Abstracts Asociación Mexicana del Hígado (AMH) 2023
More infoAdvanced liver fibrosis (ALF) is a predictor of adverse prognosis in chronic liver disease. In addition to etiological treatment, a new approach to stop or reverse residual fibrosis would be desirable. Our aim was to assess the efficacy and safety of a prolonged-release pirfenidone formulation (PR-PFD) compared to placebo, plus standardized care, in patients with compensated liver cirrhosis.
Materials and Patients180 patients with ALF (F4 by elastography) of various causes were randomly assigned to 3 groups: placebo (G1), PR-PFD: 1200 mg/d (G2) or 1800 mg/d (G3), plus standardized care, during 24 months. All participants underwent standard lab tests, quality of life assessment, elastography, fibrotest, liver US, and endoscopy at baseline and at 12 and 24 months. Ethics Committee Registry H14-004. Patients signed an informed consent, which will be in custody for 15 years. This study was funded by CellPharma Laboratory.
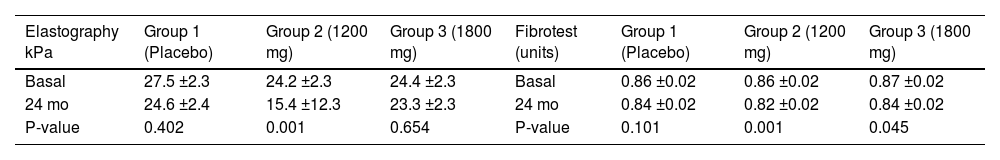
Results165 patients were eligible for the efficacy and 180 for the safety analysis. At baseline, demographics, etiology, stage of cirrhosis, Child-Pugh or MELD scores, quality of life or fatigue scales, and liver stiffness (kPa) and Fibrotest (units) scores (mean ± 1SE) were similar between groups (multivariate mixed model). The estimated fibrosis scores presented a significant reduction, mainly in G2 (Table). Decompensations were detected in 19 patients: variceal bleeding (5), encephalopathy (4), hepatocarcinoma (4) with similar distribution between groups. Ascites (12) was more frequent in the placebo group (p=0.003). G2 patients presented significant improvements between baseline and 24 months in: ALT (43.5 ± 3.8 vs. 31.3 ± 4.8 UI/L, p=0.003), albumin (4.2 ± 0.06 vs. 4.5 ± 0.07 g/dL, p<0.001); total bilirubin (0.90 ± 0.08 vs. 0.65 ± 0.10 mg/dL, p<0.001); platelets (121.7 ± 7.8 vs. 144.3 ± 9.7 × 10³/µL, p<0.001), MELD (9.73 ± 0.32 vs. 9.03 ± 0.40, p=0.022) and quality of life (83.7 ± 1.5 vs. 90.9 ± 1.9 %, p=0.002). Adverse events were mainly mild from the GI tract (n=48, 46, and 35) and skin (n=15, 22, and 12) in G1, G2, and G3, respectively.
ConclusionsProlonged-release pirfenidone at a dose of 1200 mg significantly decreased indirect fibrosis markers at 24 months and induced improvement in LFTs, MELD, and quality of life in compensated cirrhosis and without safety concerns.
Ethical statement
HI14-004
Declaration of interests
None
Funding
CellPharma Laboratory
Table. The estimated fibrosis scores presented a significant reduction, mainly in G2
| Elastography kPa | Group 1 (Placebo) | Group 2 (1200 mg) | Group 3 (1800 mg) | Fibrotest (units) | Group 1 (Placebo) | Group 2 (1200 mg) | Group 3 (1800 mg) |
|---|---|---|---|---|---|---|---|
| Basal | 27.5 ±2.3 | 24.2 ±2.3 | 24.4 ±2.3 | Basal | 0.86 ±0.02 | 0.86 ±0.02 | 0.87 ±0.02 |
| 24 mo | 24.6 ±2.4 | 15.4 ±12.3 | 23.3 ±2.3 | 24 mo | 0.84 ±0.02 | 0.82 ±0.02 | 0.84 ±0.02 |
| P-value | 0.402 | 0.001 | 0.654 | P-value | 0.101 | 0.001 | 0.045 |