteria, we included 24 observational studies with 12,882 confirmed cases of COVID-19 patients detailing comorbid liver disease or elevated liver enzymes or acute liver injury and outcomes. Meta-analysis random effects models quantified the study level impact of liver disease and liver injury on outcomes in COVID-19 hospitalized patients.
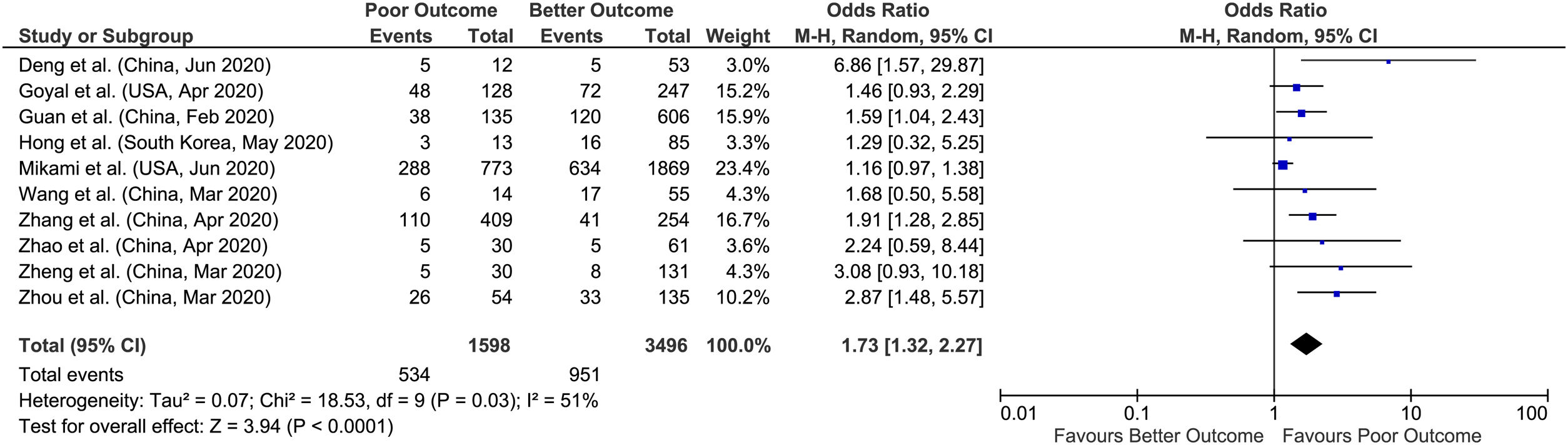
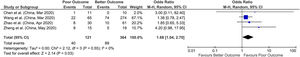
The overall prevalence of acute liver injury was 26.5% [(129/485)*100], comorbid chronic liver disease was 2.6% [(229/8800)*100], elevated AST was 41.1% [(2115/5135)* 100] and elevated ALT was 29.1% [(1485/5094)*100] in our meta-analysis (Figs. 2–5).
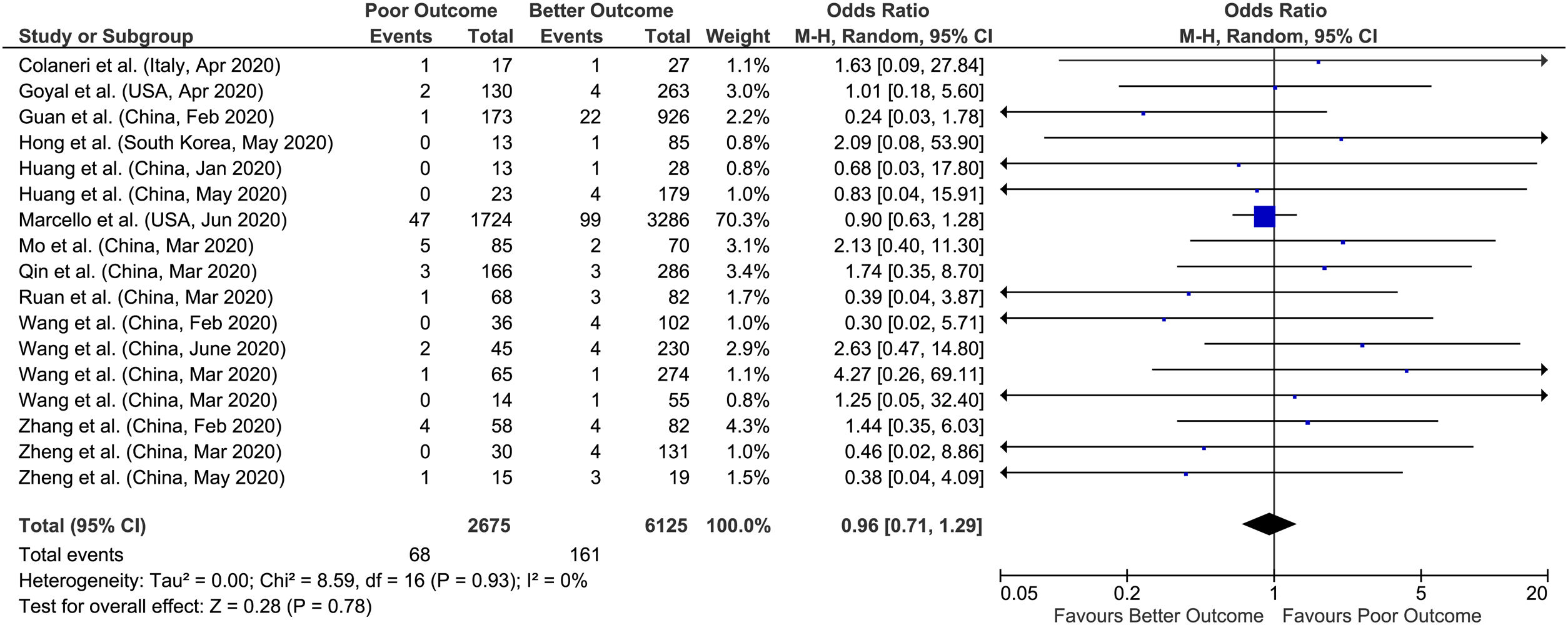
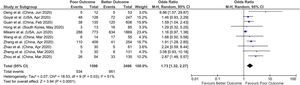
3.1Chronic liver diseaseA total of 17 studies reported data on chronic liver disease with outcomes giving a sample size of 8800 COVID-19 patients for evaluation. Meta-analysis of all 17 studies showed that chronic liver disease had no significant association with poor outcomes compared to better outcomes (pooled OR: 0.96; 95% CI: 0.71–1.29; p=0.78) with no heterogeneity in the data (p=0.93; I2=0%) (Fig. 2).
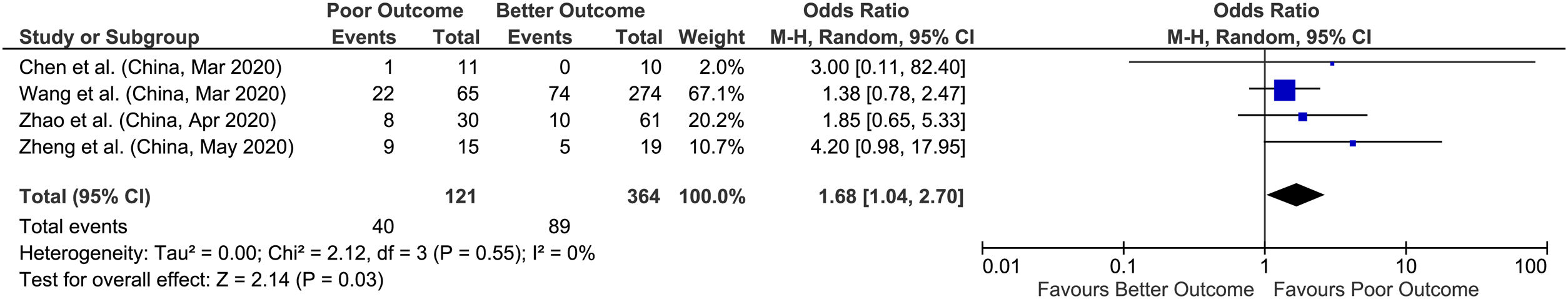
3.2Acute liver injuryWe found 4 studies that provided data on acute liver injury and outcomes with a sample size of 485 patients for evaluation. Meta-analysis of all 4 studies showed that acute liver injury had higher odds of poor outcomes compared to better outcomes with a pooled OR of 1.68 (95% CI: 1.04–2.70; p=0.03), with no heterogeneity between studies (p=0.55; I2=0%) (Fig. 3).
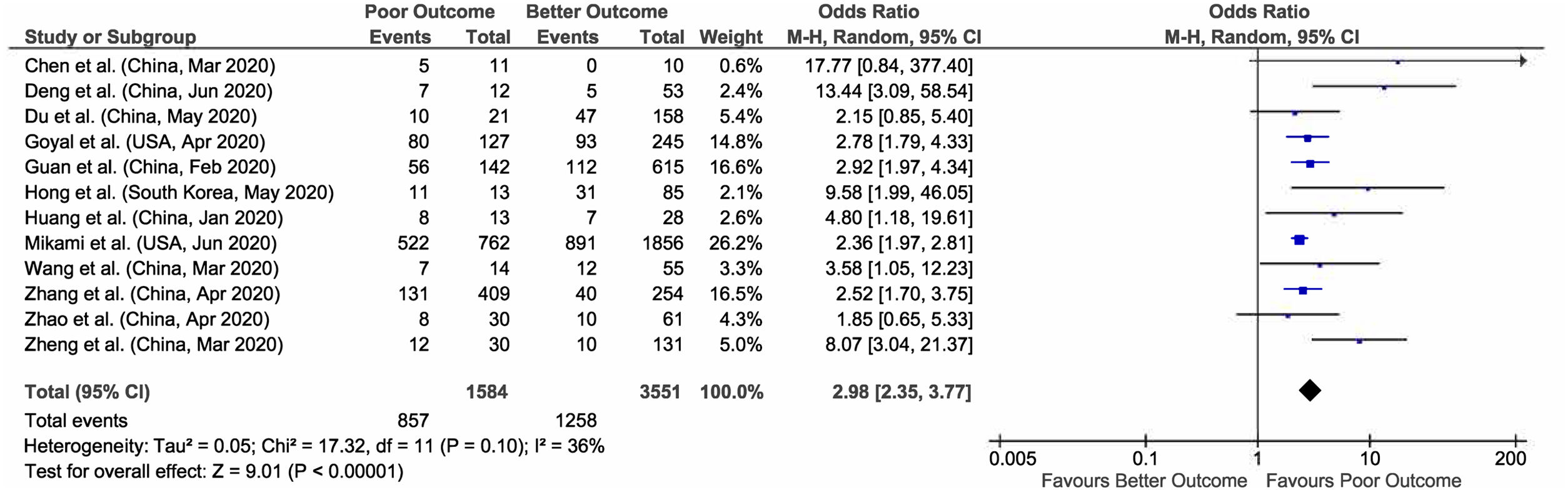
3.3Aspartate aminotransferase (AST)Out of 23 studies, meta-analysis of 12 studies who have reported data on elevated AST and outcomes giving a total sample size of 5135 patients for evaluation, showed that increased AST values are associated with 3 times more risk of poor outcomes in COVID-19 patients (pooled OR: 2.98; 95% CI: 2.35–3.77; p<0.00001), with 36% heterogeneity between studies (p=0.10). (Fig. 4).
3.4Alanine aminotransferase (ALT)Similarly, in 10 studies meta-analysis with reported elevated ALT and outcomes including 5094 patients for evaluation, we found an approx. 2-fold increased likelihood of poor outcomes (pooled OR: 1.73; 95% CI: 1.32–2.27; p<0.0001), with significant heterogeneity between studies (p=0.03; I2=51%) (Fig. 5). Sensitivity analysis was performed by removing outlying study (Mikami et al.) on funnel plots to account for heterogeneity. The results after sensitivity analysis did not change and still showed significant association of elevated ALT with poor outcomes (pooled OR: 1.85; 95% CI: 1.49–2.29; p<0.00001), with no heterogeneity between studies (p=0.50; I2=0%).
4DiscussionIn our meta-analysis, we found that acute liver injury and elevated ALT and AST levels were associated with poor outcomes. However, our study could not provide significant evidence of the effect of pre-existing chronic liver disease on COVID-19 patient outcomes. In support of our findings, a recent meta-analysis found that the frequency of underlying chronic liver disease was not statistically different between severe and non-severe diseases [14]. However, another study by Singh et al. reported that patients with preexisting liver disease, particularly cirrhosis, are at higher risk for hospitalizations and mortality [15]. Few studies have reported that the frequency of patients with liver injury was higher in severe cases compared to mild cases, consistent with our findings of liver injury associated with poor outcomes [16–18]. There are several theories proposed for liver damage in COVID-19 such as direct effect of the virus on hepatocytes or biliary epithelium via Angiotensin-converting enzyme 2 (ACE 2) receptors expression, liver injury related to increased immune response (cytokine storm) and immune mediated damage, drug toxicity (because of drugs like acetaminophen, antivirals and hydroxychloroquine), and liver failure occurring in patients having multiorgan dysfunction [19–21].
A study by Chai et al., has reported that expression of ACE 2 receptors was 57.9% in bile duct cells (Cholangiocytes) and 2.6% in hepatocytes [7]. Cholangiocytes play a vital role in liver regeneration and immune response [22]. Hence one possible theory for liver injury in COVID-19 patients is destruction of Cholangiocytes by SARS-CoV-2 virus via ACE 2 receptors. Additionally, it was also observed that expression of ACE 2 in hepatocytes increases in cases of liver injury [9]. Recently, post mortem liver biopsies of COVID-19 patients have shown moderate microvesicular steatosis and mild lobular and portal activity, indicating the injury could have been caused by either SARS-CoV-2 infection or drug-induced liver injury [23]. Whether this liver injury is caused by the virus itself or is due to a severe inflammatory response with liver damage or sepsis or multisystem organ failure or drug toxicity is not well understood [24]. Therefore, temporal relationship of COVID-19 induced liver damage cannot be established.
Furthermore, increased liver enzymes (AST and ALT) occur in the setting of hepatocyte damage (abnormal liver function). According to initial studies, more than a third of patients had elevated AST and ALT (Transaminitis) which was associated with longer hospital stay [17,25,26]. In a study done by Cai et al., 76.3% of COVID-19 patients had abnormal liver tests while 21.5% developed liver injury during hospitalization, which was defined by ALT, AST, total bilirubin and gamma-glutamyl transferase levels elevated to more than 3× the upper limit of normal [27]. The study also found that patients with abnormal liver tests had significantly higher odds of developing severe pneumonia [27]. These findings are also consistent with our study that shows a significant elevation in AST and ALT among COVID-19 patients may be helpful in predicting poor outcomes among these patients.
SARS-CoV-2 is phylogenetically similar to SARS-CoV and MERS and, studies have given evidence of association of SARS-CoV and MERS with liver injury. and elevated transaminases associated with severe disease [20,28–32]. Additionally, autopsies of SARS patients found not only virus particles in the hepatocytes and hepatic vascular endothelial cells but also significant increase in mitotic cells, with eosinophilic bodies and ballooning hepatocytes, suggesting that SARS-CoV may induce liver cells apoptosis and thus cause liver injury [33,34]. Hence, liver injury due to SARS-CoV, MERS and SARS-CoV-2 diagnosed at the early stage may be direct effect of virus or systemic inflammatory response syndrome associated with the infection is not elucidated and requires future prospective and properly designed studies.
4.1Strengths, limitations and future directionsThere are few limitations in our systematic review and meta-analysis. The most important limitation was the variability among studies in regards to definition of liver dysfunction, acute liver injury, different cut off values for elevated liver enzymes, type of chronic liver disease and different definitions of disease severity and outcomes. Additionally, due to lack of data from prospective studies, all the studies that we included were from retrospective studies. Also, there were no studies reporting changing levels of AST and ALT during the disease course, which is significant to predict the clinical course of the COVID-19 during hospital admission. Most of our studies included in analysis are from China, there may be a chance of overlap of study population as well as results may not be representative of the general population. However few strengths of the study are, our meta-analysis suggests that acute liver injury in patients and elevated AST, ALT levels are associated with poor outcomes along with their high prevalence in COVID-19 patients. Meta-analysis of 17 studies could not establish a significant effect of chronic liver diseases on outcomes in COVID-19 patients. The other main strength of this meta-analysis is the low heterogeneity of the included studies. Future prospective studies should focus on the timeline of the changes in levels of AST and ALT and the severity of the COVID-19 disease and associated liver injury. In addition, stratification of chronic liver diseases would further help to understand the relation of different chronic liver conditions and their effects on COVID-19 affected individuals. Furthermore, we suggest that COVID-19 patients with pre-existing liver disease or liver damage could possibly be treated with drugs that could both protect liver functions and inhibit inflammatory responses, which may aid in the process of disease recovery.
5ConclusionTo conclude acute liver injury, liver function abnormalities specially elevated levels of AST and ALT are not only frequent in COVID-19 but are also associated with poor outcomes. Hence, future studies should carefully investigate the cause of liver injury during COVID-19 infection especially if it is treatment drug related. From a clinical perspective, these findings may help in early triage, close monitoring of the occurrence of liver injury, and careful use of drugs which can cause liver toxicity in COVID-19 patients.AbbreviationsCOVID-19coronavirus disease 2019CM-CLDcomorbid chronic liver diseaseCOVID-19 ALICOVID-19 associated acute liver injuryASTaspartate aminotransferaseALTalanine aminotransferaseICUintensive care unit (ICU)IMVinvasive mechanical ventilationSARS-CoV2severe acute respiratory syndrome coronavirus 2ACE-2angiotensin-converting enzyme 2
AbbreviationsCOVID-19 coronavirus disease 2019 comorbid chronic liver disease COVID-19 associated acute liver injury aspartate aminotransferase alanine aminotransferase intensive care unit (ICU) invasive mechanical ventilation severe acute respiratory syndrome coronavirus 2 angiotensin-converting enzyme 2
The study had no internal or external funding source.
Authors’ contributionsConceptualization: PM, UP; Methodology: AS, PM, UP, PJ; Acquisition of data: DM, RJ; Formal analysis and investigation: PM, UP; Writing – original draft preparation: AS, PJ, AZ, AD, AK, ZM, LS, NSH, BF, YK, SP, DM, RJ; Writing – review, critical feedback, and editing: JS, PM, UP; Funding acquisition: None; Resources: AS; Supervision: UP and PM.
Ethical approvalThough this article does not contain any studies with direct involvement of human participants or animals performed by any of the authors, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentThe data used in this study is deidentified and collected from the studies published online thus informed consent or IRB approval was not needed for this study.
Availability of data and materialThe data is collected from the studies published online, publicly available, and specific details related to data and/or analysis will be made available upon request.
Conflicts of interestThe authors have no conflicts of interest to declare.