Hepatocellular carcinoma (HCC) is the second most lethal cancer around the world, with poor survival rate and high metastasis rate in patients. Long noncoding RNAs (lncRNAs) have been reported to modulate the initiation and development of liver cancer. We aimed to investigate the role of lncRNA MAGI2-AS3 in HCC and underlying mechanisms.
Materials and methodsThe expression levels of MAGI2-AS3 in plasma of HCC patients and the control participants were measured by qPCR. Hep3B and MHCC97-H cells were transfected with MAGI2-AS3 and ROCK2 expression vectors. Cell migration and invasion of HCC cells transfected with the vectors were investigated by transwell assay. In addition, flow cytometry and western blot were performed for apoptosis detection.
ResultsWe found that MAGI2-AS3 was downregulated in plasma of early stage HCC patients compared to healthy controls. After surgical resection, the expression levels of MAGI2-AS3 were increased compared to pretreatment levels on the day of discharge. During the follow-up, MAGI2-AS3 was downregulated in patients developed distant recurrence, but not in other patients compared to the levels measured on the day of discharge. In HCC cells, overexpression of MAGI2-AS3 mediated the downregulation of ROCK2. Cell invasion and migration assay showed that overexpression of MAGI2-AS3 mediated the decreased cell invasion and migration rate, while ROCK2 played an opposite role and attenuated the effects of overexpression of MAGI2-AS3.
ConclusionOur study indicated that MAGI2-AS3 was downregulated in the distant recurrence of HCC after surgical resection and affected the invasion and migration of HCC cells via ROCK2.
As the major subtype of liver cancer, hepatocellular carcinoma (HCC) is the leading cause of cancer-related mortalities, and its prevalence rate ranks the fifth place among all types of cancer [1]. Even with the efforts that have been made on cancer prevention, the incidence of HCC still showed an increasing trend in many countries, such as China [2]. At present, effective therapeutic approaches for HCC patients at advanced stages are still lacking [3,4]. Most HCC patients diagnosed at early stages are promising candidates for surgical resection, which is the only radical therapy [5]. Whereas, recurrence with inevitably occurs in many cases, leading to poor prognosis [6].
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the major risk factors for HCC [7,8]. However, the infection itself is not sufficient to initiate HCC, and multiple signaling pathways are also involved [7,8]. Rho associated coiled-coil containing protein kinase 2 (ROCK2) is a critical regulator of actomyosin cytoskeleton and plays regulatory roles in motility and invasion of cancer cells [9]. Inhibition of the expression of ROCK2 provides new insights into cancer treatment [9]. ROCK2 can be regulated by certain long (>200nt) non-coding RNAs (lncRNAs) [10], which participate in cancer biology mainly through the regulation of gene expression [11]. It is known that Fas may regulate the ROCK signaling [12]. A recent study showed that a novel lncRNA MAGI2-AS3 regulated Fas to suppress breast cancer [13]. Therefore, MAGI2-AS3 may also have crosstalk with the ROCK signaling. This study explored the interaction between MAGI2-AS3 and ROCK2 in HCC.
2Materials and methods2.1Research subjectsThe present study selected 68 HCC patients (40 males and 28 females; age range from 33 to 67 years old; mean age 49.9±6.3 years old) selected from 233 HCC patients admitted by the affiliate hospital of Qingdao University between April 2013 and January 2016. Inclusion criteria: (1) newly diagnosed HCC cases; (2) no therapies were performed; (3) early stage (Stage1, n=28; stage 2, n=40) HCC suitable for surgical resection. Exclusion criteria: (1) recurrent HCC; (2) history of malignancies; (3) with initiated therapies. During the same time period, 68 healthy volunteers (40 males and 28 females; age range from 34 to 68 years old; mean age 49.5±6.0 years old) were also enrolled. All HCC patients and healthy volunteers signed the informed consent. This study was approved by the Ethics Committee of aforementioned hospital.
2.2Plasma, treatment and a 3-year follow-upBlood (5ml) was extracted from each patient and healthy volunteer before any therapies. Patients received surgical resection combined with different doses of chemotherapies and radiotherapies according to the conditions of the patients. Blood (5ml) was also extracted on the day of discharge. After discharge, all patients were followed-up for 3 years to record the recurrence. During the follow-up, same amount of blood was extract on the day of diagnosis of recurrence or at the end of follow-up (in cases with no recurrence observed). All blood samples were centrifuged in EDTA tubes at room temperature for 14min to collect plasma (Table 1).
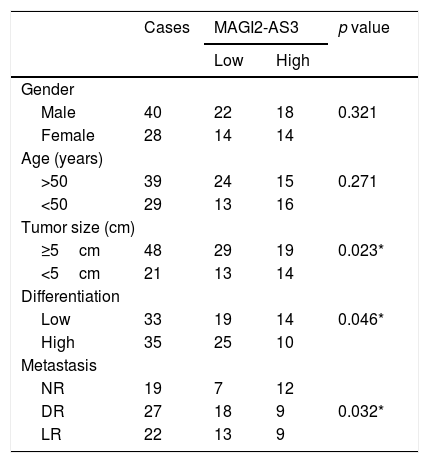
The correlation between the expression of lncRNA MAGI2-AS3 and clinicopathologic variables in patients with hepatocellular carcinoma.
| Cases | MAGI2-AS3 | p value | ||
|---|---|---|---|---|
| Low | High | |||
| Gender | ||||
| Male | 40 | 22 | 18 | 0.321 |
| Female | 28 | 14 | 14 | |
| Age (years) | ||||
| >50 | 39 | 24 | 15 | 0.271 |
| <50 | 29 | 13 | 16 | |
| Tumor size (cm) | ||||
| ≥5cm | 48 | 29 | 19 | 0.023* |
| <5cm | 21 | 13 | 14 | |
| Differentiation | ||||
| Low | 33 | 19 | 14 | 0.046* |
| High | 35 | 25 | 10 | |
| Metastasis | ||||
| NR | 19 | 7 | 12 | |
| DR | 27 | 18 | 9 | 0.032* |
| LR | 22 | 13 | 9 | |
Note: The mean expression level was used as the threshold. For analysis of association between MAGI2-AS3 levels and clinical features, Pearson's χ2 tests were used *p<0.05.
Hep3B and MHCC97-H (ATCC, USA) cells were used, which have high metastasis capacity. Cells were cultivated in RPMI-1640 medium (10% FBS) under the conditions of 5% CO2 and 37°C. MAGI2-AS3 and ROCK2 expression vectors were established using pcDNA3.1 vector (RIBOBIO, Guangzhou, China) as the backbone. Hep3B and MHCC97-H cells were collected at confluence of 70–80%, and 105 cells were transfected with 10nM MAGI2-AS3 and ROCK2 expression vector or 10nM pcDNA3.1 (negative control, NC) using lipofectamine 2000 (Invitrogen, USA). The interval between cell transfection and subsequent experiment was 24h. Hep3B and MHCC97-H cells with no transfections were used as the Control (C).
2.4RNA extractions and qPCRTrizol reagent (Invitrogen, USA) was used to extract total RNAs. In brief, 0.4ml Trizol was mixed with 105 H1993 or H1581 cells or 0.1ml plasma, followed by centrifugation and ethanol participation. All RNA samples were digested with DNase I, followed by reverse transcription using the MMLV Reverse Transcriptase (Lucigen, USA). All qPCR mixtures were prepared using the QuantiTect SYBR Green PCR Kit (Qiagen, Shanghai, China). The expression levels of MAGI2-AS3 and ROCK2 were determined with GAPDH as endogenous control. The following primer sequences were used: ROCK2 forward: 5′-CTA GGCCGG GCG AAG-3 and reverse 5′-TCC AGC TTC CTC TGA CGAC-3′; MAGI2-AS3 forward: 5′-CACCTTGCTTGACAACTTGA-3′ and reverse: 5′-CATTACAGCTCGGCTACTGC-3′; GAPDH forward:5′-AAG GTG AAG GTC GGA GTCAA-3′ and reverse: 5′-AAT GAA GGG GTC ATT GAT GG-3. All PCR reactions were prepared in triplicate manner and 2−ΔΔCT method was used to perform all data normalization.
2.5Cell migration and invasion analysisHep3B and MHCC97-H cells were harvested and 3×104 cells were mixed with RPMI-1640 medium (1% FBS) to prepare single cell suspensions. Cells were transferred to Transwell upper chamber, and each transfection group (C, NC, overexpression) included 3 replicated wells. Before the invasion assay, Matrigel (356234, Millipore, USA) was used to coat membrane to mimic in vivo invasion barrier. Transwell lower chamber was added with RPMI-1640 medium (20% FBS). Cells were cultivated for 12h under the conditions of 5% CO2 and 37°C. After staining using 1% crystal violet (Sigma–Aldrich, USA) at 22°C for 18min, a light microscope was used to count invading and migrating cells.
2.6Western blot analysisHep3B or MHCC97-H cells were harvested and counted, and 105 cells were mixed with 0.6ml RIPA solution (RIBOBIO, Guangzhou, China) to extract total proteins. Protein samples were quantified and denatured in boiled water for 6min, followed by electrophoresis using 12% SDS-PAGE gel. Gel transfer (PVDF membrane) and blocking (2h in 5% non-fate milk) were then performed, followed by incubation with primary antibodies of rabbit polyclonal ROCK2 (1:900, ab71598, Abcam), Caspase 3(1:900, ab13847, Abcam) and GAPDH antibody (1: 900, ab37168, Abcam) at 4°C for 12h. After that, membranes were further incubated with Goat anti-Rabbit IgG (H+L) HRP secondary antibody (Thermo Fisher Scientific, Inc) at 22°C for 2h. ECL (Sigma–Aldrich, USA) was used to develop signals. Gray values were normalized using the Image J v1.46 software.
2.7Statistical analysisAll data analyses were performed using the mean values of 3 biological replicates. Differences between two groups (HCC vs. Control) were explored using unpaired t test. Differences among different cell transfection groups were explored by ANOVA (one-way) and Tukey test. ROC curve was used to analyze the potentials of MAGI2-AS3 in the diagnosis of HCC. In ROC curve, true positive cases were HCC patients and true negative cases were healthy volunteers. p<0.05 was considered as statistically significant.
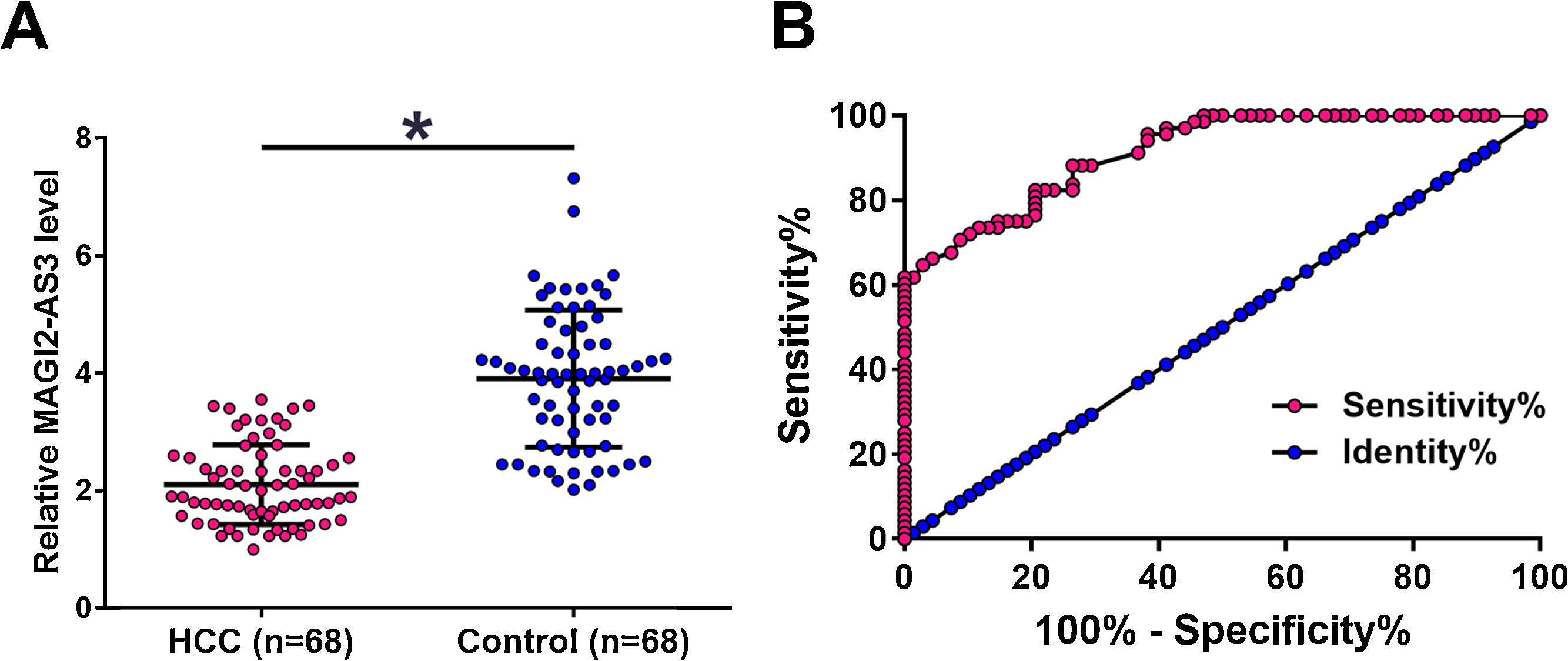
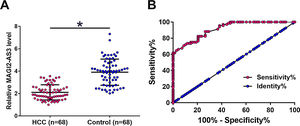
3Results3.1MAGI2-AS3 was downregulated in HCC and showed diagnostic valuesThe levels of MAGI2-AS3 in plasma of two groups of participants (HCC patients and the Control) were measured by qPCR. The results showed that the expression levels of MAGI2-AS3 were significantly lower in HCC patients compared to that in the Control group (Fig. 1A, p<0.05). ROC curve was used to analyze the potentials of MAGI2-AS3 in the diagnosis of HCC. In ROC curve, true positive cases were HCC patients and true negative cases were healthy volunteers. As shown in Fig. 1B, the area under the curve was 0.9138, with 95% confidence interval of 0.8701–0.9575 and a standard error of 0.02231, which indicated that the diagnosis model was reliable.
MAGI2-AS3 was downregulated in HCC patients and showed diagnostic values. Expression levels of MAGI2-AS3 in plasma of two groups of participants (HCC and Control) were measured by qPCR. Expression levels of MAGI2-AS3 were compared between 2 groups by performing unpaired test (A) (*, p<0.05). ROC curve was used to analyze the potentials of MAGI2-AS3 in the diagnosis of HCC. In ROC curve, true positive cases were HCC patients and true negative cases were healthy volunteers (B).
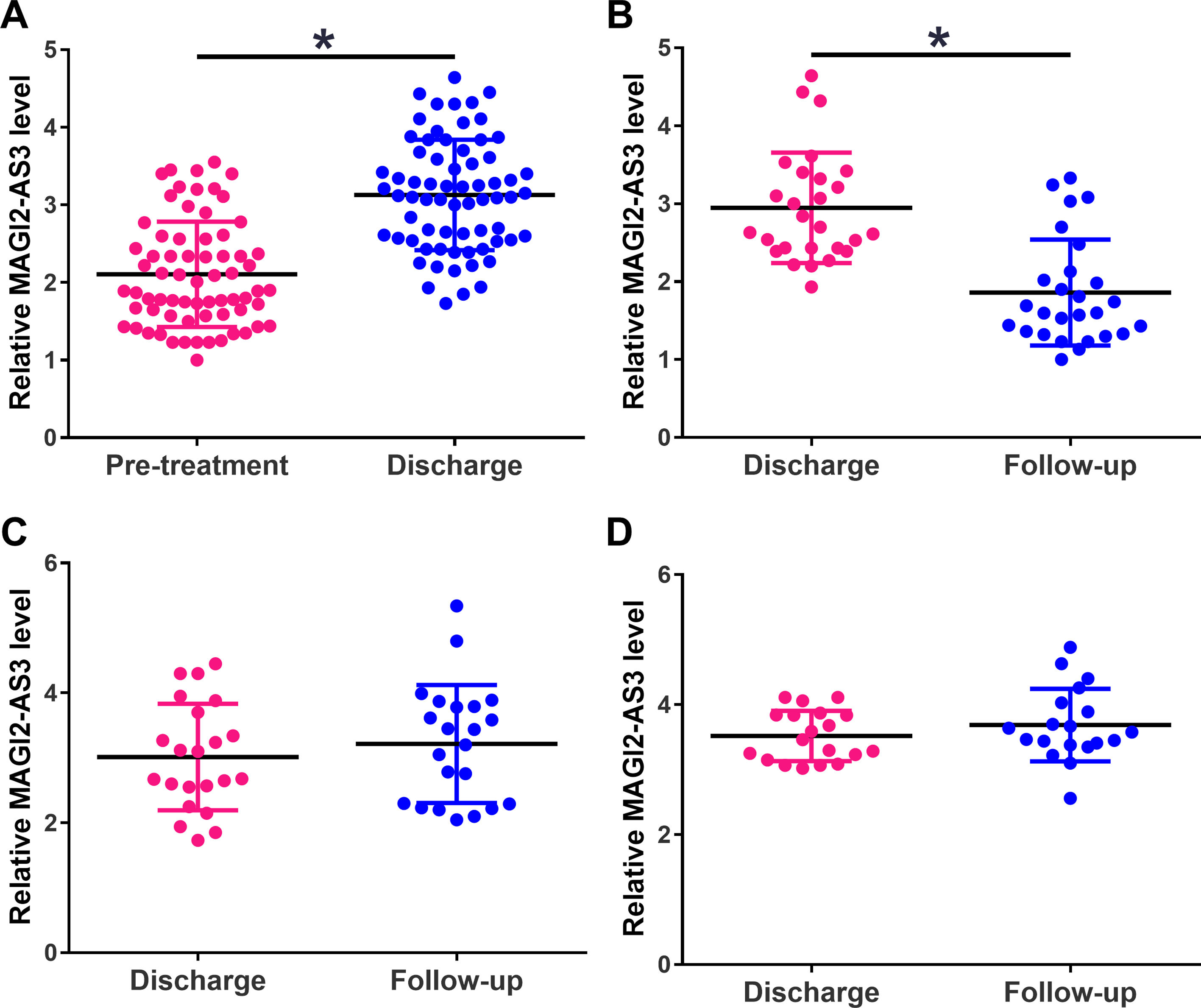
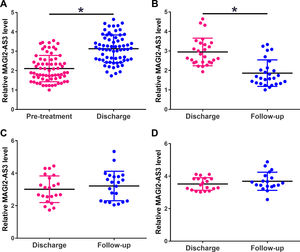
The expression levels of MAGI2-AS3 in plasma of HCC patients were measured by qPCR at 3 time points, including pre-treatment, discharge and the follow-up. Compared to pretreatment, the expression levels of MAGI2-AS3 were increased on the day of discharge (Fig. 2A, p<0.05). During follow-up, distance recurrence (combined with local recurrence in most cases) was observed in 27 cases, local recurrence (no distant recurrence) was observed in 22 cases, and the rest showed no recurrence. The expression levels of MAGI2-AS3 were significantly downregulated in patients with distant recurrence compared to that on the day of discharge (Fig. 2B, p<0.05), but not in patients with local recurrence (Fig. 2C) or non-recurrence (Fig. 2D).
Expression levels of MAGI2-AS3 were increased after resection of tumors and increased in HCC patients with distant recurrence during follow-up. Expression levels of MAGI2-AS3 in HCC patients were measured by qPCR at 3 time points – pre-treatment, discharge and follow-up. Plasma levels of MAGI2-AS3 were compared between pretreatment levels and the levels of the day of discharge (A). During follow-up, distance recurrence (combined with local recurrence in most cases) was observed in 27 cases, local recurrence (no distant recurrence) was observed in 22 cases, and the rest showed no recurrence. Comparisons of plasma levels of MAGI2-AS3 between discharge level and follow-up level were performed in patients with distant recurrence (B) patients with local recurrence (C) and patients with non-recurrence (D), (*, p<0.05).
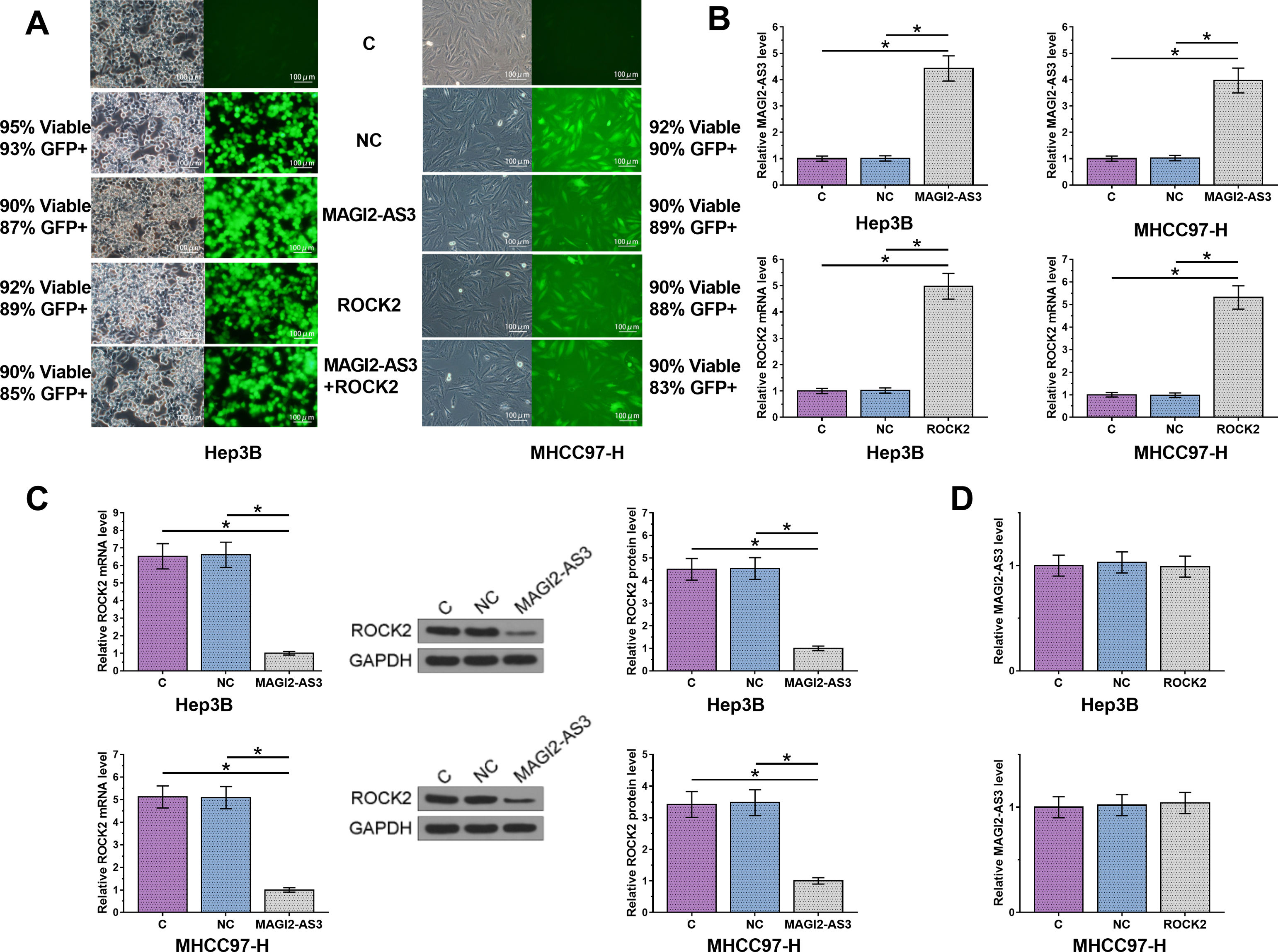
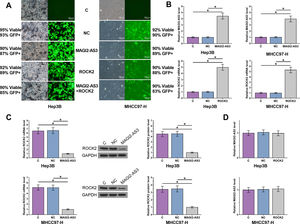
Hep3B and MHCC97-H cells were transfected with MAGI2-AS3 and ROCK2 expression vectors, with their cell viability and transfection rate shown in Fig. 3A. Compared to C and NC controls, the expression levels of MAGI2-AS3 and ROCK2 were significantly increased at 24h post-transfections (Fig. 3B, p<0.05). In addition, overexpression of MAGI2-AS3 mediated the downregulation of ROCK2 (Fig. 3C, p<0.05) at both RNA and protein levels, while the expression of MAGI2-AS3 was not affected by overexpression of ROCK2 (Fig. 3D).
MAGI2-AS3 downregulated ROCK2 in HCC cells. Hep3B and MHCC97-H cells were transfected with MAGI2-AS3 and ROCK2 expression vectors. Cell viability and transfection rates were shown (A). Overexpression of MAGI2-AS3 and ROCK2 were confirmed by qPCR (B). In addition, effects of MAGI2-AS3 on ROCK2 were analyzed by qPCR and western blot (C). Effects of overexpression of ROCK2 on the expression of MAGI2-AS3 were assessed by qPCR (D). Data were expressed as the mean±SD (n=3, *, p<0.05).
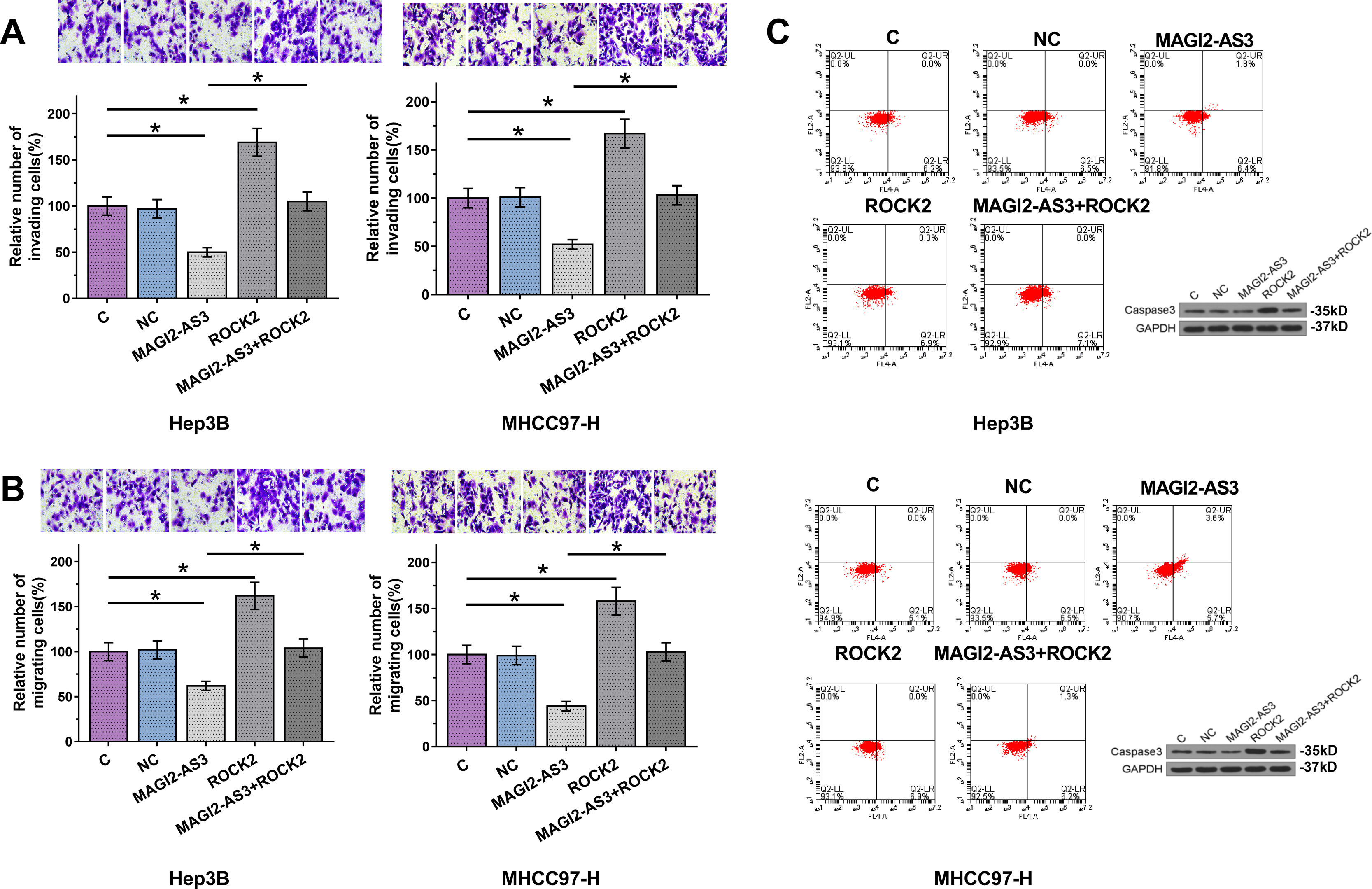
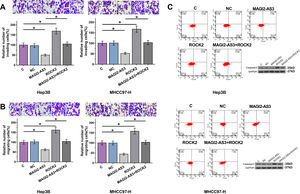
Cell invasion and migration assay showed that, compared to C and NC groups, overexpression of MAGI2-AS3 led to decreased cell invasion (Fig. 4A) and migration (Fig. 4B) rate in Hep3B and MHCC-97H cells, while ROCK2 played an opposite role and attenuated the effects of overexpression of MAGI2-AS3 (p<0.05). Flow cytometry demonstrated that overexpression of MAGI2-AS3 could induce HCC cell death while the effects were attenuated by the overexpression of ROCK2. In consistent with the apoptosis results, caspase3 splicing was activated by MAGI2-AS3 due to the decreased expression levels of total caspase3, while ROCK2 played the opposite role (Fig. 4C).
Overexpression of MAGI2-AS3 mediated the cell invasion, migration and apoptosis through ROCK2. Cell invasion and migration assays were performed to explore the effects of overexpression of MAGI2-AS3 and ROCK2 on invasion (A) and migration (B) of HCC cells. Apoptosis assays were performed by flow cytometry and caspase3 levels were measured by western blot (C). Data were expressed as the mean±SD (n=3, *, p<0.05).
In this study we detected the expression of MAGI2-AS3 in HCC. We found that MAGI2-AS3 was downregulated in HCC patients and might participate in distant recurrence of HCC after surgery by regulating invasion and migration of cancer cells through the interactions with ROCK2.
MAGI2-AS3 was closely related to cell proliferation, invasion and migration in breast cancer and bladder cancer [13,14]. In breast cancer, MAGI2-AS3 suppresses cancer cell proliferation and induces cancer cell apoptosis by activating the Fas/FasL signaling pathway [13]. In bladder cancer, MAGI2-AS3 sponges miR-15b-5p to upregulate tumor suppressive CCDC19, thereby inhibiting the growth of cancer cell [14]. In our study, we observed the downregulation of MAGI2-AS3 in HCC. In addition, inhibited invasion and migration of cancer cells were observed after the overexpression of MAGI2-AS3. Thus, MAGI2-AS3 also played a tumor suppressive role in HCC, which is consistent with previous studies.
Compared to local recurrence, distant cancer recurrence is more aggressive [15,16]. A recent study showed that the total recurrence rate of HCC patients after chemoembolization within 1 year is 68.5% [16], and a considerable portion of recurrent HCC patients developed distant recurrence. Among the 68 patients included in this study, 49 of them (72.1%) developed recurrence within 3 years after discharge. We observed that MAGI2-AS3 was upregulated after surgical resection and downregulated after distant recurrence but not local recurrence. Therefore, monitoring the expression of MAGI2-AS3 may provide insights into the prediction of treatment outcomes of HCC after surgery.
Cancer cell invasion and migration play pivotal roles in cancer distant recurrence [17]. In the present study, we showed that MAGI2-AS3 in HCC cells can inhibit the expression of ROCK2, which is highly expressed in HCC tissues [18] and promotes cancer cell motility and invasion [9]. Huang et al. reported that ROCK2 and MMP2 were markedly overexpressed in HCCs compared with that in the corresponding adjacent tissues, where a positive correlation in their expression was found. The knockdown of ROCK2 significantly decreased the expression of MMP2 and inhibited the invasion and metastasis of HCC in vitro and in vivo[19]. The MAGI2-AS3 and ROCK pathways both have crosstalk with Fas [12,13]. Therefore, Fas may mediate the interaction between them. In addition, MAGI2-AS3 can function as the sponge of miRNAs, thus mediating the regulation of ROCK2 [14]. It was reported that ROCK2 could be suppressed by miR-130a in tumor tissues of HCC patients as well as four HCC cell lines, and resulted in inhibition of proliferation, migration and invasive ability of HCC cells [20]. It is also likely that MAGI2-AS3 may sponge a miRNA to regulate the expression of ROCK2. Our future studies will further explore this possibility.
In conclusion, MAGI2-AS3 was downregulated in HCC and overexpression of MAGI2-AS3 inhibits cancer cell invasion and migration by downregulating ROCK2, thereby participating in distant recurrence of HCC. However, in vivo studies are needed to further verify our conclusions.
DeclarationsPermission has been obtained for use of copyrighted material from other sources. Journal policies detailed in the guide have been reviewed.
AbbreviationsHCC
hepatocellular carcinoma
HBVHepatitis B virus
HCVhepatitis C virus
lncRNAslong (>200nt) non-coding RNAs
Conflict of interestThe authors declare that they have no conflict of interest.
Ethical approvalThis study was approved by the ethics committee of affiliate hospital of Qingdao University. All procedures performed in participants were in accordance with the ethical standards of the Declaration of Helsinki. Informed consent was written by all participants.
We thank for the financial support from the Microwave ablation in basic research of primary small hepatocellular carcinoma (40518040221).