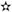Preventive effect of metformin in hepatocellular carcinoma (HCC) is not entirely clear. We aimed to evaluate the use of metformin as a protective factor of HCC in diabetic patients.
.We carried out an electronic search on PUBMED/MEDLINE, Web of Science and LILACS databases, with no limit of date, from April 2017 to January 2019. Eligible studies included cohort and case–control studies. We adressed data about the use of metformin on the risk of HCC development. Two independent reviewers extracted the data. We evaluated the quality of studies by using the Newcastle–Ottawa scale and carried out a meta-analysis using random-effects models.
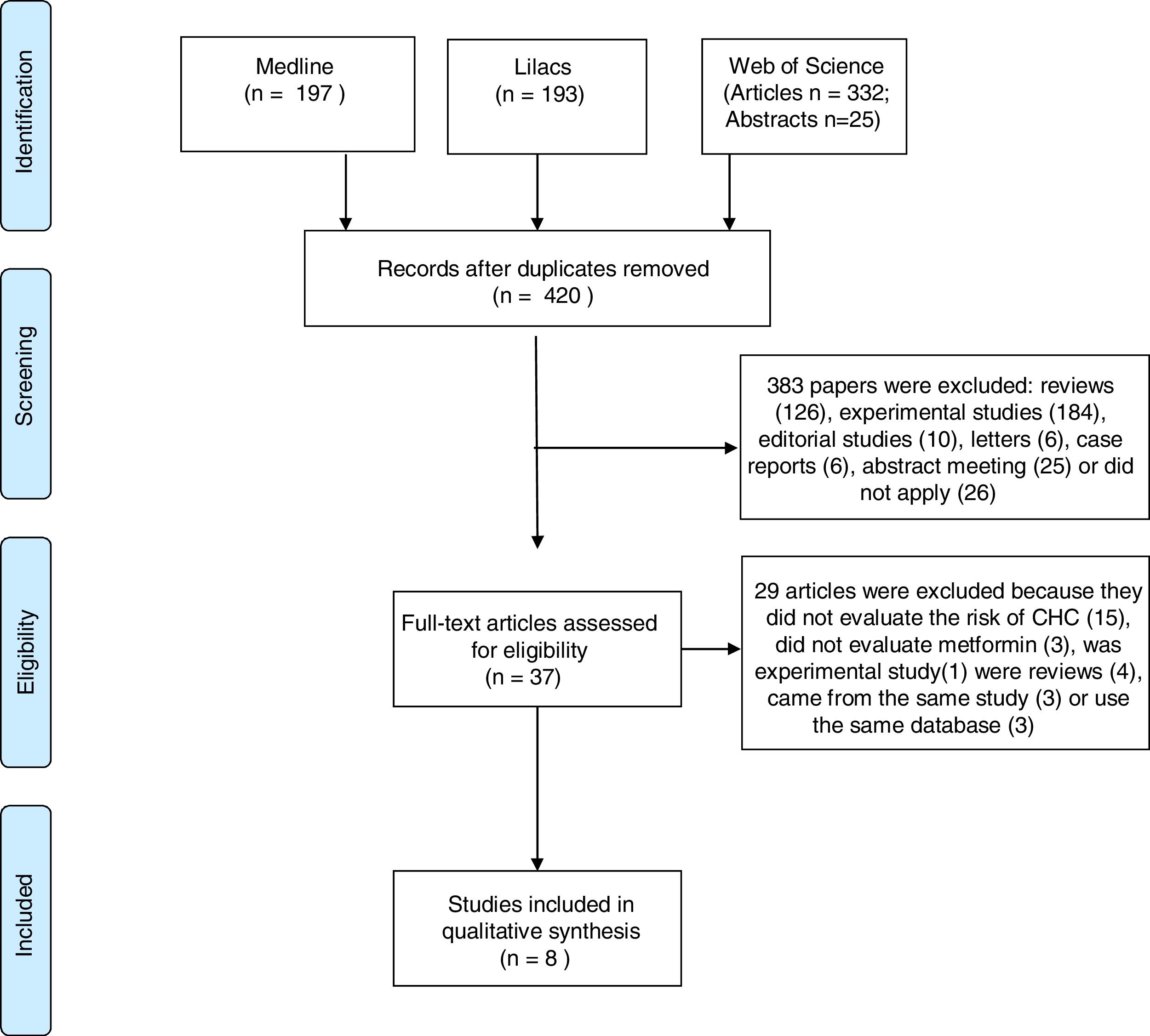
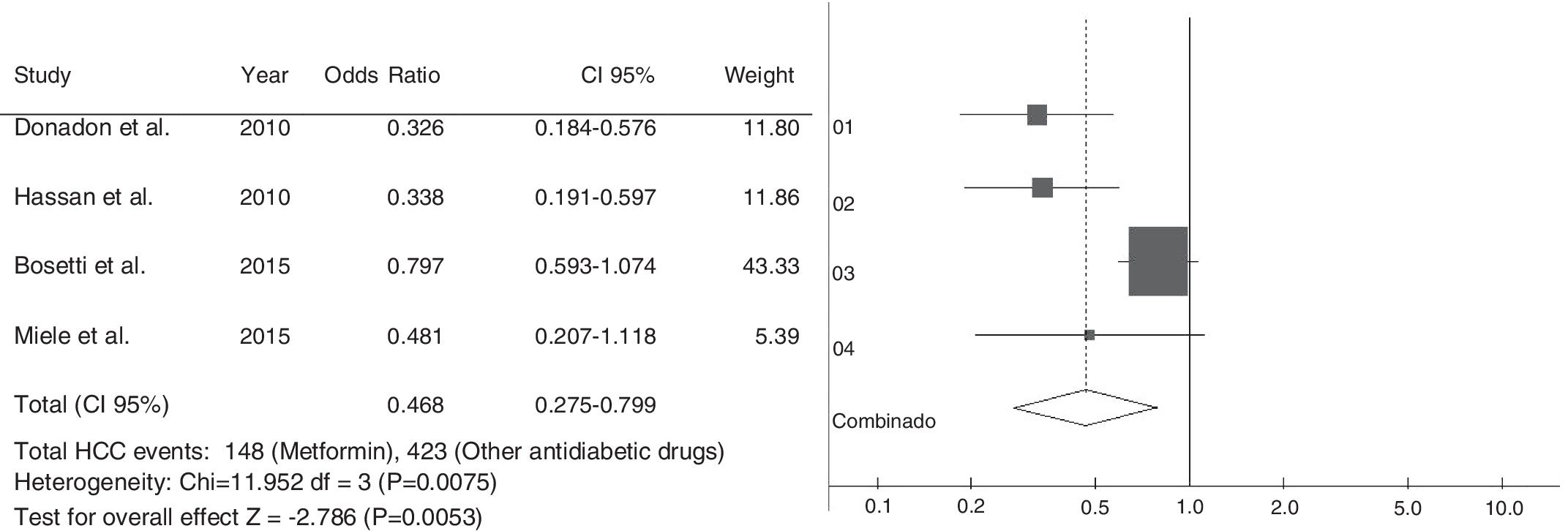
.The electronic searches identified 747 studies. After reading abstracts and titles, we excluded 327 duplicated papers and 383 irrelevant references. Eight studies were selected; four case–control and four cohort studies. All studies have observed that the therapy with metformin was associated with a lower risk of HCC, compared with non-metformin therapy. Five articles reported that patients treated with insulin, or insulin secretagogues, presented increased risk of HCC compared to those treated with metformin. One study found that not only statin but also aspirin reduced the risk of HCC, if combined with metformin. A meta-analysis, using the case–control studies, found a combined Odds Ratio of 0.468; 95% CI 0.275–0.799 for the association between HCC and the use of metformin.
.The use of metformin was associated with a reduced risk of HCC, and it may be a relevant factor for preventing HCC in diabetic patients.
Hepatocellular carcinoma (HCC) is a primary hepatic cancer that arises from a mutation of cellular genes, causing the cells to multiply in a disorderly and aberrant way. This mutagenic effect may be triggered by external agents, such as hepatotropic viruses or by excessive cellular multiplication, as it occurs in the regeneration and repair processes stimulated by successive hepatocellular lesions, interfering with and modifying DNA synthesis [1].
HCC has been diagnosed every year in more than half a million people around the world. It is an aggressive tumor and the most frequent liver cancer around the world. HCC also is the fifth most frequent type of cancer in males and the seventh in females, representing the third main cause of cancer death [2]. Among the main causes of HCC are alcoholic cirrhosis, infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), and non-alcoholic fatty liver disease (NAFLD) [3–5]. Obesity and type 2 diabetes mellitus (T2DM) are considered an independent risk factor for HCC [6]. Some studies have shown that patients with T2DM have a substantially increased risk of HCC regardless of potential confounding variables such as obesity, alcoholism and chronic viral hepatitis [6–8]. One fact that closely links T2DM to HCC is its association with NAFLD. Insulin resistance (IR) is one of the pathophysiological mechanisms of NAFLD, also strongly correlated with T2DM [9,10].
As an insulin-sensitizing drug, metformin improves insulin sensitivity, reducing plasma levels of this hormone, inhibiting hepatic gluconeogenesis and reducing glycogenolysis. Several studies have shown an inverse association with the use of metformin and the risk of developing solid tumors, such as prostate and pancreatic cancer [11–13]. Experimental studies have found evidence of the chemopreventive action of metformin from an antitumor perspective. However, the effect of this drug on HCC is not entirely clear, and further studies on the mechanisms of action of metformin in these HCC cells are needed [14].
This present review evaluated the use of metformin as a protective factor of HCC in diabetic patients.
2Material and methodsWe conducted a systematic literature review following a written pre-defined protocol based on the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [15]. We included clinical trials, observational studies; cohort or case–control studies addressing data on the association between the use of metformin and occurrence of hepatocellular carcinoma. We included papers in English, Portuguese, Spanish, Italian, French and German. We excluded review articles, updates, case reports, and editorial letters, as well as experimental studies with animals. Electronic searches were performed in databases including Medline, Web of Science, and Lilacs. The search terms included “hepatocellular carcinoma”[All Fields] AND (“metformin”[MeSH Terms] OR “metformin”[All Fields]). We reviewed included articles references and abstracts of major conferences. We had no date restriction when performing databases search and used the PRISMA checklist as guidance to conduct the review [15].
Two authors independently performed the search and extracted data in accordance with the inclusion and exclusion criteria previously determined. A third author validated data extraction, and disagreements were sorted out by consensus or by the third reviewer. Data included the country of origin, study design, the period of study, place of study, number of cases and controls, outcome measures, and adjusted confounders. We used the Newcastle Ottawa Scale (NOS) [16] to evaluate the risk of bias and quality of the included studies. According to NOS scale, we evaluated selection, comparability, and outcome of studies, and the maximum score for each study is 9. Studies having less than 5 points were excluded due to the high risk of bias [16]. We used BioEstat 5.0 to perform meta-analysis of included studies. We used the chi-square test to determine the presence of statistical heterogeneity. Due to the clinical heterogeneity of studies, we used random effects models (the DerSimonian-Laird random-effects test). Since our meta-analysis included fewer than 10 studies, we did not used tests for funnel plot asymmetry.
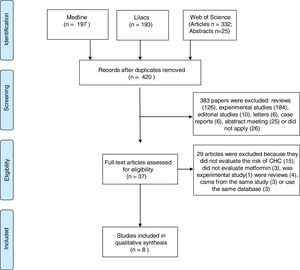
3ResultsOur search identified 747 studies. After the analyses of titles and abstracts of retrieved papers, we excluded 327 duplicated articles, and 383 according to inclusion criteria. Of the 383 excluded studies, 126 were reviews, 184 were experimental studies, 16 were editorial material, six were case reports, 26 clinical studies and 25 meeting abstracts were out of the scope of this review. Of the clinical studies, five were clinical trials; five case–control studies, 14 cohort studies, and two guidelines. None of them evaluated the effect of metformin on HCC prevention. All 37 selected papers were submitted to a full-text appraisal. Of these, 29 articles were excluded: 15 did not assess the risk of HCC, three did not evaluate metformin alone, one experimental study and four reviews which were not identified previously due to the absence of data in the abstract. Four studies used the same cohort data, and period. Out of these four studies, we included only the one with higher NOS scale score [16]. The other three studies were excluded (Fig. 1).
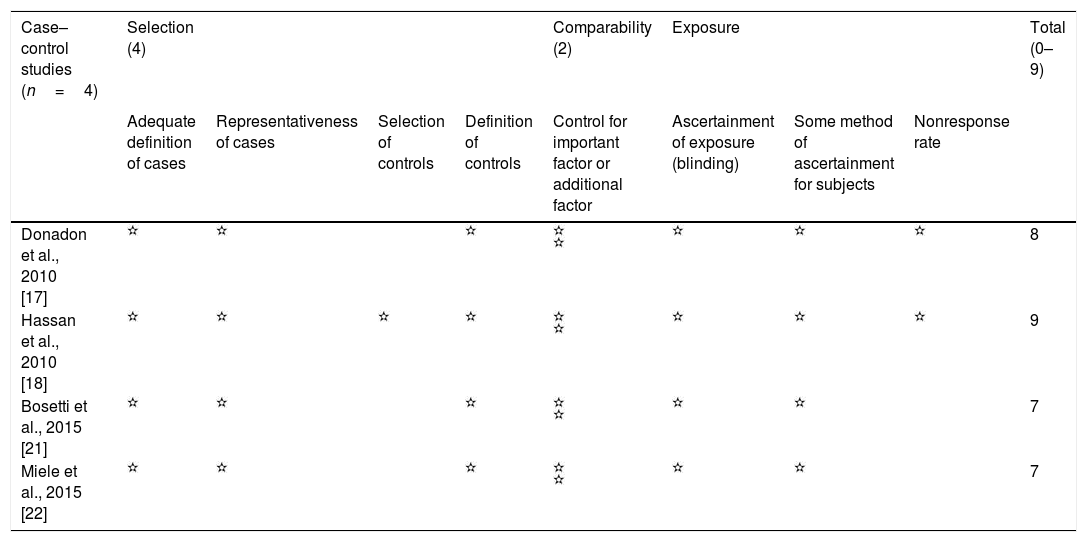
Eight observational studies were selected; four case–control studies [17,18,21,22] and four cohort studies, two retrospective cohorts [23,24] and two prospective cohorts [19,20]. Four studies were population-based [20,21,23,24], while four studies [17–19,22] used hospital populations. Three out of the four population-based studies, used national databases encompassing individuals from all over the country, while one study [21] used a regional database to collect information about included patients. All included study presented NOS score≥7 (Table 1).
Evaluation of quality of included studies by Newcastle-Ottawa scale.
| Case–control studies (n=4) | Selection (4) | Comparability (2) | Exposure | Total (0–9) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for important factor or additional factor | Ascertainment of exposure (blinding) | Some method of ascertainment for subjects | Nonresponse rate | ||
| Donadon et al., 2010 [17] | 8 | ||||||||
| Hassan et al., 2010 [18] | 9 | ||||||||
| Bosetti et al., 2015 [21] | 7 | ||||||||
| Miele et al., 2015 [22] | 7 |
| Cohort studies (n=4) | Selection (4) | Comparability (2) | Exposure | Total (0–9) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Control for important factor or additional factor | Ascertainment of outcome | Follow-up long enough form outcome s to occur | Adequacy of follow-up of cohorts | ||
| Nkontchou et al., 2012 [19] | 8 | ||||||||
| Ruiter et al., 2012 [20] | 7 | ||||||||
| Kasmari et al., 2017 [23] | 8 | ||||||||
| Tseng et al., 2018 [24] | 8 |
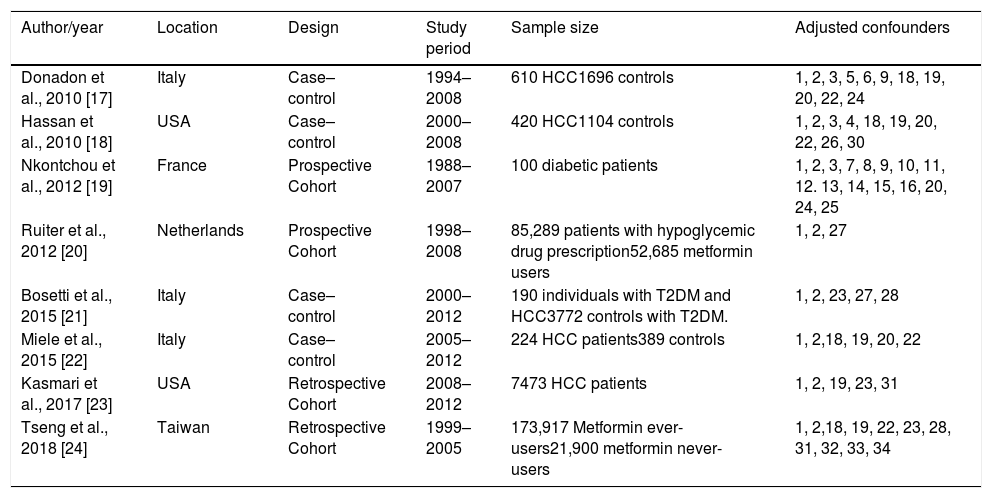
Table 2 shows the characteristics of included studies, including author, year of publication, study design, studied population, and adjusted confounders of each study. Most studies adjusted their results for the following confounding variables: age (8/8), gender (8/8), alcohol intake (4/8), HCV (4/8) and HBV (4/8).
Design and characteristics of included studies.
| Author/year | Location | Design | Study period | Sample size | Adjusted confounders |
|---|---|---|---|---|---|
| Donadon et al., 2010 [17] | Italy | Case–control | 1994–2008 | 610 HCC1696 controls | 1, 2, 3, 5, 6, 9, 18, 19, 20, 22, 24 |
| Hassan et al., 2010 [18] | USA | Case–control | 2000–2008 | 420 HCC1104 controls | 1, 2, 3, 4, 18, 19, 20, 22, 26, 30 |
| Nkontchou et al., 2012 [19] | France | Prospective Cohort | 1988–2007 | 100 diabetic patients | 1, 2, 3, 7, 8, 9, 10, 11, 12. 13, 14, 15, 16, 20, 24, 25 |
| Ruiter et al., 2012 [20] | Netherlands | Prospective Cohort | 1998–2008 | 85,289 patients with hypoglycemic drug prescription52,685 metformin users | 1, 2, 27 |
| Bosetti et al., 2015 [21] | Italy | Case–control | 2000–2012 | 190 individuals with T2DM and HCC3772 controls with T2DM. | 1, 2, 23, 27, 28 |
| Miele et al., 2015 [22] | Italy | Case–control | 2005–2012 | 224 HCC patients389 controls | 1, 2,18, 19, 20, 22 |
| Kasmari et al., 2017 [23] | USA | Retrospective Cohort | 2008–2012 | 7473 HCC patients | 1, 2, 19, 23, 31 |
| Tseng et al., 2018 [24] | Taiwan | Retrospective Cohort | 1999–2005 | 173,917 Metformin ever-users21,900 metformin never-users | 1, 2,18, 19, 22, 23, 28, 31, 32, 33, 34 |
1, age; 2, gender; 3, BMI; 4, race; 5, cholesterol; 6, triglycerides; 7, creatinine clearance; 8, GGT; 9, ALT; 10, AST; 11, plaques; 12, prothrombin activity; 13, albumin; 14, bilirubin; 15, AFP; 16, steatosis; 17, cirrhosis, 18, HBV; 19, HCV; 20, ethanol intake; 21, NAFLD; 22, smoking; 23, T2DM therapy; 24 –T2DM duration; 25, HbA1c; 26, family history of cancer; 27, another medication intake; 28, cardio/cerebrovascular disease; 29, Charlson comorbid index; 30, educational level; 31, country; 32, occupation; 33, T2DM complications; 34, potential risk factors for cancer.
None of the selected studies had totally excluded patients with positive serology for hepatotropic viruses. Two studies excluded patients with HBV but maintained HCV patients [19,23]. Six studies used information recorded in systematized databases to obtain information about the use of metformin [17,20–24]. One study used a personal interview and questionnaire application [18] and another used routine cohort management [19].
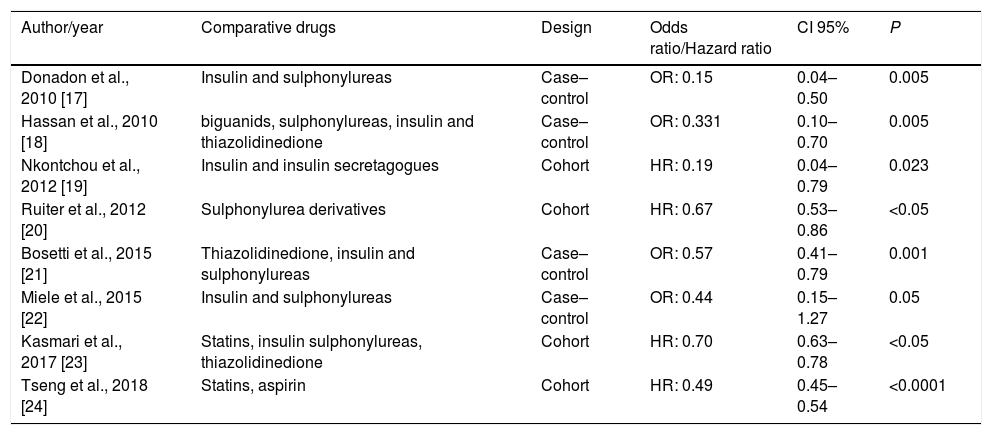
All included studies had reported reduced risk of HCC associated with metformin use (Table 3). The eight selected studies compared the influence of metformin use on the risk of HCC in relation to other drugs. Seven articles [17–23] compared the action of metformin with other insulin-sensitizing drugs and six of them observed the influence of the use of insulin secretagogues and exogenous insulin on the risk of HCC [17,18,20–23]. Compared with non-metformin therapy, metformin therapy was associated to a greater reduction in the risk of HCC in diabetic patients. Five articles found that insulin therapy increased the risk for HCC [17,18,21–23]. Three studies observed that therapy with insulin secretagogues, such as sulphonylureas, was associated with increased risk of HCC compared to those using metformin [17,18,21], while three others did not observe significant differences in the risk for HCC in users of sulphonylureas [20,22,23]. Two studies [23,24] observed that statins also had a protective action for HCC. Tseng [24] found that not only statin but also aspirin reduces the risk of HCC, especially if used in combination with metformin.
Comparative drugs and metformin therapies results.
| Author/year | Comparative drugs | Design | Odds ratio/Hazard ratio | CI 95% | P |
|---|---|---|---|---|---|
| Donadon et al., 2010 [17] | Insulin and sulphonylureas | Case–control | OR: 0.15 | 0.04–0.50 | 0.005 |
| Hassan et al., 2010 [18] | biguanids, sulphonylureas, insulin and thiazolidinedione | Case–control | OR: 0.331 | 0.10–0.70 | 0.005 |
| Nkontchou et al., 2012 [19] | Insulin and insulin secretagogues | Cohort | HR: 0.19 | 0.04–0.79 | 0.023 |
| Ruiter et al., 2012 [20] | Sulphonylurea derivatives | Cohort | HR: 0.67 | 0.53–0.86 | <0.05 |
| Bosetti et al., 2015 [21] | Thiazolidinedione, insulin and sulphonylureas | Case–control | OR: 0.57 | 0.41–0.79 | 0.001 |
| Miele et al., 2015 [22] | Insulin and sulphonylureas | Case–control | OR: 0.44 | 0.15–1.27 | 0.05 |
| Kasmari et al., 2017 [23] | Statins, insulin sulphonylureas, thiazolidinedione | Cohort | HR: 0.70 | 0.63–0.78 | <0.05 |
| Tseng et al., 2018 [24] | Statins, aspirin | Cohort | HR: 0.49 | 0.45–0.54 | <0.0001 |
Among selected cohort studies, two were prospective [19,20] and two retrospective cohorts [23,24]. All cohort studies had the NOS scale score higher than the minimal recommended one and were included in systematic analysis. However, only one study showed complete data [19]. The meta-analysis included case–control studies to evaluate the association between the use of metformin and development of HCC (Fig. 2). The chi-square test of heterogeneity was significant (p-value=0.0075). We used the DerSimonian–Laird random-effects test. The Odds Ratio was 0.468 95% Confidence Interval: 0.275–0.799 (p-value=0.0053).
4DiscussionThis systematic review found evidence that the use of metformin might reduce the risk of HCC in diabetic patients. All included studies [17–24] have found significant association between the use of metformin and lower risk of HCC, while the use of insulin [22,23] or sulphonylureas has been associated with a higher risk of HCC [17,18,21,22]. We evaluated the risk of bias by using the NOS scale, and all included study presented score higher than the minimal recommended score [16].
Literature describes type 2 diabetes mellitus as an independent risk factor for HCC [17], and that metformin reduces insulin resistance, as well as hyperinsulinemia [17]. Metformin treatment has been also associated with better outcomes in HCV cirrhotic patients with T2DM [19].
Metformin also is an insulin-sensitizing and anti-hyperglycaemic drug that promotes the reduction of plasma levels of this hormone and glucose in patients with hyperglycemia and hyperinsulinemia. It is through the activation of AMP-activated protein kinase (AMPK) that metformin reduces hepatic gluconeogenesis and increases the uptake of glucose by the skeletal muscles. Compared with exogenous insulin and insulin secretagogues, which raise plasma insulin levels and promote body weight gain, metformin has the benefit of stimulating moderate weight loss through the activation of AMPK [25,26].
The reduction generated by metformin in serum glucose levels in diabetic patients is mainly due to the reduction of hepatic gluconeogenesis and glycogenolysis [27,28]. In addition, it also increases insulin-stimulated glucose uptake into skeletal muscles, suppresses oxidation of fatty acids, and reduces triglyceride levels in patients with hypertriglyceridemia. All these effects can contribute to reduce hyperinsulinemia, improve hepatic insulin resistance, reduce steatosis, improve liver enzymes and reduce body weight [18].
Metformin treatment has been independently associated with decreasing the occurrence of HCC and liver-related deaths [19]. These observations are according a case–control study [17], which reported an 85% reduction in the chance of developing HCC in cirrhotic patients receiving metformin compared to patients using exogenous insulin and insulin secretagogues.
Some studies have suggested that the protective effect of metformin may have direct and indirect mechanisms [25–28]. The direct mechanism generated by metformin is the reduction of plasma insulin levels. Among the indirect mechanisms of inhibition of carcinogenesis, the induction of cellular apoptosis, the stimulation of the immune system, and the activation of AMPK are posed [26]. When in high intracellular concentrations in the liver, metformin can prevent the protein synthesis, cellular proliferation and angiogenesis through the activation of the AMPK pathway. AMPK is a key mediator of the tumor suppressor liver kinase B1 (LKB1) [29], working as a cellular energy sensor, essential for the metabolic process and can be suppressed in cancerous cells containing LKB1 function loss mutations or in cancer associated with the metabolic syndrome.
The prevalence of type 2 diabetes (T2DM) has increased worldwide [25] as well as obesity [26]. Both of which are risk factors for HCC and are closely associated. This association increases the risk of HCC developing. T2DM appears to precede HCC in most cases, being associated with substantial increase in risk occurrence [6,17–19]. The link between T2DM and HCC is shown by several studies [6,8,12].
Some included studies advocates the association of T2DM with an increased risk of HCC, regardless of confounding or metabolic variables, such as gender, age, BMI, hypertension, alcoholism, viral hepatitis, NAFLD and cirrhosis. Among the studies reviewed, several of them came from the Asian, European and American continents and the results remain consistent in different populations, different geographic areas and in a variety of control groups [17–22]. It has also been found that the duration of T2DM increases the risk of HCC [24] and that the concomitance of T2DM with obesity results in a synergy that increases substantially the risk [22].
Another important element is that T2DM is associated with the spectrum of NAFLD that has been considered one relevant risk factor for HCC [30,31]. Metformin may have a beneficial effect on NAFLD, enhancing liver enzymes and reducing weight, steatosis and maybe a drug protector against HCC in these patients [30,31]. NAFLD has elevated prevalence around the world and NASH (steatohepatitis) with fibrosis and cirrhosis has been considered a relevant risk factor for HCC. Since the control of viral hepatitis is in constant advance, in the next years, NAFLD and NASH will be the most frequent cause of HCC [31].
This review has some limitations. First, due to the limitation of resources, we could not include access-fee required databases that were not available by the Brazilian Government. Second, the selected studies were observational. Thus, the majority of patients exposed to metformin had some degree of insulin resistance, which prevented the evaluation of the protective effect of this drug on risk factors that were not related to T2DM. Besides, patients receiving metformin may have less severe diabetes when compared to patients receiving insulin. Gender and age were the only variables investigated in all studies. The studies did not adjust for the same set of confounding variables. Studies did not exclude patients with viral hepatitis since they are independent factors for HCC. Another limiting aspect is that only two studies have adjusted for the use of statins, which also represents a possible protective factor for HCC. Our meta-analysis included only four studies; therefore, we could not use tests for funnel plot asymmetry. When there are fewer than ten studies, the power of the tests is not able to distinguish chance from real asymmetry [32]. More prospective studies are necessary, especially clinical trials focusing on the use of metformin as a protective factor for HCC.
5ConclusionIn conclusion, despite the limitations, this systematic review shows that there is a strong association between the use of metformin and reduced risk of HCC.AbbreviationsHCC hepatocellular carcinoma hepatitis B virus hepatitis C virus non-alcoholic fatty liver disease type 2 diabetes mellitus Preferred Reporting Items for Systematic Reviews Newcastle Ottawa scale body mass index alanine aminotransferase alpha-fetoprotein confidence interval odds ratio AMP-activated protein kinase liver kinase B1
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Conflict of interestAll authors declared that there are no conflicts of interest.