Introduction. Liver biopsy is a complementary method for diagnosis, staging and therapeutic guidance in liver diseases, where chronic viral hepatitis are the most acknowledged causes for the indication of histopa-thological study. The objective is to assess the patients’ profile as well as the indication and results of percutaneous liver biopsies in a tertiary hospital.
Material and methods. A descriptive, cross-section study was carried out through the review of medical charts (retrospective cohort) of patients submitted to blind percutaneous liver biopsies (PLB) at a hospital in Porto Alegre, South Brazil, from October 1993 to December 2011.
Results. 1,955 PLB were carried out, the mean patients’ age was 44.8 years old, and 1,127 (57.65%) were men. Chronic hepatitis C was the main indication (60.5%), followed by HCV-HIV coinfection (12.2%) and chronic hepatitis B (3.5%). Seven cases (0.3%) had complications, without deaths.
Conclusion. PLB is a safe method and continues to be an important option to assist patients with chronic liver disease.
The histopathological liver exam, through liver biopsy, has become a complementary method not only for diagnosis but also for staging, treatment control and/or prognostic determination of liver diseases.1
It is known that patients diagnosed with chronic viral hepatitis by the B (HBV) or C virus (HCV) are the highest contingent in population samples submitted to liver biopsies.2.–4 Besides them, patients presenting atypical course of liver diseases; changes in liver panel and/or in abdominal imaging without established diagnosis; suspected infiltrative, infectious or granulomatous diseases, can have their diagnosis established through a histopathological liver exam.1,5,6
In Brazil, treatment for hepatitis B and C is provided by the Public Health System – Brazilian Ministry of Health, and many cases depend on the fibrosis staging through liver biopsy to determine the indication for therapy.7,8
This study aimed to assess the indications, histo-pathological results and percutaneous liver biopsy complications carried out in a tertiary public hospital in Porto Alegre, Rio Grande do Sul – South Brazil, and whether there was a change in the profile of the patients submitted to the procedure over the last two decades.
Material and MethodsA descriptive cross-section study was carried out by reviewing medical charts (retrospective cohort) of patients blindly submitted to blind percutaneous liver biopsy (PLB) in a single center from Hospital Nossa Senhora da Conceição (HNSC), Brazil - a terciary attendance Hospital and reference for liver disease, from October 1993 to December 2011.
Data regarding gender, age, indication to liver biopsy, histopathological results and complications were assessed.
The blind PLB procedures were carried out by the same team during the aforementioned study period, with training of Medical Residents. The patients should present platelet count higher than 80,000/ mm3 and prothrombin time not higher than 3 seconds above control.9 Subjects were guided about the need to stop using drugs that potentially cause hemorrhagic diathesis 10 days before the procedure, in accordance to the guidelines of the American Association for Study of the Liver (AASLD).1 All PLB were carried out under local anesthesia with 2% li-docaine and 16G or 18G Menghini needle. After the procedures, the patients remained under observation for at least 2 h before discharging.
In the viral hepatitis assessment, the fibrosis staging was performed according to Metavir score.10
This study was approved by the Research Ethics Committee of HNSC and is in accordance with the governing rules of the National Health Council/Ministry of Health and its rules that regulate research involving human beings.
The obtained results were stored in confidential database, shared only by the researchers involved in the study. The data were analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows®, version 15. Values were considered statistically significant when p < 0.05.
ResultsFrom 1993 to 2011, 1,955 blind PLB were carried out. For the comparative assessment, two groups of patients were established, according to the period in which the PLB was performed. One group included the patients who had PLB between the years of 1993 and 2002 (1,037 patients) and the other the ones who had PLB between 2003 and 2011 (918 patients) (p > 0.05).
The mean age was 44.8 years old, and 1,127 (57.6%) patients were male. No statistically difference was found when the mean ages in both distinct periods were assessed, but male prevailed over female gender in the first assessed period (Table 1).
Regarding the main indications for PLB, infection by HCV was the most prevalent, accounting for a total of 1,184 (60.5%) cases. Patients with HCV-HIV co-infection represented 12.2% of the cases (238 patients), followed by HBV infection (69 patients -3.5%). Other indications totalized 12.2% of the cases (238 patients).
Statistically significant difference was observed when the indications for PLB in both distinct periods were compared (p < 0.001), with the exception of HBV infection, as presented in figure 1. There were more indications for HCV and HIV/HCV in the last decade.
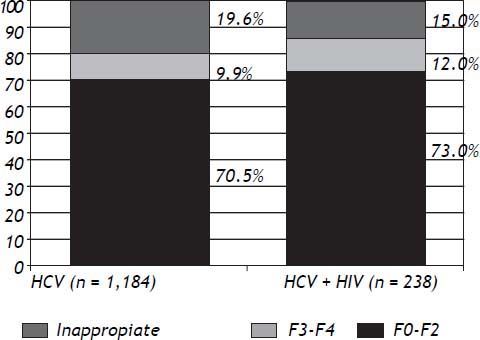
Liver fibrosis graduation was compared among patients monoinfected by HCV and those with HCV-HIV coinfection. It was observed that, in both groups, a higher proportion of patients had mild-moderate fibrosis (F0–F2) than advanced fibrosis (F3–F4), although without statistically significant difference (Figure 2).
There was an association between age and fibro-sis in the HCV monoinfected group, observing that younger patients (≤ 39-years old) had milder degree of fibrosis than older than 40–years old (Table 2).
Liver steatosis was observed in 36.3% of HCV monoinfected patients and in 31.2% of HCV-HIV co-infected ones, without statistically significant difference. On the other hand, coinfected patients presented higher intra-hepatic iron rates than mo-noinfected ones (20.0 x 8.0%) (Table 3).
The material was considered inappropriate for analysis in 205 cases (10.48% of the sample). The average size of the assessed fragments was 10.51 ± 6.8 mm.
Major complications (other than pain) related to the procedure were observed in 07 (0.3%) cases, with no cases of death: 05 punctures where bile was aspired, 01 pneumothorax and 01 hemoperitoneum, which required surgery.
DiscussionDespite the recent development of non-invasive methods to asses liver fibrosis, liver biopsy remains as an important tool to evaluate liver diseases, regarding the diagnosis, staging, prognosis and management of patients with liver disorders.1,11,12
Considering the risk of adverse events and the development of non invasive methods for evaluation of liver fibrosis, the liver biopsy should only be indicated in cases in which the definition of the histopa-thology would modify the therapeutic/prognostic conduct.1,11
In the present study all the procedures were performed without ultrassonographic (US) guidance. Some authors suggest that procedures using US would be safer than blind PLB,13 but the complication rate observed in the present casuistry was low even with blind PLB.
There was a predominance of men in the first assessed decade (63.3%), as it was also observed in other national and international studies;2,14 however, there was a reduction in the percentage of the second assessed decade (51.3%) (p < 0.001).
The mean age of the patients submitted to PLB was similar in both decades. Such findings are similar to the results of other Brazilian studies.2,12
Chronic hepatitis C was the main indication for liver biopsy in the present study, representing 55% of the cases between 1993 and 2002, and 66% of the cases between 2003 and 2011, reflecting the national reality.2,12
The second most prevalent indication for PLB was HIV infection, including coinfected patients, with 18.3% between 1993 and 2002 and 18.8% between 2003 and 2011. HIV monoinfection was more frequent in the first decade (10.8% x 1.4%), while HCV-HIV coinfection was more prevalent in the second decade (7.5% x 17.4%), as presented in figure 1. The current expected tendency is that the number of coinfected patients will decrease in the PLB pool, once the procedure is no longer required for the treatment of this subpopulation.8 However, PLB still has an important role in the diagnosis of other as-sociated conditions, such as steatosis or toxicity by antiretroviral drugs.15,16
In a French study presented in the 2012 AASLD liver meeting,13 it was observed a marked reduction in the total number of liver biopsy in the last period studied, as well as for chronic viral hepatitis C.
When the fibrosis degree was assessed in such subgroup of patients, there was no difference when compared to monoinfected HCV (Figure 2). There were higher prevalence of patients with lower degrees of fibrosis (F0–F2), when both HCV monoin-fected and HCV-HIV coinfected patients were analyzed (Figure 2). Such findings can be explained by the fact that patients with clinical diagnosis of cirrhosis can be treated without the need for PLB, in accordance with the Brazilian Ministry of Health.8
The natural history of HCV chronic hepatitis and the liver fibrosis progression in patients carrying such disease has been studied, aiming at identifying factors related to the evolution to higher fibrosis degrees (age over 40 years old in the infection, daily alcohol consumption and male gender).22 In the present study, the age group and degrees of liver fibro-sis were compared (Table 2), and a significant difference between patients over 40 years of age for higher fibrosis was observed, corroborating the lite-rature.17.–19 It was not possible to assess whether such finding were due to the longer infection time or higher age at the time of the infection.
Liver steatosis is a histological finding commonly observed in patients with chronic hepatitis C infection, with estimated prevalence of 30–70%,15,20,21 where some studies show genotype 3 as the most related to non-alcoholic fatty liver disease (NAFLD). Such finding is frequently followed by insulin resistance, alterations in the glucose and lipids metabolism.15,20,22
The incidence of liver steatosis among HCV-HIV coinfected patients is estimated between 25–75%, and is usually attributed to the use of antiretroviral drugs.23 In the present study, the prevalence of liver steatosis corroborates the findings of previous studies,15,20,21,23,24 both HCV monoinfected and HCV-HIV coinfected patients, without statistically significant difference between the groups.
Patients with chronic hepatitis C usually present greater amounts of iron storage in the liver parenchyma, related to faster progression of liver fibrosis and development of hepatocellular carcinoma.25.–27 This accumulation is attributed to several factors, such as the reabsorption of iron coming from hepatocytes necrosis, hemochromatosis-related mutations, decreased expression of hepcidin or changes in the iron liver metabolism.28 In the present casuistry, iron accumulation was observed in 8% of the monoinfected patients, which is in accordance with the literature.2,3 Moreover it was observed that 20% of the HCV-HIV coinfected patients presented iron accumulation. These metabolic disturbances can also be related to insulin resistance, progression of HIV disease, and the type of antiretroviral therapy prescribed, increasing the risk of cardiovascular disease and mortality.12
The PLB is a safe procedure, specially in experienced hands. Patients with malignant liver disease and/or advanced liver insufficiency present higher risk, and may have a mortality rate up to 19% in the first three months after biopsy.29 Overall, mortality directly related to the procedure is a rare event, varying from 0.01 and 0.1%. The main cause of death after liver biopsy is intraperitoneal hemorrhage, with 0.03 to 0.07% incidence.11,29 In the present study there were no deaths and the complications rate was low (0.3%).
In conclusion, percutaneous liver biopsy remains an important and widely used diagnostic tool in the survey of patients with liver disease, although the non-invasive methods establish a new paradigm in the diagnostic of liver diseases. The modification of the studied population over the last two decades reflects the real life, showing a greater access of the HCV and coinfected HCV/HIV patients to the public health system.