Cholangiocarcinoma (CCA) is characterized by early distant invasion and metastasis, whereas the underlying mechanism is still obscure. Increasing evidence shows that collagen type Ι alpha 1 (COL1A1) is a gene associated with the progression of multiple diseases. Here, we attempted to investigate the role of COL1A1 in CCA.
Materials and MethodsThe expression of COL1A1 between tumor tissues and adjacent normal tissues obtained from CCA patients was detected by Western blot and immunofluorescence, followed by analysis of its clinical significance. Then, the biological effects of COL1A1 overexpression or knockdown on CCA cells were evaluated in vitro and in vivo. Finally, molecular mechanism of COL1A1 in regulating the invasion and metastasis of CCA cells was determined by a series of experiments.
ResultsCOL1A1 expression was significantly higher in CCA pathological tissues than in corresponding adjacent normal tissues. Analysis of 83 CCA patients showed that higher expression of COL1A1 was correlated with poorer patient prognosis. Notably, overexpression or knockdown experiments revealed that COL1A1 contributed to the migration and invasion, as well as epithelial-to-mesenchymal transition (EMT), in CCA cells. Further investigations demonstrated that matrix metalloproteinase-2 (MMP2) promoted COL1A1 upregulation via the integrin alpha Ⅴ pathway, therefore affecting ECM remodelling and inducing EMT in CCA cells. Moreover, COL1A1 expression was positively related to PD-1 and PD-L1 in CCA, and COL1A1 increased PD-L1 expression by activating the NF-κB pathway.
ConclusionsCOL1A1 plays an important role in regulating CCA progression and may act as a promising biomarker and therapeutic target for CCA.
Cholangiocarcinoma (CCA), one of the most common malignant tumours located in the biliary tract, is prone to early distant lymph node and vascular invasion and metastasis [1,2]. CCA usually requires complex clinical surgery, but the surgical outcome is poor and prone to recurrence [1]. Therefore, research on tumour recurrence and metastasis has become a hot topic.
Tumour metastasis is considered the leading cause of death from cancer [3]. Tumour invasion and metastasis are complex biological processes involving many factors. In this process, tumour cells are separated from the primary site and form a migration channel for themselves by interacting with the extracellular matrix (ECM). Subsequently, they cross the vascular basement membrane and enter peripheral blood vessels, leading to tumour cells spreading in distant tissues and forming metastases there [3]. Previous studies have shown that high invasion and metastasis abilities are important reasons for postoperative recurrence of CCA [1]. In addition, researchers have found that the composition of the ECM in tumour growth or metastasis is important for tumours to successfully metastasize to a new location. Collagen type Ⅰ alpha 1 (COL1A1) is an important component of the ECM and participates in ECM remodelling [4]. It has been shown that COL1A1 content in adjacent normal tissue is substantially diminished [5]. Increasing COL1A1 expression can accelerate the invasion and metastasis of breast cancer cells [6]. Accumulating evidence shows that ECM remodelling is a key factor affecting tumour progression [7], and its importance has attracted much attention in cancer biology. At present, ECM remodelling has become a new target for preventing tumour invasion and metastasis.
Matrix metalloproteinases (MMPs) produced by tumour cells are involved in the invasion and migration of tumours when the ECM is degraded [8]. MMPs are associated with many cancers, including breast cancer, probably because they regulate the extracellular environment rich in collagen and ECM [9]. For example, studies have shown that MMP9 is responsible for colon cancer invasion and metastasis [10]. Meanwhile, other studies have indicated that MMP2 can promote breast cancer metastasis by modulating COL1A1 expression and promoting distant metastasis [11]. Furthermore, the mechanism by which MMP2 regulates COL1A1 to promote EMT and metastasis in CCA is not fully understood.
It has been reported that integrins can bind different kinds of ECM components, which contribute to cell‒cell and cell-matrix adhesion, cell migration, and invasion [12]. The integrin αvβ3 receptor is absent in normal tissue but is markedly upregulated in vessels with increased angiogenesis. Indeed, the expression of the αvβ3 receptor is significantly increased in several types of tumour cells, such as breast cancer and prostate cancer, and in almost all tumour vasculature [13].
This study whether the level of COL1A1 expression is closely associated with CCA benign and malignant pathologies, differentiation degree, lymph node metastasis and CCA prognosis. Furthermore, we assessed whether MMP2-induced COL1A1 synthesis via integrin alpha Ⅴ influences ECM remodelling, leading to the invasion and metastasis of CCA.
2Materials and Methods2.1CCA pathological specimensPathological specimens were obtained from 83 patients with hilar CCA who received liver tumour resection in hepatobiliary surgery at the Southwest Hospital Affiliated with the Third Military Medical University (Chongqing, China) from October 2009 to May 2016. In addition, 27 tumour tissues and adjacent normal tissues were collected from patients with CCA. It should be emphasized that 27 CCA patients were randomly selected from 83. All patients (excluding patients with history of digestive system cancers and hepatocellular carcinoma) were re-examined using upper abdominal color Doppler ultrasound and computed tomography angiography (CTA) every three months after discharge. The median follow-up period was 17 months (range,1-87 months). Overall survival was calculated from the date of surgery until the date of last contact.
2.2Cell cultureThe human CCA cell lines RBE and HCCC-9810 were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. All cells were grown in RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.) medium supplemented with 10 % foetal bovine serum (FBS; Zeta Life, Inc.) and cultured in a humidified incubator containing 5 % CO2 at 37°C.
2.3AnimalsMale BALB/c-nu mice were purchased from the Institute of Laboratory Animal Sciences of the Chinese Academy of Sciences. A total of 10 male nude (BALB/c-nude) mice (age, 4-6 weeks; weight, 18-22 g) were randomly divided into 2 groups and housed under specific pathogen-free conditions (temperature, 24-26°C; humidity, 40-60 %; ventilation, 15 times/h; 12:12-h light/dark cycle; free access to food and water). The euthanasia of all mice was carried out using 1 % pentobarbital sodium 150 mg/kg (Sigma Aldrich; Merck KGaA) by intraperitoneal injection, and a 1 cm abdominal median incision was made under the xiphoid process.
2.4ImmunoblottingImmunoblotting analysis was conducted as reported previously [14]. Total protein was extracted from cells and CCA tissue samples using RIPA lysis buffer (Sigma Aldrich; Merck KGaA) containing protease inhibitors (Roche Diagnostics); the protein concentration was measured using a BCA reagent kit (Beyotime Institute of Biotechnology). Antibodies against MMP2 (ab97779; Abcam), COL1A1 (ab34710; Abcam), PD-1 (ab52587; Abcam), PD-L1 (ab213524; Abcam), E-cadherin (Cell Signaling Technology, Inc.; #3195), N-cadherin (Cell Signaling Technology, Inc.; #13116), vimentin (Cell Signaling Technology, Inc.; #5741), integrin αV (Cell Signaling Technology, Inc.; #4711), NF-κB p65 (Cell Signaling Technology, Inc.; #8242) and phosphorylated (p-) NF-κB p65 Ser536 (Cell Signaling Technology, Inc.; #3033) were used at a 1:1,000 dilution. Membranes were washed with TBST for 30 min and incubated with an HRP-conjugated anti-rabbit or anti-mouse secondary antibody (1:4,000; cat. no. ab6721; Abcam) for 2 h at room temperature.
2.5ELISATotal protein was extracted from CCA tissue samples as reported previously [14]. The levels of COL1A1 were determined using an ELISA kit (CUSABIO Systems, USA) following the manufacturer's instructions.
2.6Invasion and migration assayTumour cell invasion assays were performed using Transwell chambers (8 μm, 24-well format) (Corning Costar, NY) coated with 30 μl of Basement Membrane Matrigel (diluted 1:6 in 1640; Corning Life Science, USA) for 5 h in a 37°C incubator. Then, 200 μl of serum-free 1640 containing 2 × 105 cells and 800 μl of 1640 containing 10 % FBS were added to the upper and lower wells of the chamber, respectively. After 24 h (RBE) or 36 h (HCCC-9810), cells remaining in the upper part of the Transwell insert were removed with a cotton swab. Migrated cells were then stained with 0.5 % crystal violet, and the number of cells was counted per high field (x200) with an Olympus microscope.
Tumour cell migration assay: Transwell migration assays were performed according to the protocol of the cell invasion assay, except that the 24-well chambers (Corning Costar, NY) were not coated with Matrigel. HCCC-9810 and RBE cells were incubated for an additional 12 h for migration studies. Migrated cells were then stained with 0.5 % crystal violet, and the number of cells per field was determined using a light microscope (Olympus Corporation; magnification, × 200).
2.7Immunofluorescence stainingFresh CCA tissues and paired adjacent pathological tissues were embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; Sakura Finetek USA, Inc.). After washing with PBS, pathological sections (8 µm thickness) on the cover slips were fixed with 4 % paraformaldehyde for 20 min. The cover slips were rinsed 3 times with PBS for 3 min each. Then, the tissue slides were incubated with a blocking solution of 5 % goat serum in PBS for 30 min. The tissue slides were incubated with COL1A1 antibody (1:400, ab34710, Abcam), PD-1 antibody (1:400, ab52587, Abcam) or PD-L1 antibody (1:400, ab213524, Abcam) overnight at 4°C. The cover slips were washed with PBS 3 times for 3 min each. Antibodies against COL1A1 (1:200; cat. no. ab180735; Abcam), PD-1 (1:200; cat. no. ab52917; Abcam) and PD-L1 (1:200; cat. no. ab52917; Abcam) were used for 1 h in a moist, dark environment. After washing with PBS, 10 μl of antifade mounting medium containing DAPI (VECTASHIELD, USA) was added to the cover slips for nuclear staining. Images were then taken using an Olympus confocal microscope or a fluorescence microscope.
2.8Lentivirus and stable cell linesHuman full-length MMP2 and COL1A1 complementary DNA (cDNA) was amplified by RT‒PCR and cloned and inserted into a lentiviral vector to establish an RBE cell line that stably expressed MMP2 and COL1A1. Two clones were tested, and clone-1 was selected for subsequent experiments. For MMP2 and COL1A1 knockdown, lentivirus encoding a specific shRNA against MMP2 and COL1A1 was used to establish a stable HCCC-9810 cell line. Two clones were tested, and clone-1 was selected for subsequent experiments (MMP2 target sequence: CCGGGCGACAAGAAGTATGGCTTCTCTCGAGAGAAGCCATACTTCTTGTCGCTTTTTG and COL1A1 target sequence: CCGGCCCAT-TGGTAATGTTGGTGCTCTCGAGAGCACCAACATTACCAATGGGTTTTTG).
MMP2 and COL1A1 overexpression (LV-MMP2(27177-1) and LV-COL1A1(70914-3)), and interference
Lentiviruses (LV-MMP2-RNAi(101659-31) and LV-COL1A1-RNAi(98436-1)) were purchased from Shanghai Genechem Co., Ltd., HCCC-9810 and RBE cells were seeded onto 6-well plates, and lentiviral infection was carried out at 50‑70 % confluency. For infection, the cells were provided with 1 ml of fresh culture medium, and 30 µl of lentivirus (1 × 108 transforming units/ml) was added to each well. After 48-72 h, the infection rate was observed using a fluorescence microscope. MMP2 and COL1A1 expression was assessed by western blot analysis.
In particular, in the knockout and overexpression of MMP2 and COL1A1, the composition of NC is described below.
Knockdown (MMP2-NC): si-NC (MMP2), catalogue number: LVCON313; CON313 (hu6-MCS-CBh-gcGFP-IRES-puromycin); contract number: GIEL0288689.
Overexpression (MMP2-NC): Vector-NC (MMP2), LVCON335, CON335 (UBi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin); contract number: GOSL0272230.
Knockdown (COL1A1-NC): si-NC (COL1A1), catalogue number: LVCON313; CON313 (hu6-MCS-CBh-gcGFP-IRES-puromycin); contract number: GIEL0275812.
Overexpression (COL1A1-NC): Vector-NC (COL1A1), catalogue number: LVCON522; CON522 (CMV enhancer-MCS-3FLAG-EF1a-ZsGreen1-T2A-puromycin); contract number: GOSL0293616. NC represents the negative control.
2.9Integrin αV knockdownSmall interfering (si) RNAs against integrin αV (siIntegrin αV) and a negative control (siNC) were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). All siRNA sequences targeting integrin αV are shown in Supplementary Table 1. siRNAs (5 µg/ml) were transfected into HCCC-9810 and RBE cells according to the manufacturer's instructions. After incubation at 37°C for 2 days, the knockdown efficiency was assessed.
Notably, the sequence of si-NC (integrin αV) was not made available by the company, but they did provide the catalogue number (D203105255).
2.10Immunohistochemistry (IHC) stainingThis assay was performed as previously described [15]. The following antibodies were used: MMP2 (1:200; cat. no. ab97779; Abcam), COL1A1 (1:200; cat. no. ab34710; Abcam), E-cadherin (1:300; Cell Signaling Technology, Inc.; #3195) and vimentin (1:200; Cell Signaling Technology, Inc.; #5741). Haematoxylin and eosin (H&E) staining was carried out as previously described [16]. The immunostained tissue slides were scored using our previously described methods [2]. Finally, images were obtained with an Olympus microscope.
2.11ReagentsThe NF-κB inhibitor ammonium pyrrolidinedithiocarbamate (PDTC; cat. no. HY-18738) was purchased from MedChemExpress, and the concentration used in this study was 40 µmol/l [17].
2.12Wound healing assayThe diluted cell suspension was prepared and seeded into 6-well plates at a rate of 2 × 105 cells/well and incubated at 37°C until the cell fusion degree was 100 %. A 10 μl pipette was used to draw a straight line on the cell surface to create a wound. Then, the cells were washed three times with PBS to remove the cell fragments. The cells were cultured in a serum-free 1640 cell incubator. The migration of cells along the wound line was monitored under an inverted microscope at 0 and 24 hours after the scratch.
2.13ECM degradation assayThe ECM degradation assay was performed according to a previously described protocol [18,19] and the manufacturer's instructions. The QCM™ Gelatine Invadopodia Assay (Red) (cat. no. ECM671; Millipore) was used. Briefly, 2 % gelatine was prelabelled with 0.2 mg/ml FITC in 50 mM sodium borate and 40 mM sodium chloride before dialysis into PBS. A thin layer of 150 μl preheated (37°C) FITC gelatine was coated on the glass cover glass slide and immediately crosslinked with 0.8 % glutaraldehyde (electron microscopy Science) at 4°C for 15 min and then crosslinked in the room for 30 min. The cover slides were quenched with 1640 for 1 hour in PBS (3 × 5 min), 5 mg/ml sodium bisulfite/PBS (1 × 3 min), PBS (3 × 5 min) and 70 % ethanol (1 × 10 min). For the ECM degradation assay, 2 × 104 cells were suspended in medium and added to plates for 36 h (HCCC-9810) and 24 h (RBE) of incubation. Then, the cover glass was treated with a standard fluorescence microscope program, and the degradation area of ECM was quantified by ImageJ software.
Quantification of ECM degradation is carried out by measuring the area of degradation under and near each cell. The values obtained are expressed as total degradation area per cell and are thus a direct readout of degradation ability that accounts for most foreseeable variables.
2.14In vivo experimentsThe metastasis of CCA cells was assessed after xenotransplantation into nude mice via intrasplenic injection, as previously described [20,21]. After anaesthetizing with 1 % sodium pentobarbital 75 mg/kg (Sigma‒Aldrich; Merck KGaA) by intraperitoneal injection, a median abdominal incision of 1 cm was made under the xiphoid of nude mice. To assess experimental liver metastasis, HCCC-9810 and HCCC-9810 (KD-COL1A1) cells were trypsinized and resuspended in PBS, and 5 × 105 cells or the indicated number of cells (in 0.1 ml of PBS) were injected into the spleens of the mice.
Mice were sacrificed at 45 days after injection, and livers were isolated and fixed in 10 % neutral‑buffered formalin for 24 h at room temperature and then dehydrated and embedded in paraffin. Metastatic foci on the surface of the livers were counted under a dissecting microscope, and the nodule size was measured. To assess tumour volumes, the longest (length) and shortest (width) diameters of the metastatic tumour foci in the liver were measured, and the volumes were calculated using the following formula: Length x width 2 × 0.52. The maximum volume of liver tumours was 104 mm3, and the maximum diameter was 6 mm.
2.15Statistical analysisStatistical analyses were performed using SPSS 19.0 software (IBM Corp.), and graphs were generated using Prism 7.0 (GraphPad Software, Inc.). Categorical data were analysed by chi-square analysis or Fisher's exact test. Kaplan‒Meier and Cox proportional hazards regression analyses were used to assess overall survival. For normally distributed data, the mean ± standard error is presented, and paired or independent Student's t test was applied to compare the difference between groups for paired samples or random samples, respectively. ANOVA followed by Bonferroni post hoc correction was deployed to analyse the difference among groups over two. Nonnormally distributed data were compared using the Mann‒Whitney U test for random samples or using the Wilcoxon signed-rank test for paired samples. The association of the expression levels of MMP2 and COL1A1 and between COL1A1 and PD-L1 were determined by the Spearman rank correlation coefficient. The assay was performed in duplicate and repeated three times independently. P<0.05 was considered a statistically significant difference.
2.16Ethical statementAll subjects signed an informed consent form. The present study was approved by the Ethics Committee of the Third Military Medical University and followed the guidelines of the Declaration of Helsinki. We obtained each patient's written informed consent. The animal research was approved by The Institutional Animal Use and Care Committee and complied with the Animal Research Ethics Committee of the Third Military Medical University.
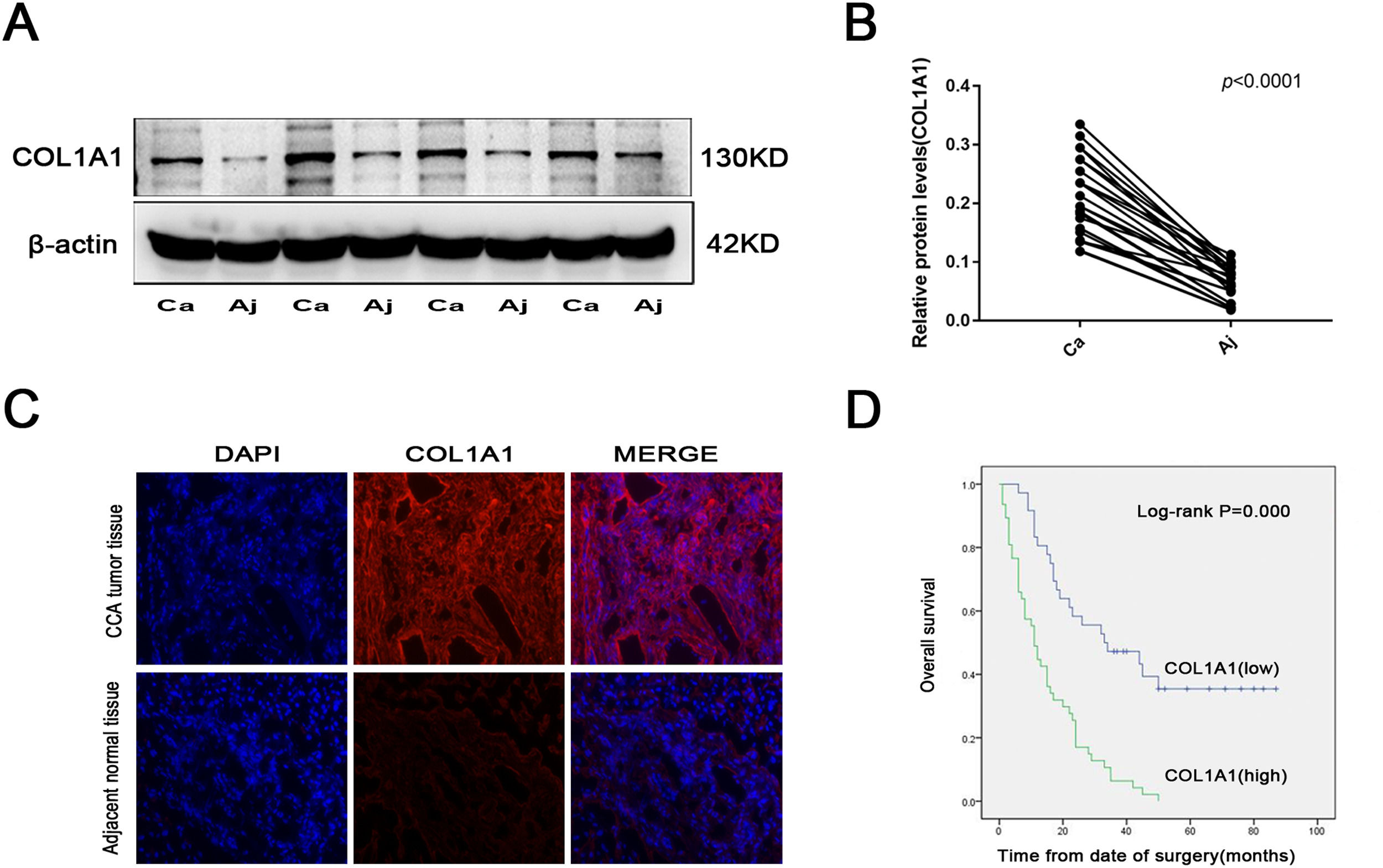
3Results3.1COL1A1 is significantly unregulated in CCA, and its expression is closely related to CCA severityTo explore the role of COL1A1 in CCA, tumour tissues and corresponding adjacent normal tissues were obtained from 27 CCA patients. The expression of COL1A1 was higher in CCA tissues than in adjacent normal tissues (Fig. 1A and B). At the same time, the results of immunofluorescence staining of pathological tissues of CCA were consistent with the above results (Fig. 1C).
COL1A1 is significantly unregulated in CCA, and its expression is closely related to CCA severity (A) Protein expression levels of COL1A1 were determined by immunoblotting analysis in 27 tumour tissues and adjacent normal tissues from patients with CCA. Representative blots from 4 paired samples are shown. (B) COL1A1 protein expression levels in 27 tumour tissues and adjacent normal tissues. (C) Visualization of COL1A1 (red) staining in tumour tissues and adjacent normal tissues from patients with CCA by immunofluorescence microscopy. (D) Kaplan‒Meier plot for overall survival of 83 patients with CCA with low or high COL1A1 expression. Patients were divided into two groups based on the mean expression level. Experiments were repeated at least three times independently.
Our study found that higher COL1A1 expression was significantly associated with poorer tumour differentiation and increased lymph node metastasis (Table 1). Moreover, multivariate Cox proportional hazards regression analysis revealed that COL1A1 expression levels were also significantly associated with poor prognosis in patients with CCA (Table 2). Consistent with this finding, CCA patients with high COL1A1 expression had a significant reduction in overall survival (Fig. 1D). These results suggested that COL1A1 expression is closely related to the severity of CCA.
COL1A1 Expression and clinical characteristics in 83 cholangiocarcinoma patients.
COL1A1, Collagen type Ⅰ alpha 1.
Multivariate Cox proportional hazards regression analysis for COL1A1 expression levels and overall survival in 83 patients with cholangiocarcinoma
COL1A1, Collagen type Ⅰ alpha 1; CI, confidence interval.
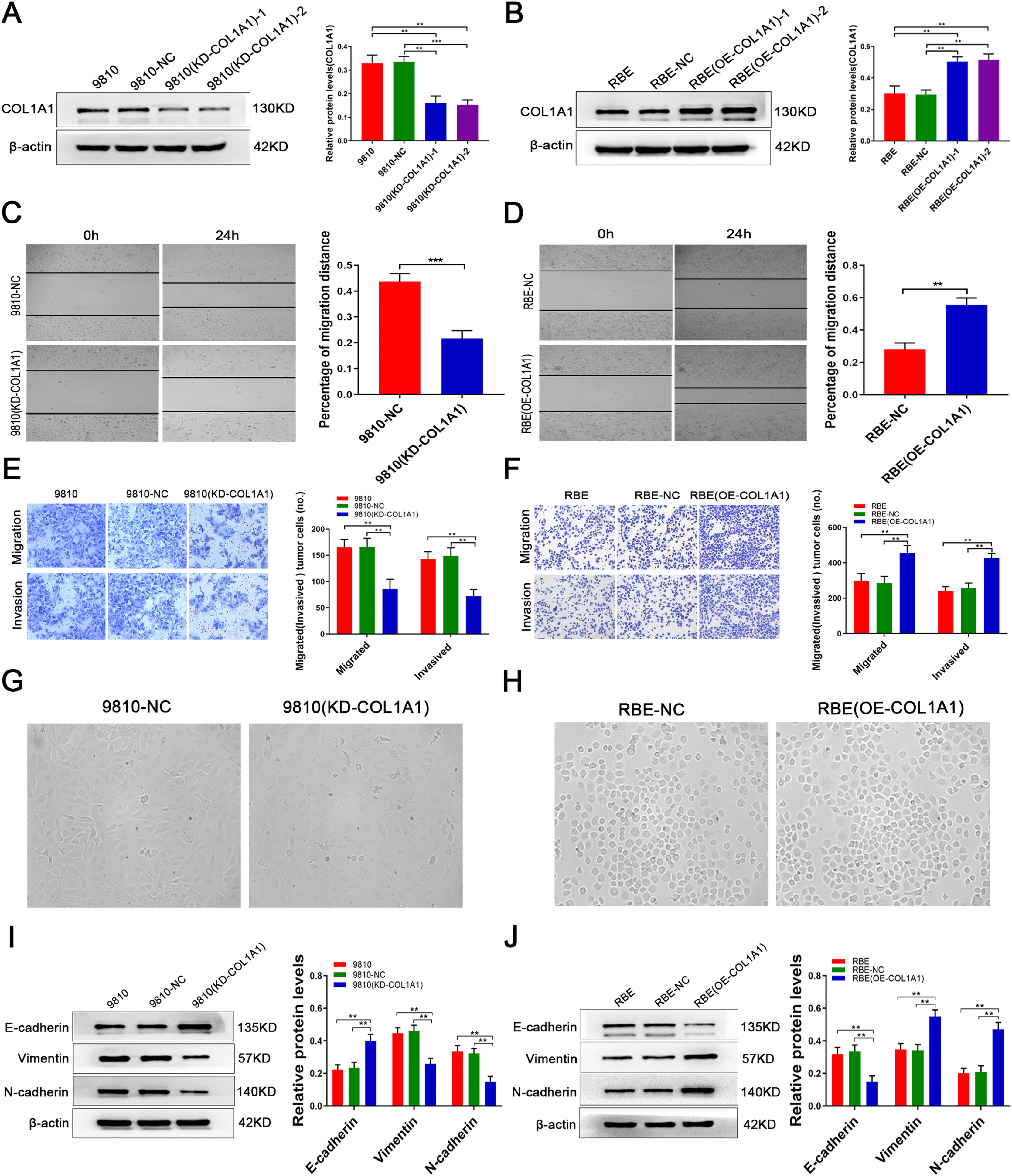
The above data prompted us to speculate that COL1A1 may be involved in the pathological process of CCA. To investigate this, the HCCC-9810 CCA cell line, which has high intrinsic COL1A1 protein expression, was transduced with lentivirus against COL1A1, and the transduction efficiency was verified by immunoblotting (Fig. 2A; clone 1 was selected for subsequent experiments). However, in the RBE CCA cell line, the expression of COL1A1 was significantly reduced. Overexpression of COL1A1 was performed in the CCA cell line RBE (Fig. 2B; clone 1 was selected for further experiments). Importantly, transwell and wound healing assays showed that silencing COL1A1 significantly impaired the invasion and migration ability of HCCC-9810 cells (Fig. 2C and E). In contrast, overexpression of COL1A1 in RBE cells had the opposite effect (Fig. 2D and F).
COL1A1 promotes invasion and migration and induces EMT in CCA cells in vitro. (A) Knockdown of COL1A1 in the HCCC‑9810 CCA cell line following lentivirus transduction was confirmed by western blotting. Two separate knockdown clones, (KD‑COL1A1)‑1 and (KD-COL1A1)-2, were tested. (B) Overexpression of COL1A1 in the RBE CCA cell line following lentivirus transduction was confirmed by western blotting. Two separate overexpressing clones, (OE‑COL1A1)‑1 and (OE-COL1A1)-2, were tested. (C) COL1A1 knockdown significantly reduced the migration ability of HCCC-9810 cells compared with the control after incubation for 12 h. (D) COL1A1 overexpression significantly enhanced the migration ability of HCCC-9810 cells compared with the control after incubation for 24 h. (E) Transwell migration and invasion assays of control HCCC-9810 and HCCC-9810 (KD-COL1A1) cells after incubation for 12 h and 36 h. (F) Transwell migration and invasion assays of control RBE and RBE (OE-COL1A1) cells after incubation for 12 h and 24 h. (G) Phase-contrast micrographs of the morphological changes of tumour cells in control HCCC-9810 and HCCC-9810 (KD-COL1A1) cells were observed at 36 h. (H) Phase-contrast micrographs of the morphological changes of tumour cells in control RBE and RBE (OE-COL1A1) cells were observed at 24 h. (I) E-cadherin, vimentin and N-cadherin protein expression levels were determined by western blotting in control HCCC-9810 and HCCC-9810 (KD-COL1A1) cells incubated for 36 h. (J) E-cadherin, vimentin and N-cadherin protein expression levels were determined by western blotting in control RBE and RBE (OE-COL1A1) cells incubated for 24 h. **P<0.01 and ***P<0.001. Experiments were repeated at least three times independently. 9810, HCCC-9810; OE, overexpression; KD, knockdown; NC, negative control.
Increasing the invasion and migration ability may cause morphological changes in tumour cells, which are required to induce epithelial-to-mesenchymal transition (EMT). Our experimental results showed that knockdown of COL1A1 resulted in a loss of spindle-shaped and fibroblast-like phenotypes in HCCC-9810 cells, while overexpression of COL1A1 had the opposite effect in RBE cells (Fig. 2G, H). In our study, immunoblotting analysis revealed that knockdown of COL1A1 led to the elevation of E-cadherin expression and the reduction of vimentin and N-cadherin expression in HCCC-9810 cells (Fig. 2I). Opposite results were observed in RBE cells following COL1A1 overexpression (Fig. 2J). Taken together, these data illustrated that COL1A1 may be implicated in promoting CCA invasion and migration and inducing EMT.
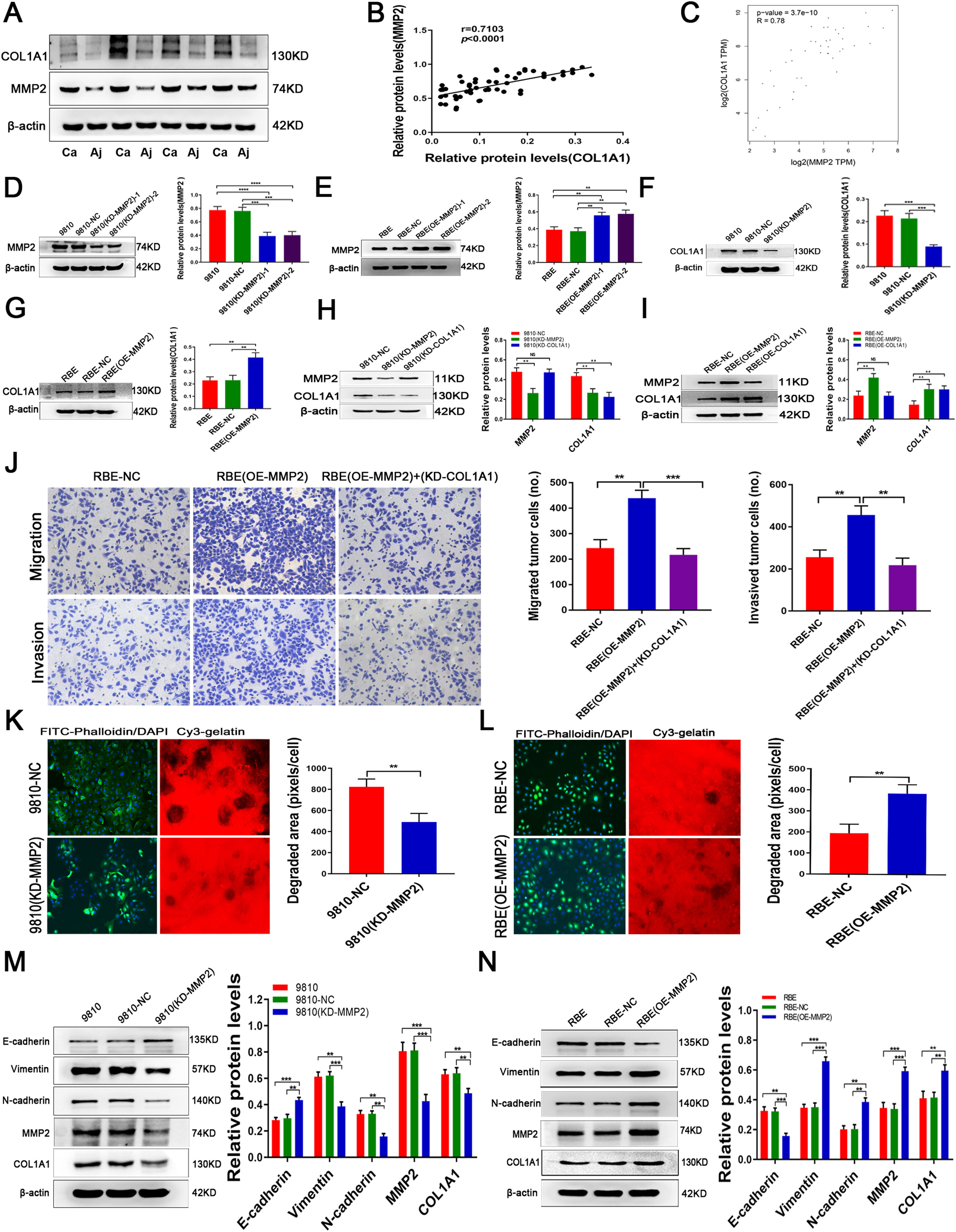
3.3MMP2 induces COL1A1 synthesis and affects ECM remodelling in CCAPrevious studies found that MMP2 is the main protein involved in ECM degradation, which is needed for invasive growth and metastasis of most malignant tumours. Indeed, in addition to COL1A1, we found that the expression of MMP2 was significantly increased in CCA tumour tissues compared with adjacent normal tissues (Fig. 3A). Interestingly, there was a significant correlation between MMP2 and COL1A1 expression in CCA specimens (Fig. 3B). Additionally, we used mRNA data from the TCGA database. The results of Spearman correlation analysis revealed a strong correlation between MMP2 and COL1A1 (Fig. 3C). Furthermore, our study found that the expression of MMP2 is similar to that of COL1A1 in HCCC-9810 and RBE cell lines. Given the above facts that MMP2 can regulate collagen expression, we then tested COL1A1 expression in MMP2 knockdown and overexpression CCA cells (Fig. 3D and E; clone 1 was selected for further experiments; see Materials and Methods for details). It was found that knockdown or overexpression of MMP2 could significantly decrease or increase the expression of COL1A1, respectively (Fig. 3F and G). However, knockdown or overexpression of COL1A1 did not affect the expression of MMP2 in CCA cells (Fig. 3H, I). Of note, knockdown of COL1A1 weakened the invasion and migration of RBE (OE-MMP2) cells (Fig. 3J), suggesting that COL1A1 may mediate the main effects of MMP2 on CCA cells.
MMP2 induces COL1A1 synthesis and affects ECM remodelling in CCA. (A) Protein expression levels of MMP2 and COL1A1 were detected by western blotting in 27 tumour tissues and paired adjacent normal tissues from patients with CCA. Representative blots from 4 paired samples are shown. (B) The expression levels of MMP2 and COL1A1 were semiquantitatively analysed by western blotting in 27 tumour tissues and paired adjacent normal tissues from patients with CCA, and the correlation between these two proteins was evaluated with Spearman's rank correlation method. (C) Correlation between MMP2 and COL1A1 mRNAs in the TCGA database. (D) Knockdown of MMP2 in the HCCC‑9810 CCA cell line following lentivirus transduction was confirmed by western blotting. Two separate knockdown clones, (KD‑MMP2) ‑1 and (KD-MMP2)-2, were tested. (E) Overexpression of MMP2 in the RBE CCA cell line following lentivirus transduction was confirmed by western blotting. Two separate overexpressing clones, (OE‑MMP2) ‑1 and (OE-MMP2)-2, were tested. (F) COL1A1 protein expression levels were determined by western blotting in HCCC-9810 parental, NC and HCCC-9810 (KD‑MMP2) cells incubated for 36 h. (G) COL1A1 protein expression levels were determined by western blotting in RBE parental, NC and RBE (OE‑MMP2) cells incubated for 24 h. (H) MMP2 and COL1A1 protein expression levels were determined by western blotting in HCCC-9810-NC, HCCC-9810 (KD‑MMP2), and HCCC-9810 (KD‑COL1A1) cells incubated for 36 h. (I) MMP2 and COL1A1 protein expression levels were determined by western blotting in RBE-NC, RBE (OE‑MMP2), and RBE (OE‑COL1A1) cells incubated for 24 h. (J) Transwell migration and invasion assays of control RBE-NC, RBE (OE‑MMP2) and RBE (OE‑MMP2) cells with knockdown of COL1A1 incubation for 12 h and 24 h. (K) ECM degradation analysis of control HCCC-9810-NC and HCCC-9810 (KD-MMP2) cells ware plated on Cy3 gelatine matrices for 36 h. The degradation under the cells was quantified as described in the Materials and Methods, and the area per cell was plotted (right). (L) ECM degradation analysis of control RBE-NC and RBE (OE-MMP2) cells ware plated on Cy3 gelatine matrices for 24 h. The degradation was described as in (K). (M) E-cadherin, vimentin, N-cadherin, MMP2 and COL1A1 protein expression levels were determined by western blotting in control HCCC-9810 parental, NC and HCCC-9810 (KD‑MMP2) cells incubated for 36 h. (N) E-cadherin, vimentin, N-cadherin, MMP2 and COL1A1 protein expression levels were determined by western blotting in control RBE parental, NC and RBE (OE‑MMP2) cells incubated for 24 h. **P<0.01; ***P<0.001 and ****P<0.0001. Experiments were repeated at least three times independently. 9810, HCCC-9810; OE, overexpression; KD, knockdown; NC, negative control.
To study the role of MMP2 in ECM remodelling, HCCC-9810 (KD-MMP2) and RBE (OE-MMP2) cells were plated on a glass slide coated with a thin layer of cross-linked fluorescent gelatine for 24 hours. Specifically, RBE (OE-MMP2) cells degraded more gelatine than control cells (Fig. 3L), while HCCC-9810 (KD-MMP2) cells showed the opposite effect (Fig. 3K). Furthermore, knockdown and overexpression experiments revealed that MMP2 promoted the expression of E-cadherin and inhibited the expression of vimentin, N-cadherin and COL1A1 in CCA cells (Fig. 3M, N). Thus, our results suggested that MMP2 is essential for COL1A1-mediated EMT in CCA cells.
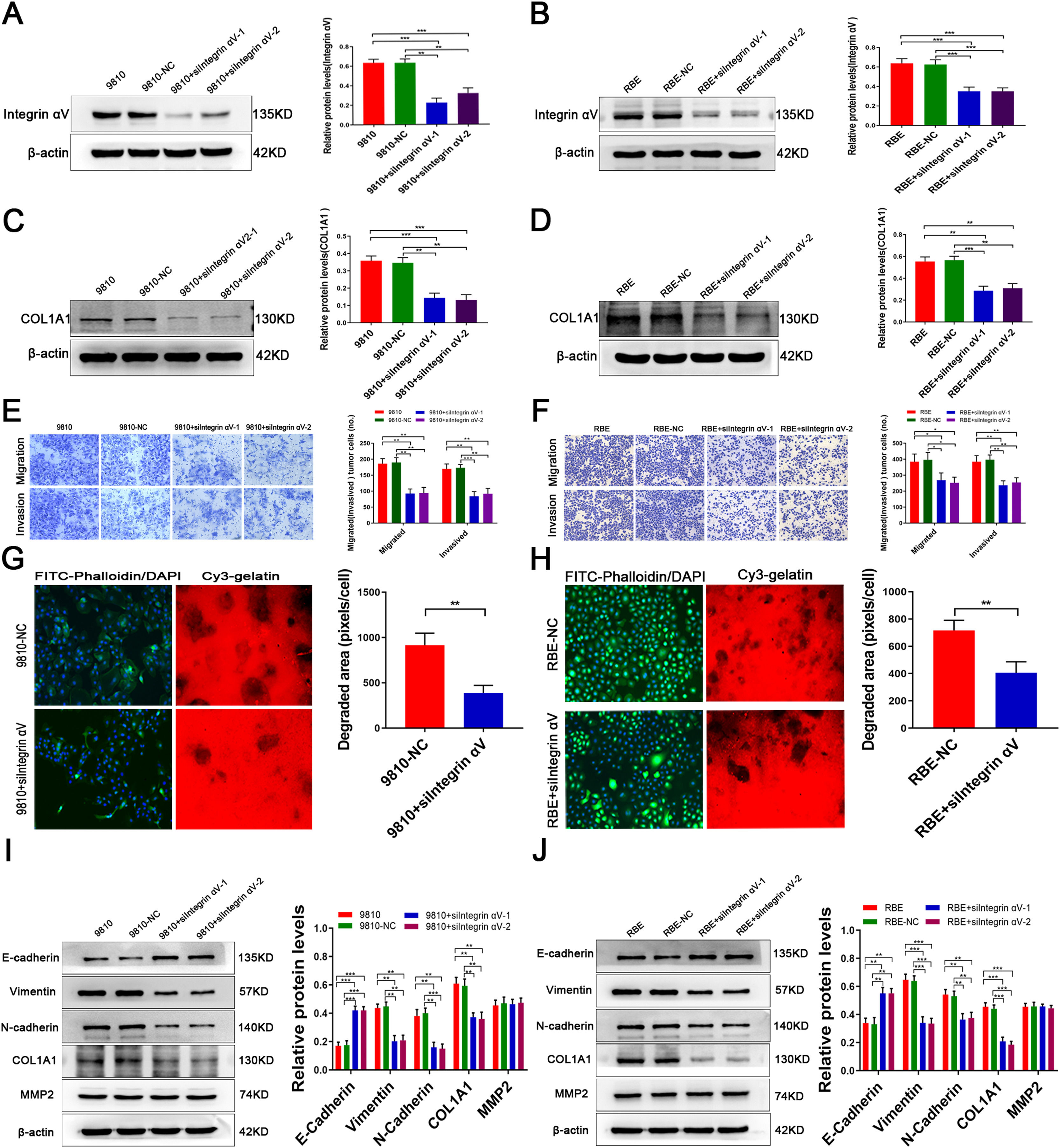
3.4MMP2 regulates COL1A1 via the integrin alpha Ⅴ pathway and affects ECM remodellingTo further study whether the regulation of COL1A1 by MMP2 is mediated by integrin αV, we knocked down integrin αV expression in HCCC-9810 and RBE cells (Fig. 4A and B). Compared with the controls, the expression of COL1A1 was significantly decreased following integrin αV knockdown (Fig. 4C and D). In addition, Transwell assays showed that the invasion and migration abilities of CCA cells were significantly reduced after integrin αV knockdown (Fig. 4E and F). Taken together, these results demonstrated that the integrin αV pathway mediates the role of MMP2 in facilitating COL1A1 expression and the invasion and metastasis of CCA cells.
MMP2 regulates COL1A1 via the integrin alpha Ⅴ pathway and affects ECM remodelling. (A) Integrin αV protein expression levels were determined by western blotting analysis after transfection of HCCC-9810 cell lines with integrin αV-siRNA. (B) Integrin αV protein expression levels were determined by western blotting analysis after transfection of RBE cell lines with Integrin αV-siRNA. (C) COL1A1 protein expression levels were determined as in (A). (D) COL1A1 protein expression levels were determined as in (B). (E) Transwell migration and invasion assays of HCCC-9810 parental, NC and HCCC-9810 cells with Integrin αV-siRNA incubation for 12 h and 36 h. (F) RBE cells as in (E) were incubated for 12 h and 24 h. (G) ECM degradation analysis of control HCCC-9810-NC and HCCC-9810 cells with Integrin αV-siRNA ware plated on Cy3 gelatine matrices for 36 h. The degradation under the cells was quantified as described in the Materials and Methods, and the area per cell was plotted (right). (H) RBE cells as in (G) ware plated on Cy3 gelatine matrices for 24 h. The degradation was described as in (G). (I) E-cadherin, vimentin, N-cadherin, MMP2 and COL1A1 protein expression levels were determined by western blotting in control HCCC-9810 parental, NC and HCCC-9810 cells with Integrin αV-siRNA incubation for 36 h. (J) RBE cells as in (I) were incubated for 24 h. *P<0.05; **P<0.01 and ***P<0.001. Experiments were repeated at least three times independently. 9810, HCCC-9810; OE, overexpression; KD, knockdown; NC, negative control.
To study the effect of MMP2 on ECM remodelling induced by integrin αV, we performed an ECM degradation experiment. The results showed that integrin αV knockout cells degraded less gelatine than wild-type cells (Fig. 4G and H). On the other hand, knockdown of integrin αV enhanced the expression of E-cadherin and decreased the expression of vimentin, N-cadherin and COL1A1 in CCA cells, while there was no significant decrease in MMP2 expression (Fig. 4I and J). Hence, MMP2-induced tumour cell EMT is dependent on integrin αV signalling.
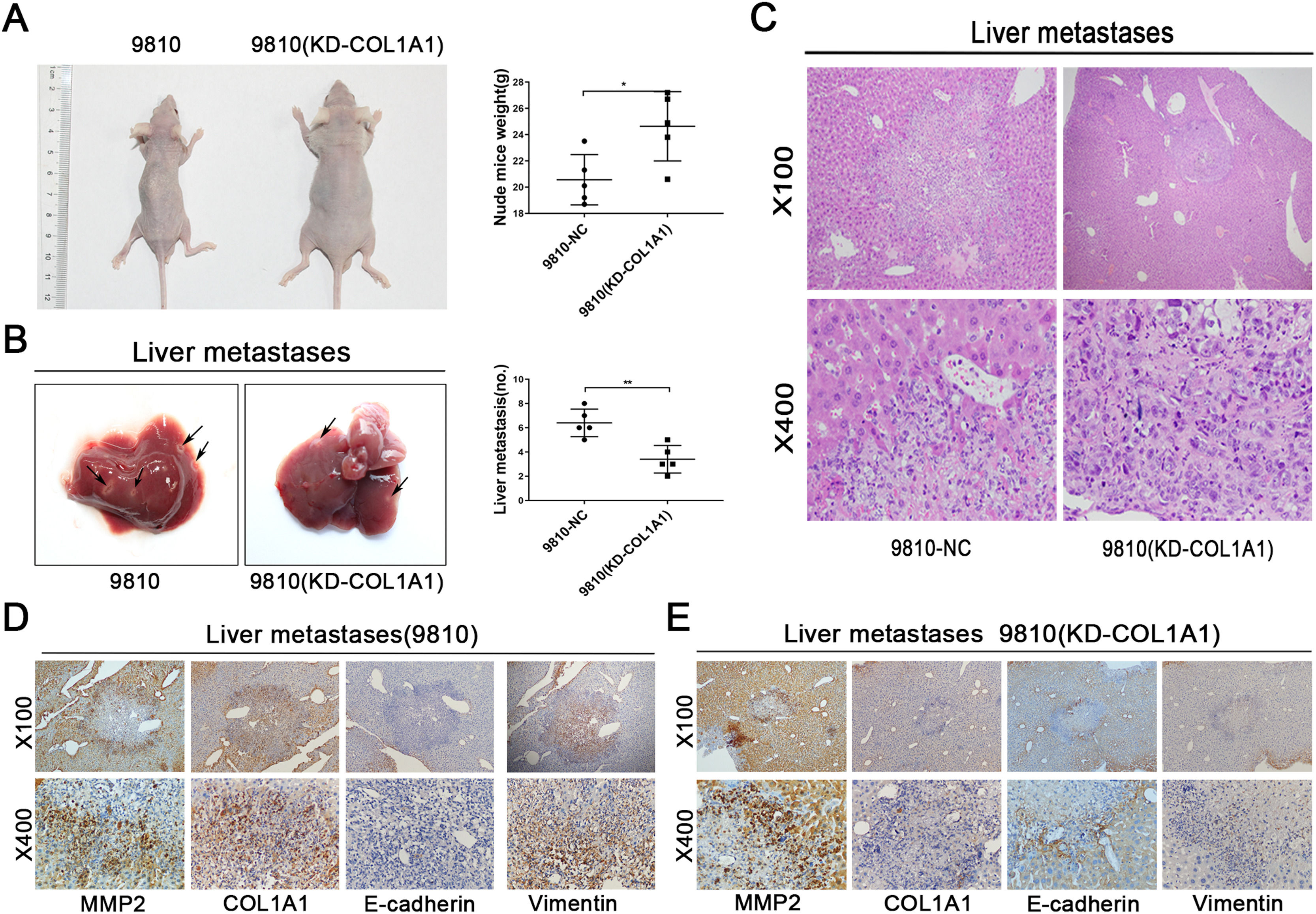
3.5COL1A1 contributes to the invasion and metastasis of CCA in vivoWe next evaluated the effect of COL1A1 on liver metastasis using a nude mouse model. HCCC-9810 cells with or without COL1A1 knockdown were implanted into the spleens of nude mice (see Materials and Methods). After 45 days of monitoring, we noticed that the weight of mice injected with HCCC-9810 (KD-COL1A1) cells was evidently higher than that of the controls (Fig. 5A). Additionally, mice injected with COL1A1-knockdown HCCC-9810 cells formed fewer metastatic foci (Fig. 5B, C). To determine whether COL1A1 influences metastatic growth, tumour volume in the liver of mice was detected. However, no significant difference was observed between the two groups (data not shown). Moreover, the IHC data showed that knockdown of COL1A1 enhanced the expression of E-cadherin and decreased the expression of vimentin in liver metastatic tumours, accompanied by largely unchanged expression of MMP2 (Fig. 5D and E). Altogether, these findings indicated that COL1A1 may drive the invasion and metastasis of CCA cells in vivo.
COL1A1 contributes to the invasion and metastasis of CCA in vivo. (A) Nude mice were injected with tumour cells through the spleen. (n = 5 per group). The weights of the mice were counted (right). (B) Representative photographs of liver metastasis foci in mice 45 days after splenic injection of HCCC-9810 cells with or without COL1A1 knockdown (n=5 per group). Quantification of the number of liver metastatic foci is shown on the right. (C) Representative images of metastatic foci (magnification, x100 and x400) in haematoxylin and eosin-stained liver tissue sections from mice injected with control or COL1A1 knockdown HCCC-9810 cells. (D) Paraffin-embedded liver metastasis nodules were stained with anti-MMP2, anti-COL1A1, anti-E-cadherin or anti-Vimentin antibodies using CCA. (E) Paraffin-embedded liver metastasis nodules (HCCC-9810(KD-COL1A1)) were stained with anti-MMP2, anti-COL1A1, anti-E-cadherin or anti-Vimentin antibodies using CCA. *P<0.05 and **P<0.01. Experiments were repeated at least three times independently. 9810, HCCC-9810; KD, knockdown; NC, negative control.
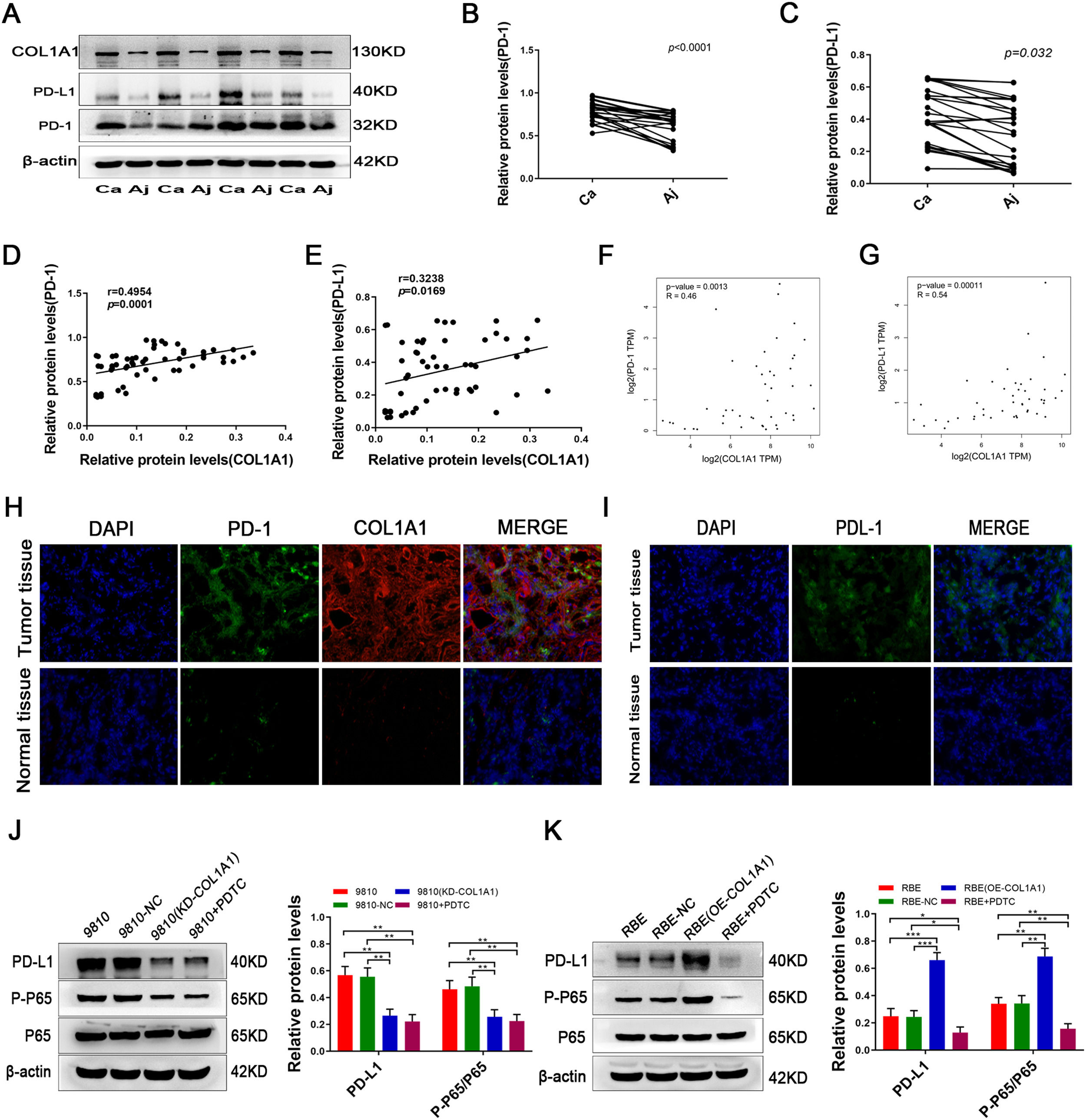
Notably, a recent study reported that the expression of COL1A1 is positively correlated with PD-L1 levels in gastric cancer. We next tested the expression of PD-1/PD-L1 in CCA and adjacent tissues. Intriguingly, PD-1/PD-L1 was expressed at high levels in CCA tissues and at low levels in adjacent tissues (Fig. 6A, B and C). Further analysis showed that COL1A1 and PD-1/PD-L1 protein expression levels were positively correlated in CCA samples (Fig. 6D and E). Similar results were observed at the mRNA level by analysing the data from the TCGA cohort (Fig. 6F and G). In addition, immunofluorescence staining further confirmed this finding in CCA specimens (Fig. 6H and I).
COL1A1 upregulates PD-L1 expression in CCA cells through activating the NF-κB pathway. (A) Protein expression levels of COL1A1, PD-L1 and PD-1 were detected by western blotting in 27 tumour tissues and paired adjacent normal tissues from patients with CCA. Representative blots from 4 paired samples are shown. (B) PD-1 protein expression levels in 27 tumour tissues and adjacent normal tissues. (C) PD-L1 protein expression levels as in (B). (D) Correlation between COL1A1 and PD-1 protein levels in 27 tumour tissues and adjacent normal tissues with CCA, as determined by western blotting. (E) Correlation between COL1A1 and PD-L1 protein levels as in (D). (F) Correlation between COL1A1 and PD-1 mRNAs in the TCGA database. (G) Correlation between COL1A1 and PD-L1 mRNAs in the TCGA database. (H) Visualization of PD-1 (green) and COL1A1 (red) staining in tumour tissues and adjacent normal tissues from patients with CCA by immunofluorescence microscopy. (I) Visualization of PD-1 (green) staining in tumour tissues and adjacent normal tissues from patients with CCA by immunofluorescence microscopy. (J) PD-L1, p-p65 and total p65 protein expression levels were determined by western blotting in NC, HCCC-9810 (KD-COL1A1) and HCCC-9810 cells with or without treatment with PDTC inhibitors (40 μmol/l) for 12 h. (K) PD-L1, p-p65 and total p65 protein expression levels were determined by western blotting in NC, RBE (OE-COL1A1) and RBE cells with or without treatment with PDTC inhibitors (40 μmol/l) for 24 h. *P<0.05; **P<0.01 and ***P<0.001. Experiments were repeated at least three times independently. 9810, HCCC-9810; OE, overexpression; KD, knockdown; NC, negative control.
Finally, we sought to determine whether COL1A1 modulates PD-L1 in CCA. In fact, overexpression of COLA1 increased PD-L1 expression, while knockdown of COL1A1 suppressed COL1A1 expression in CCA cells (Fig. 6J and K). Considering that PD-L1 is downstream of NF-κB signalling [22,23], we speculated that COL1A1 might promote PD-L1 expression through the NF-κB pathway. As anticipated, the NF-κB pathway was decreased in COL1A1-knockdown HCCC-9810 cells (Fig. 6J). In contrast, activation of the NF-κB pathway was found in RBE cells after overexpression of COL1A1 (Fig. 6K). More importantly, the increase in the expression of PD-L1 in RBE cells after COL1A1 overexpression was completely abrogated by NF-κB inhibitor treatment (Fig. 6 K). Collectively, the present findings demonstrated that COL1A1 promoted PD-L1 expression in CCA by regulating the NF-κB pathway. These experimental data provide a strong scientific basis for immunotargeting therapy in patients with CCA.
4DiscussionCCA is a highly malignant epithelial tumour that originates from bile duct epithelial cells or bile duct cells in intrahepatic or extrahepatic bile duct systems. In recent years, the morbidity and mortality rates of CCA have increased worldwide. The extracellular matrix, especially collagen, is increasingly considered to play an indispensable role in CCA occurrence, development and prognosis [24,25]. It has been well accepted that tumour cells prefer to move along collagen fibres, which is an important way for their invasion and metastasis [26,27].
In the present study, we found that CCA is associated with the accumulation of COL1A1 due to an imbalance in extracellular matrix synthesis and degradation. Research confirms that COL1A1 is an important part of ECM remodelling [4]. Compared with tumour tissues, the content of COL1A1 is substantially decreased in adjacent normal tissues [5,28]. Additionally, COL1A1 is implicated in promoting the invasion and metastasis of breast cancer [6].Nevertheless, the exact role of COL1A1 in malignant bile duct tumours is still unknown. We demonstrated for the first time that COL1A1 was overexpressed in CCA tissues and that its upregulation could predict a poor clinical outcome for CCA patients. In addition, in vitro and in vivo experiments demonstrated that knockdown or overexpression of COL1A1 significantly changed the morphology of tumour cells and reduced or enhanced the invasion and metastasis of tumour cells. Additionally, COL1A1 expression displayed a significant association with CCA severity. We also identified that COL1A1 promoted EMT in vitro and in vivo[29–31].
Static fibroblasts exposed to activation induction cannot be activated without MMP2, which indicates that MMP2 is necessary for the complete activation of fibroblasts. Previous studies found that MMP2 is the main protein involved in ECM degradation, which is needed for invasive growth and metastasis of most malignant tumours [32].Studies on breast cancer have shown that increased expression of MMP2 is correlated with collagen in the stroma from breast cancer patients. They reveal a new mechanism by which MMP2 facilitates the progression of breast cancer through increasing collagen expression. As we reported previously, MMP2 upregulation can significantly promote CCA progression [33]. Here, our observations suggested that COL1A1 participated in tumour aggressiveness following MMP2 overexpression. Decreasing or increasing MMP2 expression resulted in COL1A1 upregulation or downregulation, thus affecting the invasion and migration ability of tumour cells and the degradation of ECM, respectively. In addition, MMP2 overexpression suppressed E-cadherin expression and induced vimentin and N-cadherin expression in CCA cells. In contrast, MMP2 knockdown reversed the induction of EMT. In addition, knockdown of COL1A1 in MMP2-overexpressing tumour cells significantly reversed the invasion and migration abilities. These data are of significance for elucidating the role of MMP2 in tumour collagen dynamics, pathogenesis and metastasis of CCA.
Integrins, as a group of cell surface receptors, are involved in mediating cell‒cell and cell-extracellular matrix protein adhesion [34]. In addition, integrin αV has been implicated in the pathophysiological process of the ECM [35–37]. It has been shown that MMP2 can directly bind to integrin alpha (V) beta3 on the surface of tumour cells, eventually facilitating their invasion [38–40].Our results further confirmed the notion that MMP2 induced COL1A1 synthesis via integrin αV. We showed that knockdown of integrin αV significantly reduced COL1A1 expression, the invasion and migration of CCA cells and ECM degradation. In addition, we noticed that the expression of EMT-associated proteins, such as N-cadherin and vimentin, was significantly decreased after knockdown of integrin αV in CCA cell lines, accompanied by the increased expression of the epithelial marker E-cadherin. The present study proves that COL1A1 might promote CCA cell migration mainly via the integrin αV signalling pathway. However, additional studies will be necessary to further disclose this.
It is well established that immunotherapy with anti-PD-1/PD-L1 antibodies is an effective strategy against a variety of malignancies [41–44]. The expression of PD-1/PD-L1 in tumours has been defined as a vital indicator of immunotherapy effects [45,46]. Notably, a recent study reported that the expression of COL1A1 is positively correlated with PD-L1 levels in gastric cancer [47]. Moreover, our work reported that PD-1/PD-L1 was more abundant in CCA tissues than in adjacent normal tissues, which was similar to the COL1A1 data. The results of our own patient cohort study and data from the TCGA database showed that COL1A1 levels were positively related to PD-1/PD-L1 levels. Numerous studies have confirmed that the NF-κB pathway participates in the modulation of the progression of many types of cancer [48]. The present study indicated that COL1A1 contributed to CCA cell migration probably by stimulating the NF-κB signalling pathway, but the exact mechanism needs to be further studied. Many studies have revealed that the combination of anti-PD-1/PD-L1 therapy with other therapies, including surgery, chemotherapy, radiotherapy, and molecular targeted therapy, can effectively slow the progression of malignant tumours [49]. Our current research provides a strong scientific theoretical basis for the use of immune-targeted treatment for this disease.
5ConclusionsIn summary, this study reveals that COL1A1 promotes cell migration and invasion, which is mediated by the MMP2/integrin αV axis. Moreover, COL1A1 promotes PD-L1 expression in CCA cells by activating the NF-κB pathway. As a consequence, our findings not only clarify the underlying molecular mechanism of COL1A1 in affecting the progression of CCA but also provide a deep understanding of tumour-related ECM, which can be used as a predictor of the clinical prognosis of CCA.
FundingThis work was supported by grants from the National Natural Science Fund of China (NO. 81725019 and NO. 81270535), Southwest Hospital (grant No. SWH2016JCYB-10) and the Scientific Research Project (NO. 201502014-3,4174DD3).
Author contributionsYu He and Junping Wang designed and supervised all experiments. Mo Chen and Ping Zheng provided auxiliary reagents and animal purchases. Ying Hu, Jiejuan Lai, YuJun Zhang and Ling Shuai assisted with animal and molecular biology experiments. Lang Gan and Yan Jiang analysed the data. Shuguang Pan completed most of the experiments and drafted the manuscript. All the authors have read and discussed the final manuscript.
Data availability statementThe datasets used during the current study are available from the corresponding author upon reasonable request.
We thank the Institute of Hepatobiliary Surgery of Southwest Hospital for providing human cholangiocarcinoma tissue.