Non-alcoholic fatty liver disease (NAFLD) represents a global public health burden. Despite the increase in its prevalence, the disease has not received sufficient attention compared to the associated diseases such as diabetes mellitus and obesity. In 2020 it was proposed to rename NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD) in order to recognize the metabolic risk factors and the complex pathophysiological mechanisms associated with its development. Furthermore, along with the implementation of the proposed diagnostic criteria, the aim is to address the whole clinical spectrum of the disease, regardless of BMI and the presence of other hepatic comorbidities. As would it be expected with such a paradigm shift, differing viewpoints have emerged regarding the benefits and disadvantages of renaming fatty liver disease. The following review aims to describe the way to the MAFLD from a historical, pathophysiological and clinical perspective in order to highlight why MAFLD is the approach to follow.
Nowadays, metabolic dysfunction-associated fatty liver disease (MAFLD) is the most common cause of chronic liver disease (CLD), affecting one-quarter up to one third of the worldwide adult population [1,2]. The term nonalcoholic fatty liver disease (NAFLD) was originally used 37 years ago to define fatty liver disease (FLD) unrelated to excessive alcohol intake that has been conceptualized as a minority outlier group of patients at that time [3,4]. Although new knowledge has been developed, and the soaring burden of the disease has been expanded, this primitive term and accompanied diagnostic criteria have remained in use.
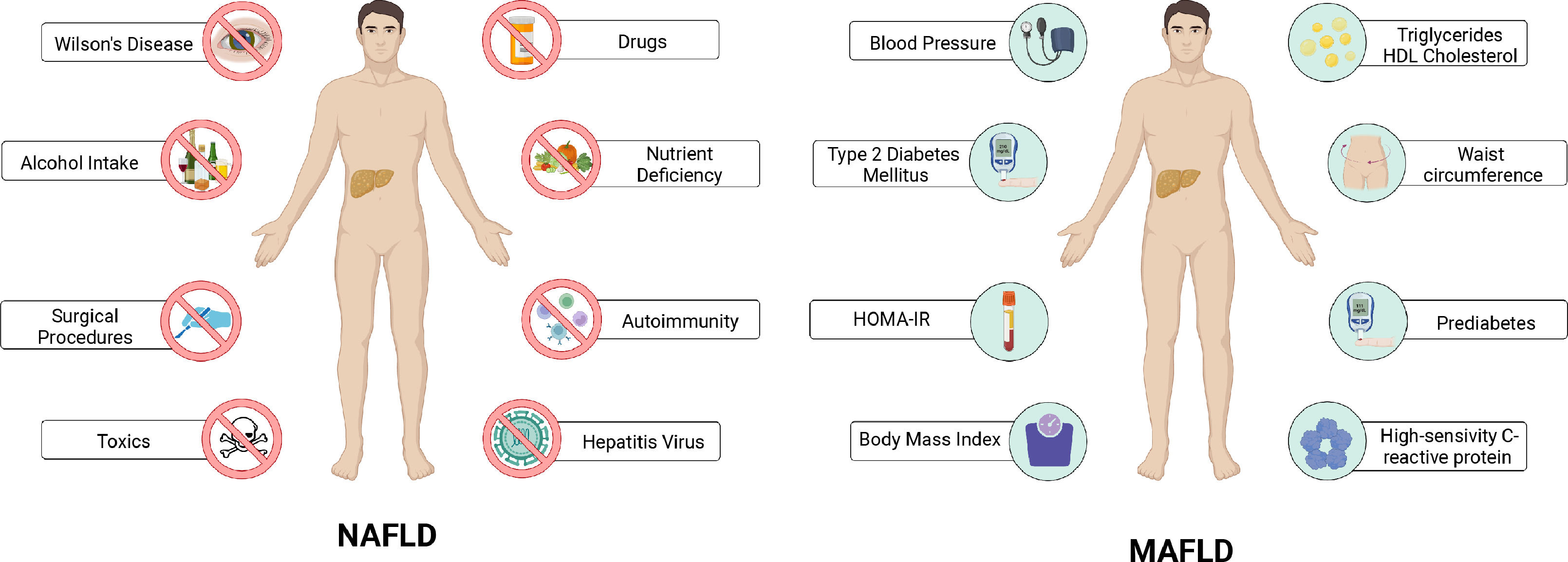
In a paradigm shift, the term MAFLD and associated simple positive criteria were conceptualized as an outcome of a consensus-driven process by an international panel of experts in 2020 [5–7]. The set of MAFLD positive diagnostic criteria includes overweight or obesity, type 2 diabetes, or evidence of metabolic dysfunction [6]. These changes aim to reflect the complexity of the disease pathophysiology as well as the central role of the underlying metabolic factors (Fig. 1). This review aims to highlight the historical basis of this change and the role of metabolic dysfunction in FLD.
Factors considered for the diagnosis of NAFLD and MAFLD. Evidence of hepatic steatosis (intrahepatic fat of at least 5% of liver weight) by imaging, histology or serum biomarkers is required to integrate the diagnosis of non-alcoholic fatty liver disease (NAFLD). In addition, the presence of any other cause of chronic liver disease must be ruled out. In contrast to NAFLD, the diagnosis of metabolic-associated fatty liver disease (MAFLD) is integrated by the combination of different clinical and biochemical parameters that evidence metabolic dysregulation in the patients (Created with BioRender).
Medical terms, as well as the names of the multiple and diverse diseases we currently know, have been subject of many changes and modifications through time and history, when there is a need for this. NAFLD is not an exception. In the 19th century Thomas Adisson, a renowned physician, described for the first-time liver histological changes related to FLD in patients with a background of excessive alcohol intake [8]. In the subsequent years, pathologists from different parts of the world reported numerous cases of liver histologic changes mostly in obese and diabetic patients. These histologic changes were characterized by the presence of liver fat accumulation, perilobular fibrosis and cirrhosis [9]. Thereafter, many names for this disease emerged based on the histological characteristics of the disorder. Including fatty infiltration of the liver, hepatic steatosis, fatty liver hepatitis and cirrhosis, among others [9].
Nonetheless, it was until 1980 that Ludwig et al. introduced the term nonalcoholic steatohepatitis (NASH) for the first time, by finding histological alterations marked by lobular hepatitis, focal necrosis, inflammatory infiltrate, and Mallory bodies in liver biopsies from patients who denied excessive alcohol intake [10,11]. Importantly, most of these patients were obese and had obesity-related diseases. Lastly, the term NAFLD was introduced in 1986 by Schaner & Thaler to describe a lighter form of liver steatosis [12]. Simultaneously, in those patients with such histological features, systemic metabolic alterations were also observed. Some of these included obesity, hyperlipidemia, hyperglycemia, hyperinsulinemia, and hypertension [9].
1.1.2NAFLD and metabolic diseases, the development of MAFLDWith the arrival of the 21st century, concerns about the use of NAFLD surfaced. In the previous years many proposals for renaming the disease were made (e.g. bright liver syndrome [13], non-alcoholic steatosis syndromes [14], etc.). Each of them highlighted the clinical and histological features that had been identified over the course of time. Nevertheless, not even one emphasized the different metabolic factors that were early identified and associated with the disease [15]. The term MAFLD emphasizes the role that metabolic dysfunction has in the disease.
2Defining metabolic dysfunctionNowadays, there is not an universal consensus on the definition of metabolic dysfunction. Although some authors have proposed a definition based on the criteria for metabolic syndrome (MetS) [16]. The term MetS refers to the co-occurrence of different known cardiovascular risk factors, including insulin resistance, obesity, atherogenic dyslipidemia, and hypertension [17]. The association between various types of metabolic disorders was first described a century ago, including hypertension, hyperglycemia, and hyperuricemia [18]. Years later, it was described that obesity, principally male or android phenotype obesity, was associated with cardiovascular disease (CVD) and type 2 diabetes mellitus [19]. With the course of time, the associations between adiposity, metabolic alterations (insulin resistance, hyperinsulinemia, high plasma triglycerides and low HDL cholesterol levels and hypertension) and CVD were strongly documented [9]. It was not until 1988 that Reaven named these associations as Syndrome X [20]. In 1999 the world health organization (WHO) proposed the term MetS and diagnostic criteria based on insulin resistance [21]. Nevertheless, the criteria for MetS proposed by the National Cholesterol Education Program (NCEP) were chosen to harmonize the definition of MetS worldwide [22]. In the middle of 2023, there is not a universal consensus or definition about metabolic health [23]. Some definitions are supported by the absence of diagnostic criteria for MetS [24–26], meanwhile others use insulin sensitivity to define it [26,27].
The sedentary lifestyle, intake of hypercaloric and nutritionally unbalanced diets and related environmental factors, along with genetics and epigenetics contribute to progressive development of MetS [28–30]. Multiple pathophysiological mechanisms are implicated in MetS. Insulin resistance, adipose tissue dysfunction, gut dysbiosis and macrophage activation and chronic inflammation have been proposed to be key players in MetS [31].
3Understanding metabolic dysfunction in MAFLDAt the end of the 1990s Day proposed the "two-hit hypothesis" to explain the pathophysiology of MAFLD [32]. This proposed that the “first hit” of the disease is hepatic steatosis characterized by the accumulation of hepatic triglycerides and insulin resistance. Once the “first hit” is established, it provides susceptibility for the “second hit” to develop. The” second hit” involves the interaction of various processes and molecules which include proinflammatory cytokines, adipokines, mitochondrial dysfunction and oxidative stress leading eventually to necroinflammation, fibrosis and lastly to cirrhosis [33]. The two-hit hypothesis did not capture the complex relationship among environmental, genetic and metabolic factors and the development and progression of MAFLD. This led to the introduction of the multiple-hit hypothesis (Fig. 2). Which reflects the importance of metabolic dysfunction, nutritional factors, gut microbiota and genetic and epigenetic factors in the development of MAFLD [34,35].
The multiple hits theory. The multiple hits theory highlights the diverse factors associated with metabolic-associated fatty liver disease (MAFLD) and their interaction in the development and progression of the disease. Such factors extend from the dietary habits of individuals to the activation of complex signaling pathways. Providing an overview of the complex pathophysiology of MAFLD (Created with BioRender).
The connection between MetS and MAFLD has been evidenced. Technological advances have allowed us to recognize the pathophysiological mechanisms involved in this association and determine how obesity, MetS and MAFLD interact with each other [12]. Obesity is a complex disease resulting from interactions between environmental, genetic, socioeconomic, and internal factors of each individual. The prolonged state of imbalance of energy uptake and energy expenditure leads to metabolic alterations, mainly in adipose tissue [36]. The metabolic alterations induced by this disease, nutrient overload and a sedentary lifestyle have been associated with the development of MAFLD [37–39].
Recently, it has been observed that alcohol intake also plays an important role in the development of MAFLD, due to the fact that moderate drinkers are susceptible to develop liver steatosis. This increased risk is not necessarily attributable only to the direct toxic effects of ethanol. Although heavy alcohol intake is a well-known risk factor for alcoholic liver disease (ALD), recent studies have highlighted the role of increased caloric intake from alcohol as a significant contributing factor to MAFLD. Alcohol itself is high in calories, and its regular intake can contribute to a positive energy balance, leading to weight gain and metabolic disturbances. Furthermore, alcohol intake can induce insulin resistance and impair lipid metabolism, enhancing lipid synthesis and impairing fatty acid oxidation in the liver. These mechanisms, combined with individual susceptibility to MAFLD, may contribute to the development of liver steatosis even in individuals with moderate alcohol intake [40,41].
Under physiological conditions insulin is the hormone responsible for the inhibition of lipolysis and gluconeogenesis at the adipose tissue level in response to high levels of glucose in the blood. In the presence of insulin resistance (IR), that develops as a consequence of obesity, lipolysis inhibition is impaired. Consequently, there is an increase in circulating free fatty acids (FFA) that simultaneously leads to a greater (IR) [28]. The uncontrolled lipolysis in adipose tissue generates excessive mobilization of FFAs to the liver [42]. In the liver, the presence of FFAs promotes gluconeogenesis and lipogenesis de novo, key processes in the onset of MAFLD [43]. In addition, IR induces an alteration in the production and secretion of adipokines and proinflammatory cytokines [36].
3.2LipotoxicityLipotoxicity corresponds to the dysregulation of lipid metabolism and the alterations in their intracellular composition that leads to accumulation of harmful lipids in the liver [44]. In response to elevated levels of circulating FFAs, hepatocytes increase the uptake of FFAs, which results in an increased accumulation of intrahepatic lipid droplets (hepatic steatosis). Increased intrahepatic FFA levels are the main trigger of lipotoxicity. Nevertheless, FFAs are not the only lipids implicated in lipotoxicity. It has been observed that triglycerides, free cholesterol, lysophosphatidyl cholines, ceramides and bile acid also have an important role in the development of lipotoxicity and its deleterious effects on liver cells [45]. The accumulation of harmful lipids triggers the liver damage mechanisms characteristic of lipotoxicity, such as endoplasmic reticulum (ER) stress, oxidative stress, mitochondrial dysfunction and alteration of the electron transport chain, generating an excessive production of reactive oxygen species (ROS), leading to the development of steatohepatitis and liver fibrosis.
The unsuccessful disposal of excess FFA by hepatocytes leads to lipoapoptosis, an active process of steatohepatitis [46]. Apotosis in hepatocytes can occur through an intrinsic pathway activated by intracellular stress (oxidative stress, endoplasmic reticulum (ER) stress and mitochondrial permeabilization) in which multiple genes involved in different apoptosis signaling pathways are up-regulated, including p53 upregulated modulator of apoptosis (PUMA), PKR-like ER kinase (PERK), c-Jun NH2-terminal kinase-1 (JNK), CCAAT/enhancer-binding homologous protein (CHOP) and Bcl-2 interacting mediator (BIM). Or through an extrinsic pathway activated by cell death-associated ligands, both pathways culminate in the activation of caspases and finally in the hepatocyte death [45].
These alterations trigger the activation of Kupffer cells, responsible for liberating proinflammatory cytokines and initiating an inflammatory process. Ultimately the inflammatory process and ROS stimulate hepatic stellate cells, which in response excessively produce extracellular matrix leading to hepatic fibrosis [47,48].
In addition, the liver is not the only tissue affected by lipotoxicity. Adipose tissue, skeletal muscle, heart and pancreas are identified as targets of lipotoxicity. In muscle, lipotoxicity is associated with insulin resistance and an increase in intramyocellular lipids [43]. Furthermore, patients with MAFLD have abnormal endothelial function associated with high levels of circulating lipids and aminotransferases, which confers susceptibility to the development of cardiovascular disease (CVD) [49].
3.3The role of programmed cell death in MAFLDMinerals such as iron are crucial for maintaining the body's oxidative/redox balance. Recently, impaired mineral homeostasis has been observed in patients with MAFLD. Hepatic iron accumulation has been identified in patients with MAFLD. Iron accumulation produces liver injury through the release of free radicals (Fe2+) and generation of ROS produced during the Fenton's reaction enhanced the inflammatory response oxidative stress and the occurrence of ferroptosis [50]. Ferroptosis is defined as a form of programmed cell death caused by iron-dependent lipid peroxide accumulation, which leads to mitochondrial membrane injury. Ferroptosis can be triggered by two pathways, by disruption of the system Xc−/GSH/GPX4 axis and by lipid peroxidation caused by the increase in Fe2+ levels and the enhancement of arachidonic acid metabolism. Finally, the two pathways converge in the production of lipid peroxides. The accumulation of lipid peroxides leads to an increased formation of lipid droplets and eventually to ferroptosis, causing exacerbation of MAFLD and contributing to disease progression [51,52].
Pyroptosis is a type of programmed cell death caused by inflammasomes. There are three types of pyroptosis, each one is triggered by a different signaling pathway. The canonical pathway is activated when inflammasome sensors, such as NOD-like receptor family and pyrin domain-containing-1 and 3 (NLRP1, NLRP3) are stimulated by pathogens, pathogen-associated molecular patterns (PAMPs), and DAMPs. As a consequence, CASP1 is recruited and activates Gasdermin D (GSDMD) [53]. Activated GSDMD binds to membrane phospholipids and initiates pore formation. Presence of pores in the cell membrane allows release of intracellular proteins, ion decompensation, water influx, and cell swelling, leading eventually to cell death [54]. Recently, it has been studied how pyroptosis influences the development and progression of MAFLD. Activated GSDMD induces the expression of proinflammatory cytokines, activates NF-kB signaling pathway, increases lipogenesis and decreases lipolysis [55]. Furthermore, unrepressed NLRP3 activation has been found to increase inflammation and promote HSC activation [54]. The combination of these mechanisms contributes to the development of steatohepatitis and liver fibrosis. Nevertheless, further evidence is still being searched to elucidate the underlying mechanisms linking pyroptosis and MAFLD.
4Implications of renaming fatty liver diseaseNow we can realize this change in FLD terminology is supported by the historic and current knowledge of the disease. Over the last few years, there has been an increase in the worldwide prevalence of MAFLD, most recently reported at 29.8% [56]. FLD is traditionally associated with excessive alcohol intake, the diagnosis of this disease according to the American Association for the Study of Liver Diseases (AASLD) is based on the exclusion of other causes of CLD, mainly excessive alcohol intake [57]. Recent evidence suggests that MAFLD can develop as a result of various factors, including total parenteral nutrition (TPN), viral infections of the liver tissue, and exposure to xenobiotics. These findings highlight the importance of reclassify the current terminology surrounding FLD. While the term NAFLD has been widely used, the evolving understanding of MAFLD encompasses a broader range of causative factors. TPN, a life-saving method of providing nutrition intravenously, can inadvertently contribute to metabolic dysfunction and subsequent MAFLD due to the excessive supply of nutrients, particularly glucose and lipids [58]. Furthermore, viral infections such as hepatitis B and C can induce liver inflammation and metabolic dysregulation, leading to the development of MAFLD [59]. Additionally, exposure to xenobiotics, including drugs, environmental toxins, and certain chemicals, can disrupt liver function and trigger metabolic dysfunction [60]. A more comprehensive classification system would facilitate accurate diagnosis, appropriate management, and targeted interventions for individuals affected by MAFLD. With the renaming of NAFLD to MAFLD and the implementation of the new positive criteria for diagnosis, a higher prevalence of the disease is expected [61]. Making this disease a worldwide public health problem.
4.1MAFLD in the context of NAFLDMultiple research studies have been undertaken to evaluate and validate the use of MAFLD instead of NAFLD. These studies have robustly shown the superior utility of the MAFLD criteria in identifying high risk patients compared to the former NAFLD criteria [62–66]. It has observed that patients who meet the definition of MAFLD tend to have a more severe fibrosis, higher risk of disease progression, higher risk of extra-hepatic complications and mortality compared to those who meet the NAFLD definition [65].
The spectrum of clinical MAFLD extends from patients with low or normal BMI who are metabolically dysfunctional to metabolically dysfunctional overweight/obese patients. A study conducted in 2018 showed that metabolically unhealthy patients have a higher amount of steatohepatitis and fibrosis despite their BMI. Furthermore, the prevalence of these more severe forms of MAFLD was observed to be similar among non-obese and obese patients [67]. The renaming of the disease is intended to encompass all affected individuals, especially for those with high risks of metabolic disorders, regardless of their BMI [68]. Although the advantages of using MAFLD have been identified, there is a lot of controversy about its use [69].
The MAFLD definition has been endorsed by multiple stakeholders from different medical disciplines and fields of health sciences from more than 134 countries around the world [70]. Despite this self-speaking evidence, a debate rebound and several questions have been raised particularly regarding the methodology that was used to develop the consensus.
4.2MAFLD: the new approach for metabolic syndrome and fatty liverRecent studies have been investigating the use of machine-learning algorithms and artificial intelligence (AI) approaches to predict and diagnose metabolic syndrome, as well as its association. These innovative techniques have shown great potential in identifying robust prediction and diagnostic markers for both conditions. By leveraging large-scale datasets and sophisticated algorithms, machine learning models can analyze diverse clinical and genetic factors associated with metabolic syndrome and MAFLD. These factors include anthropometric measurements, blood biomarkers, liver function tests, genetic variants, and imaging data. The integration of machine learning and AI approaches allows for the development of accurate prediction models that can identify individuals at risk of developing metabolic syndrome and subsequent MAFLD. These models also provide valuable insights into the underlying mechanisms and pathophysiology of both conditions. By identifying high-risk individuals and understanding the complex interplay between metabolic syndrome and MAFLD, these studies offer opportunities for early intervention, personalized treatment strategies, and improved patient outcomes [71–74].
4.3MAFLD: the perspective of patientsFor decades the term NAFLD has contributed to stigma, trivialization and confusion among NAFLD patients. Such factors have been observed to negatively affect patient´s life quality, adherence to treatment and the individual's perception of the disease. Increased disease burden reflects the bidirectional interactions existing between FLD and metabolic disorders and diseases. Generating a positive impact on the perception and understanding of the disease [75]. Removing the word "alcohol" from the FLD definition has been found to reduce stigmatization among patients about the disease. Compared to NAFLD, it has been observed that MAFLD increases awareness of patients about the disease and the risks associated with it [76,77]. Empowering patients and physicians to acquire a clearer picture of the disease is the cornerstone of improving the care and management of patients with MAFLD.
5ConclusionsThe relationship between FLD and metabolic alterations has been evidenced since decades ago. In the last few years, the mechanisms by which this relationship is produced have been understood. Recognizing the heterogeneity of factors associated with MAFLD as well as the broad spectrum of clinical manifestation is only the first step in our understanding of this complex disease. In this review we have addressed the change from NAFLD to MAFLD from a historical, pathophysiological and clinical perspective in order to explain why this change is needed. The evidence strongly demonstrates the positive impact of the use of MAFLD in day-to-day clinical practice. Acknowledging the underlying metabolic factor in MAFLD is crucial to address the burden of the disease worldwide. The research field is now wide open and the use of MAFLD will contribute to explore new areas of knowledge.
Author contributionsRamírez-Mejía M: study concept and design, literature review, drafting of the manuscript. Qi X: study concept and design, literature review, drafting of the manuscript. Abenavoli L: study concept and design, literature review, drafting of the manuscript. Romero-Gómez M: study concept and design, literature review, drafting of the manuscript. Eslam M: study concept and design, literature review, drafting of the manuscript. Méndez-Sánchez N: study concept and design, literature review, drafting of the manuscript, integrity of the work and critical revision of the manuscript for important intellectual content.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.