Background and aim: Chronic hepatitis C infection, develops cirrhosis with all its complications, the arrival in our country of direct-acting antivirals (DAAs), gave the opportunity to have safer and more effective drugs.
Material and methods: Retrospective, cross-sectional study of patients who received AAD in hospitals in the Mexican Republic. 20 hospital centers. Variables analyzed: gender, age, genotype, degree of fibrosis, initial and final viral load of ADA treatment. SVR and side effects were documented. Descriptive statistics were performed.
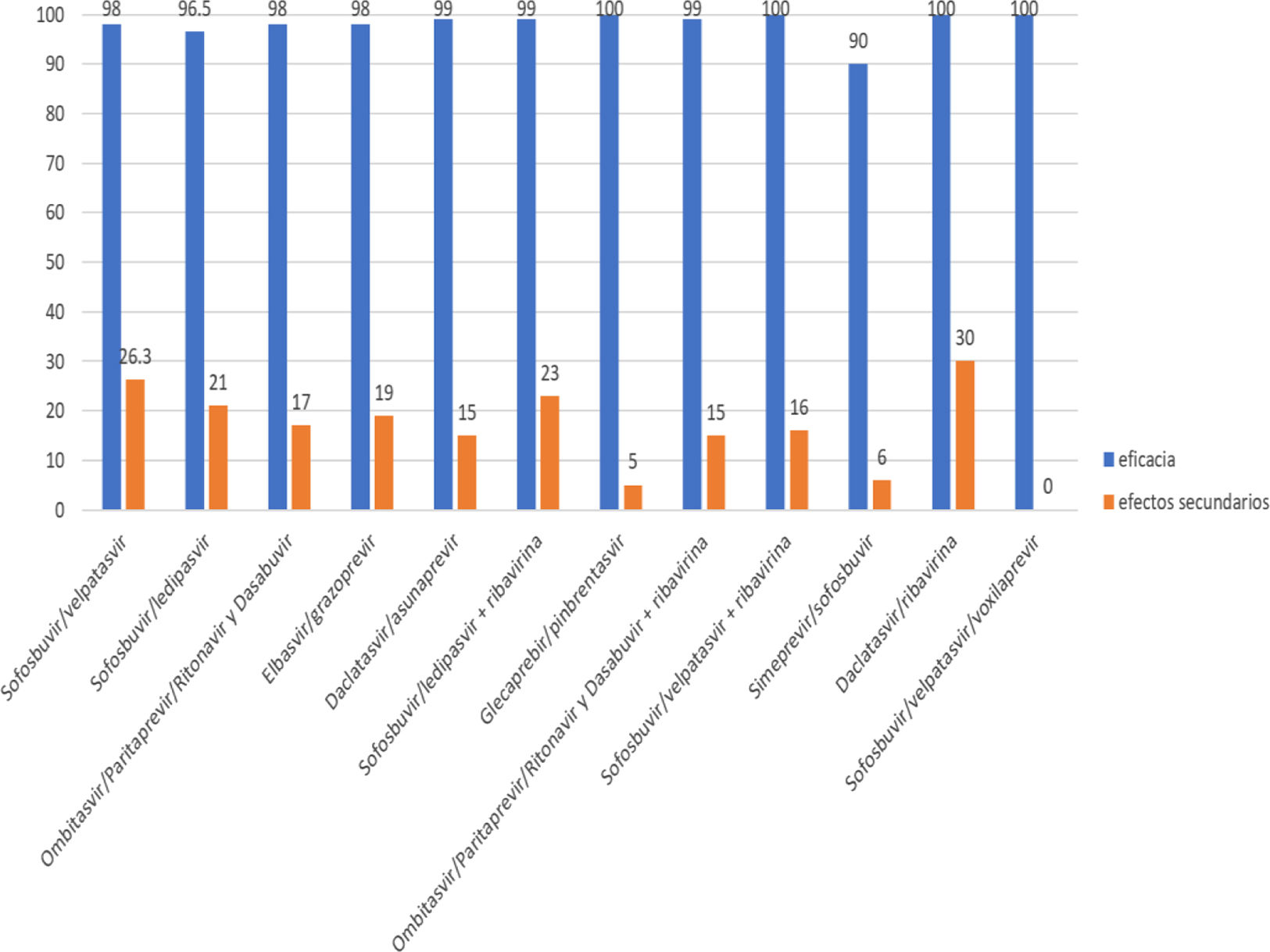
Results: Were included 813 patients, 529 women and 284 men, age 58.88±12.10 years were included. Genotype 1: 647 patients (1A: 316, 1B: 318, 1A / B: 11 and 1 A / C: 2), genotype 2: 145, genotype 3:19 and genotype 4: 2. By degree of fibrosis: F0: 93 F1: 88, F2; 86, F3: 95 and F4; 451. Patients with F4 (451), Child Pugh were classified as A: 363 and B: 88. From the Child Pugh group A 7 (1.9%) did not respond and from the group B 1 (1.1%). There were 561 näive and 252 no näive, the percentage that presented SVR was from 90 to 100%. The most frequent side effects: headache 16% and fatigue 22%, nausea (3%), muscle pain (1%), abdominal pain (1%), with ribavirin, anemia was documented in 22%.
Conclusions: Direct Action Antivirals is an effective and safe option in the Mexican population studied. Adverse events were not significant.
Conflicts of interest: The authors have no conflicts of interest to declare.