Hepatitis B surface antigen (HBsAg) clearance, indicating functional cure or resolved chronic hepatitis B (CHB), remains difficult to achieve via nucleos(t)ide analogue monotherapy. We investigated whether metformin add-on therapy could help achieve this goal in entecavir-treated patients with hepatitis B e antigen (HBeAg)-negative CHB.
Patients and MethodsPatients with HBeAg-negative CHB who met eligibility criteria (entecavir treatment for > 12 months, HBsAg < 1000 IU/mL) were randomly assigned (1:1) to receive 24 weeks of either metformin (1000 mg, oral, once a day) or placebo (oral, once a day) add-on therapy. The group allocation was blinded for both patients and investigators. Efficacy and safety analyses were based on the intention-to-treat set. The primary outcome, serum HBsAg level (IU/mL) at weeks 24 and 36, was analysed using mixed models.
ResultsSixty eligible patients were randomly assigned to the metformin (n = 29) and placebo (n = 31) groups. There was no substantial between-group difference in the HBsAg level at week 24 (adjusted mean difference 0.05, 95% confidence interval -0.04 to 0.13, p = 0.278) or week 36 (0.06, -0.03 to 0.15, p = 0.187), and no significant effect of group-by-time interaction on the HBsAg level throughout the trial (p = 0.814). The occurrence of total adverse events between the two groups was comparable (9 [31.0%] of 29 vs. 5 [16.1%] of 31, p = 0.227) and no patient experienced serious adverse events during the study.
ConclusionAlthough it was safe, metformin add-on therapy did not accelerate HBsAg clearance in entecavir-treated patients with HBeAg-negative CHB.
Hepatitis B remains a major public health problem, with approximately 257 million individuals infected with hepatitis B virus (HBV) and more than 94 million people suffering from chronic hepatitis B (CHB) worldwide [1]. In 2016, the World Health Assembly endorsed the Global Health Sector Strategy on viral hepatitis, which calls for the elimination of viral hepatitis as a public health threat by 2030 [2,3]. To achieve this goal, there is still a huge obstacle that hepatitis B surface antigen (HBsAg) clearance, an indicator of functional cure, remains difficult to achieve through current treatment agents, including nucleos(t)ide analogue (NA) and interferon [4-8]. Hence, it is necessary to develop new agents which have the potential to promote HBsAg clearance [9-13].
In addition to the traditional hypoglycaemic effect, metformin has been proven to play an inhibitory role in HBV life cycle [14]. Xun et al. demonstrated that in HepG2.2.15 cells, metformin significantly suppressed HBsAg expression by down-regulating the transcriptional activities of cccDNA promoters. Furthermore, metformin synergistically acted with lamivudine in antiviral effects [15]. Honda et al. confirmed that in hepAD38 cells, metformin successfully repressed HBsAg synthesis by down-regulating the HBV-related host transcriptional factors. Moreover, metformin and entecavir synergistically inhibited HBV DNA replication [16].
Simultaneously, metformin also plays a modulatory role in host immune response, which helps reconstitute the immune defence [17]. Kunisada et al. found that metformin reprogrammed cellular metabolism pattern by inhibiting the mammalian target of rapamycin pathway, promoted the differentiation of Naïve CD4+ T cells into helper T cells rather than regulatory T cells, and prevented the formation of an immunotolerant environment in the liver [18]. Cha et al. revealed that metformin phosphorylated S195 of programmed death ligand-1 (PD-L1) via activating the adenosine 5′-monophosphate (AMP)-activated protein kinase pathway, induced endoplasmic reticulum-associated degradation of PD-L1, and hindered the exhaustion of CD8+ T cells in the liver [19].
Based on these findings, it is expected that metformin add-on therapy could facilitate the functional cure of CHB. However, no clinical studies on this topic have been reported to date. Therefore, we conducted a randomised, double-blind, placebo-controlled trial to investigate whether metformin as an adjunct to entecavir therapy, the most common NA therapy in China, could accelerate HBsAg clearance in patients with hepatitis B e antigen (HBeAg)-negative CHB.
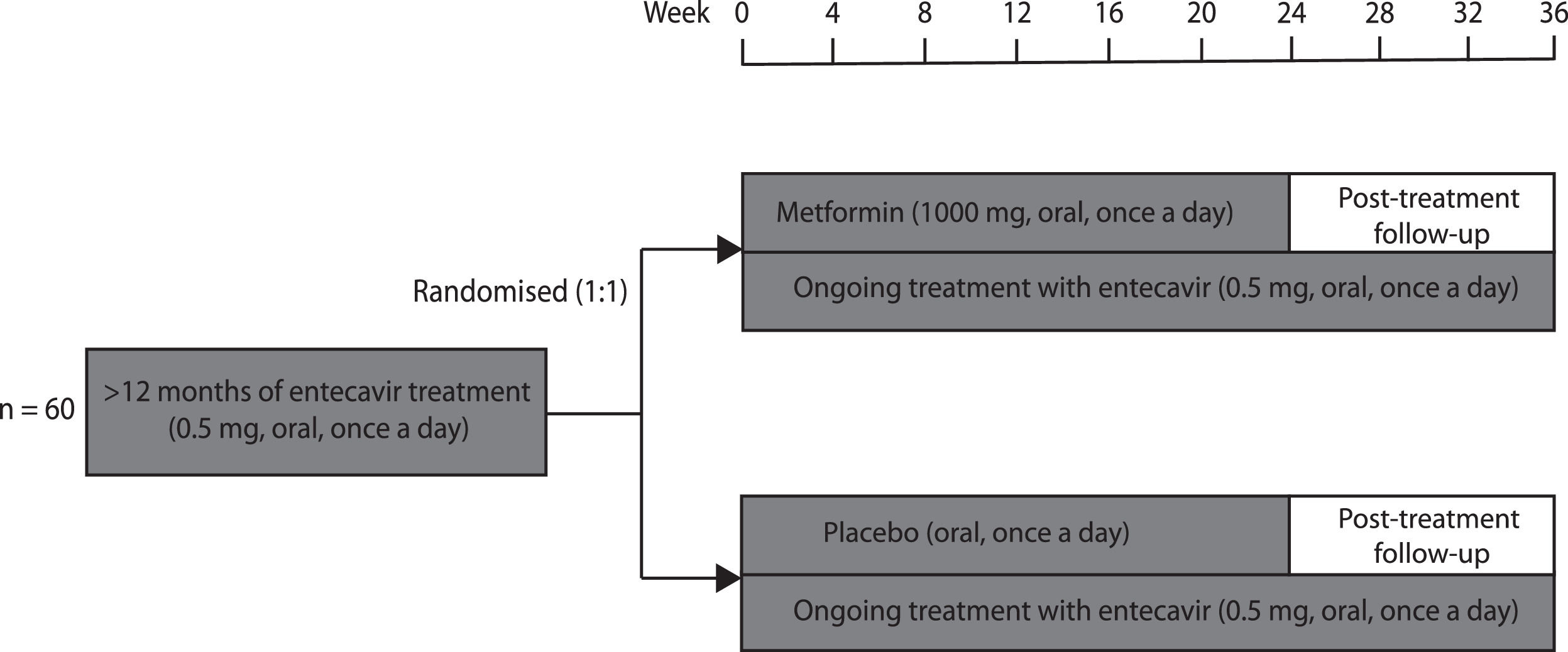
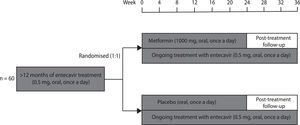
2Patients and methods2.1Study designThis randomised, double-blind, placebo-controlled trial was conducted at the 960th Hospital of Chinese PLA Joint Logistics Support Force and guided by the Fifth Medical Center of Chinese PLA General Hospital. Patients with CHB who met eligibility criteria were randomly assigned (1:1) to receive either metformin or placebo for 24 weeks in addition to their ongoing entecavir therapy (Fig. 1). Patients and investigators were both blinded to group allocation. The primary and secondary outcomes were analysed using mixed models. The intention-to-treat populations were included in efficacy and safety analyses.
2.2Ethics approval and informed consentThe study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Medical Research Ethics Committee of the 960th Hospital of Chinese PLA Joint Logistics Support Force (approval number 960-2019-84). This trial was registered at ClinicalTrials.gov (registration number NCT04182321). Written informed consent was obtained from each patient included in the study.
2.3ParticipantsWe recruited entecavir-treated patients aged 18–60 with HBeAg-negative CHB and a relatively low HBsAg level at the 960th Hospital of Chinese PLA Joint Logistics Support Force (Tai'an, Shandong, China). After obtaining written informed consent, we screened patients via medical history analysis, physical examination, and relevant laboratory tests. The inclusion criteria were as follows: entecavir treatment for > 12 months, HBV DNA undetectable, HBeAg-negative, HBsAg < 1000 IU/mL, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) < 80 U/L (i.e., two times the upper limit of normal). The exclusion criteria were diabetes mellitus, alcoholic liver disease, drug-induced liver injury, autoimmune liver disease, decompensated liver cirrhosis, HCC, and liver transplantation.
2.4InterventionsThe participants were randomly assigned (1:1) to receive 24 weeks of either sustained-release metformin hydrochloride (1000 mg, oral, once a day) or placebo (oral, once a day) add-on therapy. Metformin dosage was determined after literature review and expert consultation. The post-treatment follow-up period was 12 weeks after discontinuation of metformin or placebo. Entecavir was continuously administered, both during and after the trial.
The participants visited the outpatient clinic every 4 weeks, between weeks 0 and 36, after trial commencement. Weight and height were measured, body mass index (BMI) was calculated, blood was drawn, and serum HBsAg level and fasting glucose were measured at all visits. In addition, glycated haemoglobin (HbA1c), triglycerides, total cholesterol, ALT, AST, hepatitis B surface antibody (anti-HBs), HBeAg, hepatitis B e antibody (anti-HBe), and HBV DNA load were measured at weeks 12, 24, and 36. All clinical data were entered into the electronic data-capturing system by LQ and MMS and were regularly monitored by a qualified clinical research associate.
All adverse events that occurred during this trial were registered, reported, and treated appropriately. The procedure for managing serious adverse events (SAEs) entailed reporting relevant information to the Medical Research Ethics Committee and opening emergency unblinding envelopes for appropriate treatment. Unblinded subjects were removed from the trial.
2.5OutcomesThe primary outcome was serum HBsAg level (IU/mL) at weeks 24 and 36 after the addition of metformin or placebo. Serum HBsAg level was measured by Jinan Kingmed Center for Clinical Laboratory Co., Ltd (Jinan, China), an appropriately qualified third-party organisation.
Secondary outcomes were weight (kg), BMI (kg/m2), fasting glucose (mmol/L), HbA1c (%), triglycerides (mmol/L), and total cholesterol (mmol/L) at weeks 24 and 36 after adding metformin or placebo. Weight was measured using the same digital body weight scale for all measurements and BMI was calculated using the same computer algorithm for all calculations. All other outcomes were measured in the medical laboratory centre of our hospital. Fasting glucose, triglycerides, and total cholesterol were measured using standard laboratory assays (Biobase 8000; Biobase Biotech, Jinan, China). The HbA1c level was measured using an immunochemical assay (Cobas C 311; Roche Shanghai, Shanghai, China).
All diverse events during the study, including SAEs, non-serious clinical events, and non-serious laboratory events, were included in the safety assessment. All these adverse events were clearly defined in the study protocol.
2.6Sample size calculationThe sample size was calculated using the mixed models (simulation) procedure in Power Analysis and Sample Size (PASS) software, version 15 (Number Cruncher Statistical Systems, Kaysville, Utah, USA). For the primary outcome, we determined that a sample size of 30 patients in each group (a total of 60 patients) would provide the trial with a power of 85% (95% confidence interval [CI] 76% to 91%) to detect a 5% difference in HBsAg levels (log IU/mL) between the metformin and placebo groups at the 5% significance level. Some of the parameters needed to complete sample size calculation, such as the detectable difference in factors separating subjects into groups (0.108), the detectable difference in factors with multiple levels within a subject (0.108), the detectable difference in interactions (0.215), baseline mean (2.15), variance of random effect (0.020), and variance of residuals (0.008), were obtained from previous studies on the kinetics of HBsAg levels in patients with CHB during entecavir treatment [20,21]. Other options for sample size calculation followed the mixed models (simulation) procedure tutorial of the PASS software, version 15.
2.7Randomisation and blindingEligible patients were randomly assigned (1:1) to receive either sustained-release metformin hydrochloride or placebo add-on therapy in the block size of four using a computer algorithm. Sequentially numbered containers were used to implement random allocation concealment. A qualified contract research organisation generated a random allocation sequence, QHS recruited patients, and WZ assigned them to different treatment groups. Patients and investigators involved in outcome assessments and statistical analyses were all blinded to random allocation. Both sustained-release metformin hydrochloride and placebo tablets were produced by HUANGHAI Pharmaceutical Corporation Limited (Qingdao, China) under good manufacturing practice conditions. Their appearance, size, colour, weight, odour, taste, and packaging were identical for blinding purposes. Blinding success was assessed using Bang's index.
2.8Statistical analysisContinuous variables are reported as mean (standard deviation [SD]) if normally distributed or as median (inter-quartile range [IQR]) if non-normally distributed. Differences in continuous variables between groups were evaluated using Student's t-test if normally distributed or the Mann–Whitney test if non-normally distributed. Categorical variables are reported as n (%). Differences in categorical variables between groups were assessed using χ2 tests or Fisher's exact test.
Mixed models were used to evaluate the effect of different factors and their interactions on the primary or secondary outcomes. These models included baseline value, group (metformin and placebo), time (number of weeks since baseline), group-by-time interaction as fixed effects, subject variation as the random effect, and repeated measurement as residual. The fitness of mixed models was evaluated by the Akaike information criterion and the Bayesian information criterion, whereas the parameters of fixed and random effects were estimated using the restricted maximum likelihood method. All reported p-values were two-sided, and results with p < 0.05 were considered significant. All analyses were performed, and graphics were generated using Statistical Product and Service Solutions (SPSS) software, version 25 (International Business Machine, Armonk, New York, USA), and R software, version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analyses were performed to assess the robustness of results for the primary outcome among five different mixed models. Five different covariance structure types, namely diagonal, compound symmetry, autoregressive (first-order), Toeplitz, and unstructured, were chosen to construct five mixed models. Analysis for the primary outcome was repeated using these mixed models.
2.9Role of the funding sourceThe funder of the study had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication. All authors had access to the study data, reviewed and approved the final manuscript.
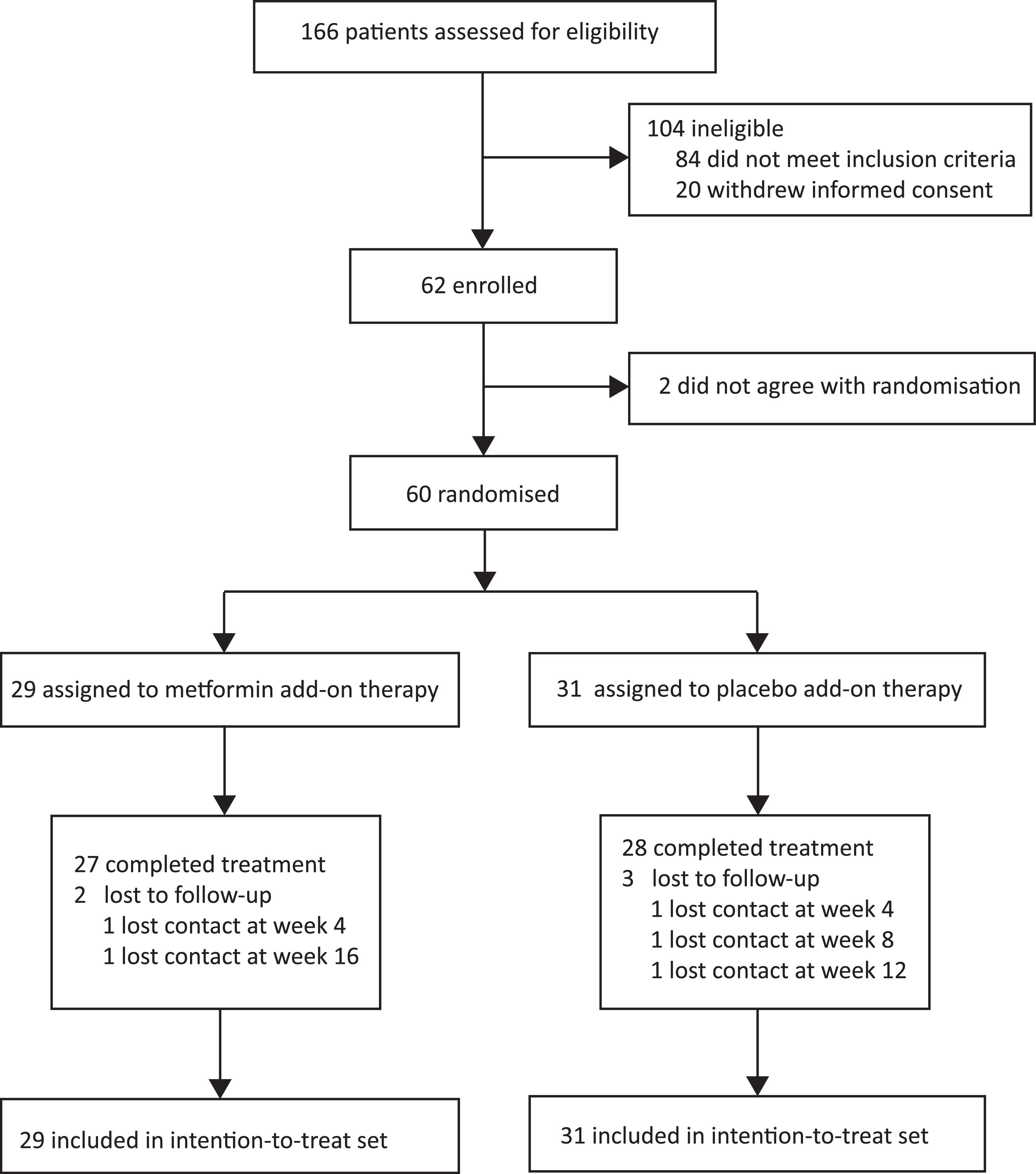
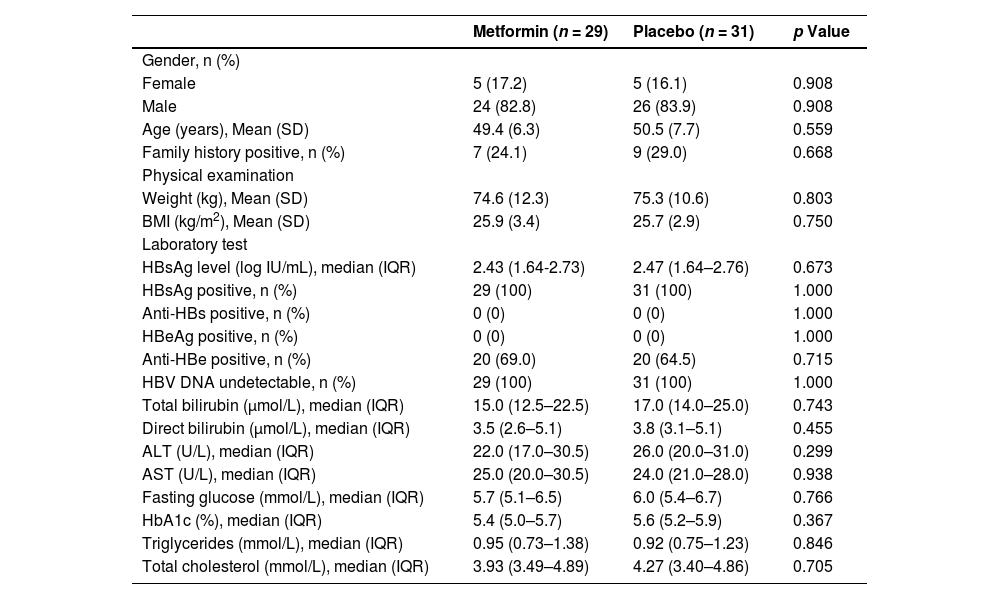
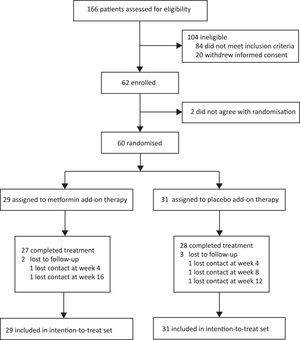
3Results3.1Participant flow and baseline characteristicsBetween January 13, 2020, and September 30, 2020, 166 consecutive patients provided informed consent and were assessed for eligibility (Fig. 2). One hundred and six patients were excluded (84 did not meet inclusion criteria, and 22 withdrew informed consent). Sixty eligible patients were enrolled and randomly assigned to the two groups (29 patients to the metformin group and 31 patients to the placebo group) and finally included in the intention-to-treat set. Two patients dropped out of the metformin group and three dropped out of the placebo group in the follow-up course. The baseline characteristics of the intention-to-treat set did not differ between the groups (Table 1).
Baseline characteristics of the intention-to-treat set.
| Metformin (n = 29) | Placebo (n = 31) | p Value | |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 5 (17.2) | 5 (16.1) | 0.908 |
| Male | 24 (82.8) | 26 (83.9) | 0.908 |
| Age (years), Mean (SD) | 49.4 (6.3) | 50.5 (7.7) | 0.559 |
| Family history positive, n (%) | 7 (24.1) | 9 (29.0) | 0.668 |
| Physical examination | |||
| Weight (kg), Mean (SD) | 74.6 (12.3) | 75.3 (10.6) | 0.803 |
| BMI (kg/m2), Mean (SD) | 25.9 (3.4) | 25.7 (2.9) | 0.750 |
| Laboratory test | |||
| HBsAg level (log IU/mL), median (IQR) | 2.43 (1.64-2.73) | 2.47 (1.64–2.76) | 0.673 |
| HBsAg positive, n (%) | 29 (100) | 31 (100) | 1.000 |
| Anti-HBs positive, n (%) | 0 (0) | 0 (0) | 1.000 |
| HBeAg positive, n (%) | 0 (0) | 0 (0) | 1.000 |
| Anti-HBe positive, n (%) | 20 (69.0) | 20 (64.5) | 0.715 |
| HBV DNA undetectable, n (%) | 29 (100) | 31 (100) | 1.000 |
| Total bilirubin (µmol/L), median (IQR) | 15.0 (12.5–22.5) | 17.0 (14.0–25.0) | 0.743 |
| Direct bilirubin (µmol/L), median (IQR) | 3.5 (2.6–5.1) | 3.8 (3.1–5.1) | 0.455 |
| ALT (U/L), median (IQR) | 22.0 (17.0–30.5) | 26.0 (20.0–31.0) | 0.299 |
| AST (U/L), median (IQR) | 25.0 (20.0–30.5) | 24.0 (21.0–28.0) | 0.938 |
| Fasting glucose (mmol/L), median (IQR) | 5.7 (5.1–6.5) | 6.0 (5.4–6.7) | 0.766 |
| HbA1c (%), median (IQR) | 5.4 (5.0–5.7) | 5.6 (5.2–5.9) | 0.367 |
| Triglycerides (mmol/L), median (IQR) | 0.95 (0.73–1.38) | 0.92 (0.75–1.23) | 0.846 |
| Total cholesterol (mmol/L), median (IQR) | 3.93 (3.49–4.89) | 4.27 (3.40–4.86) | 0.705 |
ALT, alanine aminotransferase; anti-HBe, hepatitis B e antibody; anti-HBs, hepatitis B surface antibody; AST, aspartate aminotransferase; BMI, body mass index; HbA1c, glycated haemoglobin; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; IQR, inter-quartile range; SD, standard deviation.
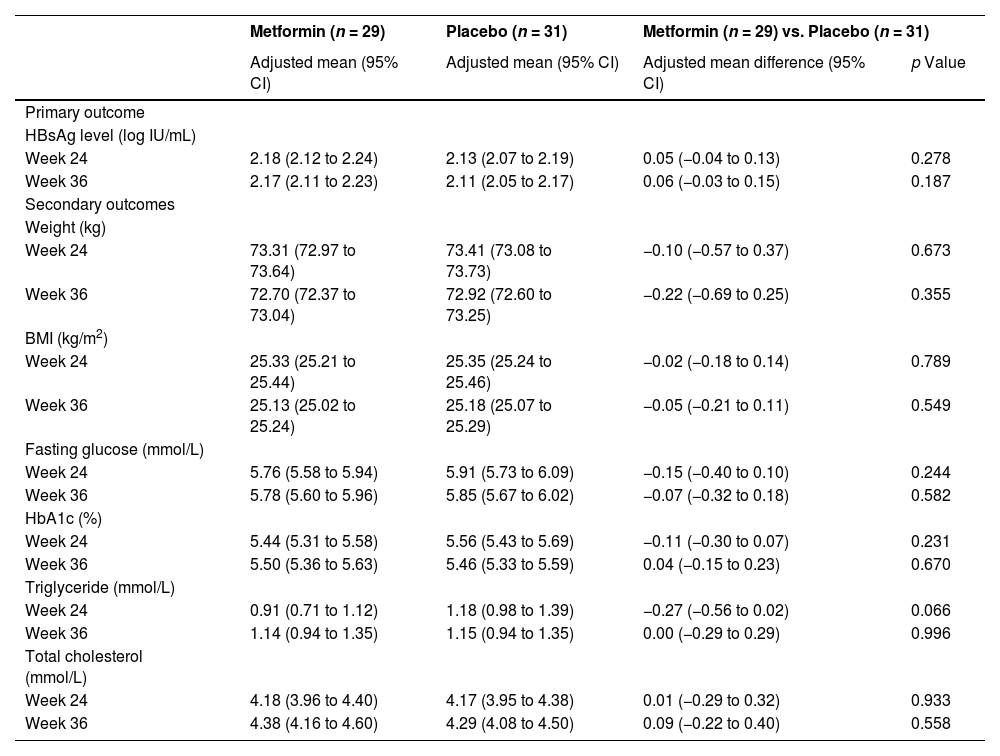
After analysis with mixed models and baseline adjustment, we noted no substantial between-group differences in the HBsAg level of the intention-to-treat set at week 24 (adjusted mean difference 0.05, 95% CI -0.04 to 0.13, p = 0.278) or week 36 (0.06, -0.03 to 0.15, p = 0.187) (Table 2). Further analysis showed no significant effect of group-by-time interaction on the HBsAg level (p = 0.814) throughout the trial (Fig. 3).
Outcome assessments of the intention-to-treat set.
| Metformin (n = 29) | Placebo (n = 31) | Metformin (n = 29) vs. Placebo (n = 31) | ||
|---|---|---|---|---|
| Adjusted mean (95% CI) | Adjusted mean (95% CI) | Adjusted mean difference (95% CI) | p Value | |
| Primary outcome | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.12 to 2.24) | 2.13 (2.07 to 2.19) | 0.05 (−0.04 to 0.13) | 0.278 |
| Week 36 | 2.17 (2.11 to 2.23) | 2.11 (2.05 to 2.17) | 0.06 (−0.03 to 0.15) | 0.187 |
| Secondary outcomes | ||||
| Weight (kg) | ||||
| Week 24 | 73.31 (72.97 to 73.64) | 73.41 (73.08 to 73.73) | −0.10 (−0.57 to 0.37) | 0.673 |
| Week 36 | 72.70 (72.37 to 73.04) | 72.92 (72.60 to 73.25) | −0.22 (−0.69 to 0.25) | 0.355 |
| BMI (kg/m2) | ||||
| Week 24 | 25.33 (25.21 to 25.44) | 25.35 (25.24 to 25.46) | −0.02 (−0.18 to 0.14) | 0.789 |
| Week 36 | 25.13 (25.02 to 25.24) | 25.18 (25.07 to 25.29) | −0.05 (−0.21 to 0.11) | 0.549 |
| Fasting glucose (mmol/L) | ||||
| Week 24 | 5.76 (5.58 to 5.94) | 5.91 (5.73 to 6.09) | −0.15 (−0.40 to 0.10) | 0.244 |
| Week 36 | 5.78 (5.60 to 5.96) | 5.85 (5.67 to 6.02) | −0.07 (−0.32 to 0.18) | 0.582 |
| HbA1c (%) | ||||
| Week 24 | 5.44 (5.31 to 5.58) | 5.56 (5.43 to 5.69) | −0.11 (−0.30 to 0.07) | 0.231 |
| Week 36 | 5.50 (5.36 to 5.63) | 5.46 (5.33 to 5.59) | 0.04 (−0.15 to 0.23) | 0.670 |
| Triglyceride (mmol/L) | ||||
| Week 24 | 0.91 (0.71 to 1.12) | 1.18 (0.98 to 1.39) | −0.27 (−0.56 to 0.02) | 0.066 |
| Week 36 | 1.14 (0.94 to 1.35) | 1.15 (0.94 to 1.35) | 0.00 (−0.29 to 0.29) | 0.996 |
| Total cholesterol (mmol/L) | ||||
| Week 24 | 4.18 (3.96 to 4.40) | 4.17 (3.95 to 4.38) | 0.01 (−0.29 to 0.32) | 0.933 |
| Week 36 | 4.38 (4.16 to 4.60) | 4.29 (4.08 to 4.50) | 0.09 (−0.22 to 0.40) | 0.558 |
BMI, body mass index; HbA1c, glycated haemoglobin; HBsAg, hepatitis B surface antigen.
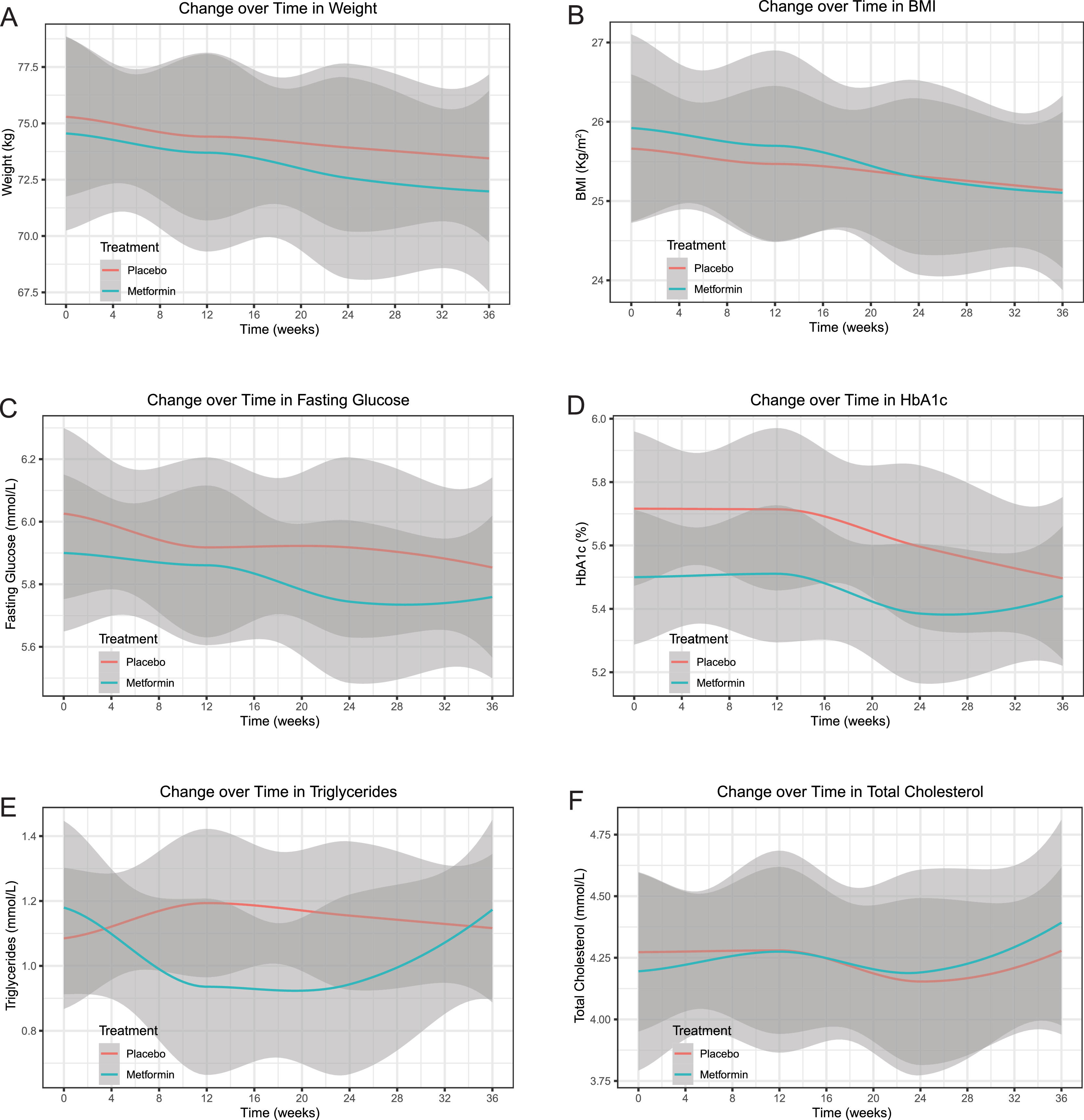
All secondary outcomes of the intention-to-treat set did not significantly differ between the groups after analysis with the same models and baseline adjustments at week 24 (weight: adjusted mean difference -0.10, 95% CI -0.57 to 0.37, p = 0.673; BMI: -0.02, -0.18 to 0.14, p = 0.789; fasting glucose: -0.15, -0.40 to 0.10, p = 0.244; HbA1c: -0.11, -0.30 to 0.07, p = 0.231; triglycerides: -0.27, -0.56 to 0.02, p = 0.066; total cholesterol: 0.01, -0.29 to 0.32, p = 0.933) or week 36 (weight: -0.22, -0.69 to 0.25, p = 0.355; BMI: -0.05, -0.21 to 0.11, p = 0.549; fasting glucose: -0.07, -0.32 to 0.18, p = 0.582; HbA1c: 0.04, -0.15 to 0.23, p = 0.670; triglycerides: 0.00, -0.29 to 0.29, p = 0.996; total cholesterol: 0.09, -0.22 to 0.40, p = 0.558) (Table 2). Further analysis showed no significant effect of group-by-time interaction on the weight (p = 0.830), BMI (p = 0.821), fasting glucose (p = 0.687), triglycerides (p = 0.082), or total cholesterol (p = 0.768) throughout the trial, except for a significant effect of group-by-time interaction on the HbA1c (p = 0.008) (Fig. 4).
Changes over time (smooth curve [95% CI]) in secondary outcomes of the intention-to-treat set. A, weight. B, BMI. C, fasting glucose. D, HbA1c. E, triglycerides. F, total cholesterol. There was no significant effect of group-by-time interaction on the weight (p = 0.830), BMI (p = 0.821), fasting glucose (p = 0.687), triglycerides (p = 0.082), or total cholesterol (p = 0.768) throughout the trial, except for a significant effect of group-by-time interaction on the HbA1c (p = 0.008). Abbreviations: BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin.
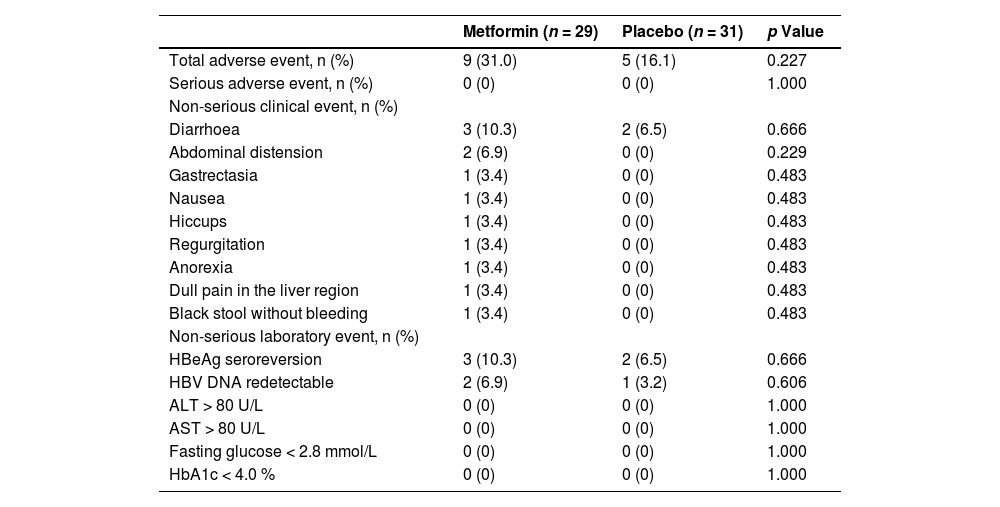
The incidence of total adverse events between the metformin and placebo groups was comparable (9 [31.0%] vs. 5 [16.1%], p = 0.227) and no patient experienced SAEs during this trial (Table 3). The incidences of non-serious clinical events did not differ between the groups (diarrhoea: 3 [10.3%] vs. 2 [6.5%], p = 0.666; abdominal distension: 2 [6.9%] vs. 0 [0%], p = 0.229; gastrectasia: 1 [3.4%] vs. 0 [0%], p = 0.483; nausea: 1 [3.4%] vs. 0 [0%], p = 0.483; hiccups: 1 [3.4%] vs. 0 [0%], p = 0.483; regurgitation: 1 [3.4%] vs. 0 [0%], p = 0.483; anorexia: 1 [3.4%] vs. 0 [0%], p = 0.483; dull pain in the liver region: 1 [3.4%] vs. 0 [0%], p = 0.483; black stool without bleeding: 1 [3.4%] vs. 0 [0%], p = 0.483). Furthermore, the incidences of non-serious laboratory events showed no substantial between-group differences (HBeAg seroreversion: 3 [10.3%] vs. 2 [6.5%], p = 0.666; HBV DNA > 500 IU/mL: 2 [6.9%] vs. 1 [3.2%], p = 0.606; ALT > 80 U/L: 0 [0%] vs. 0 [0%], p = 1.000; AST > 80 U/L: 0 [0%] vs. 0 [0%], p = 1.000; fasting glucose < 2.8 mmol/L: 0 [0%] vs. 0 [0%], p = 1.000; HbA1c < 4.0 %: 0 [0%] vs. 0 [0%], p = 1.000).
Adverse events during the trial.
| Metformin (n = 29) | Placebo (n = 31) | p Value | |
|---|---|---|---|
| Total adverse event, n (%) | 9 (31.0) | 5 (16.1) | 0.227 |
| Serious adverse event, n (%) | 0 (0) | 0 (0) | 1.000 |
| Non-serious clinical event, n (%) | |||
| Diarrhoea | 3 (10.3) | 2 (6.5) | 0.666 |
| Abdominal distension | 2 (6.9) | 0 (0) | 0.229 |
| Gastrectasia | 1 (3.4) | 0 (0) | 0.483 |
| Nausea | 1 (3.4) | 0 (0) | 0.483 |
| Hiccups | 1 (3.4) | 0 (0) | 0.483 |
| Regurgitation | 1 (3.4) | 0 (0) | 0.483 |
| Anorexia | 1 (3.4) | 0 (0) | 0.483 |
| Dull pain in the liver region | 1 (3.4) | 0 (0) | 0.483 |
| Black stool without bleeding | 1 (3.4) | 0 (0) | 0.483 |
| Non-serious laboratory event, n (%) | |||
| HBeAg seroreversion | 3 (10.3) | 2 (6.5) | 0.666 |
| HBV DNA redetectable | 2 (6.9) | 1 (3.2) | 0.606 |
| ALT > 80 U/L | 0 (0) | 0 (0) | 1.000 |
| AST > 80 U/L | 0 (0) | 0 (0) | 1.000 |
| Fasting glucose < 2.8 mmol/L | 0 (0) | 0 (0) | 1.000 |
| HbA1c < 4.0 % | 0 (0) | 0 (0) | 1.000 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HbA1c, glycated haemoglobin; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus.
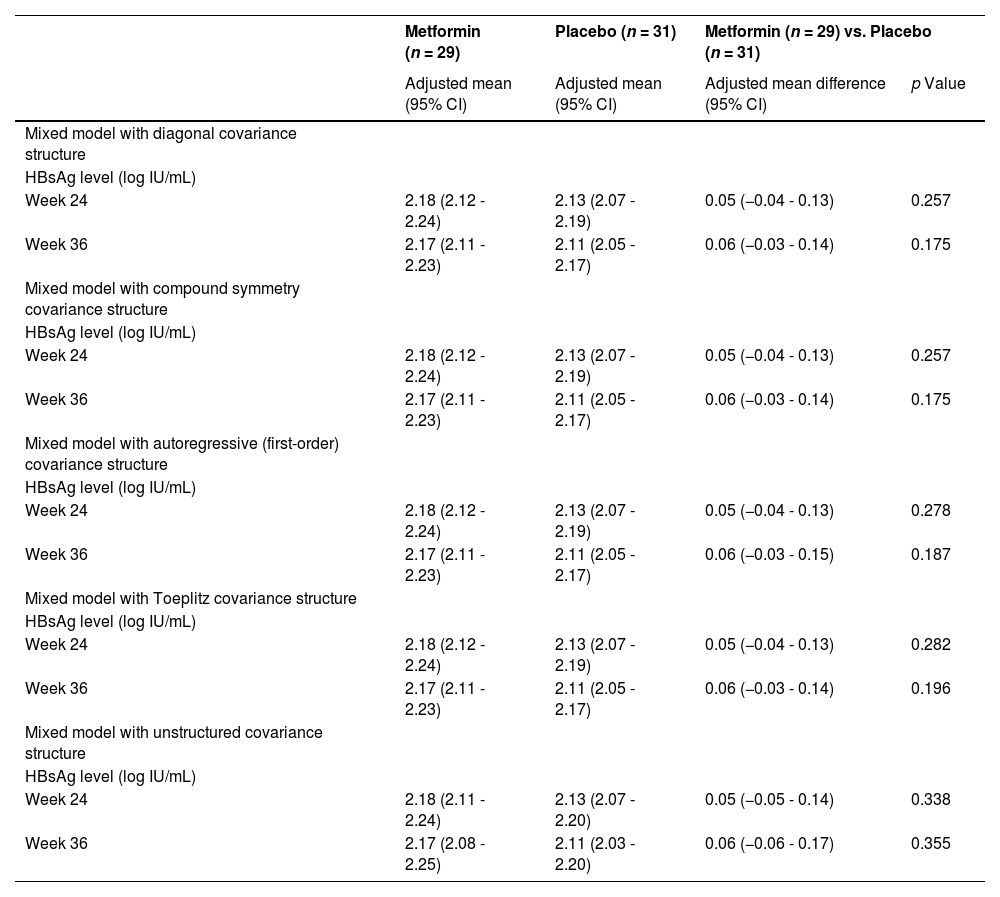
Sensitivity analyses were performed to assess the robustness of the results for the primary outcome (Table 4). We repeated the analysis for the HBsAg level of the intention-to-treat set at week 24 and 36 using the five mixed models constructed and obtained consistent results (mixed model with diagonal covariance structure: p = 0.257 and 0.175; mixed model with compound symmetry covariance structure: p = 0.257 and 0.175; mixed model with autoregressive (first-order) covariance structure: p = 0.278 and 0.187; mixed model with Toeplitz covariance structure: p = 0.282 and 0.196; mixed model with unstructured covariance structure: p = 0.338 and 0.355).
Sensitivity analyses for the primary outcome.
| Metformin (n = 29) | Placebo (n = 31) | Metformin (n = 29) vs. Placebo (n = 31) | ||
|---|---|---|---|---|
| Adjusted mean (95% CI) | Adjusted mean (95% CI) | Adjusted mean difference (95% CI) | p Value | |
| Mixed model with diagonal covariance structure | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.12 - 2.24) | 2.13 (2.07 - 2.19) | 0.05 (−0.04 - 0.13) | 0.257 |
| Week 36 | 2.17 (2.11 - 2.23) | 2.11 (2.05 - 2.17) | 0.06 (−0.03 - 0.14) | 0.175 |
| Mixed model with compound symmetry covariance structure | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.12 - 2.24) | 2.13 (2.07 - 2.19) | 0.05 (−0.04 - 0.13) | 0.257 |
| Week 36 | 2.17 (2.11 - 2.23) | 2.11 (2.05 - 2.17) | 0.06 (−0.03 - 0.14) | 0.175 |
| Mixed model with autoregressive (first-order) covariance structure | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.12 - 2.24) | 2.13 (2.07 - 2.19) | 0.05 (−0.04 - 0.13) | 0.278 |
| Week 36 | 2.17 (2.11 - 2.23) | 2.11 (2.05 - 2.17) | 0.06 (−0.03 - 0.15) | 0.187 |
| Mixed model with Toeplitz covariance structure | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.12 - 2.24) | 2.13 (2.07 - 2.19) | 0.05 (−0.04 - 0.13) | 0.282 |
| Week 36 | 2.17 (2.11 - 2.23) | 2.11 (2.05 - 2.17) | 0.06 (−0.03 - 0.14) | 0.196 |
| Mixed model with unstructured covariance structure | ||||
| HBsAg level (log IU/mL) | ||||
| Week 24 | 2.18 (2.11 - 2.24) | 2.13 (2.07 - 2.20) | 0.05 (−0.05 - 0.14) | 0.338 |
| Week 36 | 2.17 (2.08 - 2.25) | 2.11 (2.03 - 2.20) | 0.06 (−0.06 - 0.17) | 0.355 |
HBsAg, hepatitis B surface antigen.
Our study results demonstrated that although it was safe, metformin add-on therapy did not accelerate HBsAg clearance in entecavir-treated patients with HBeAg-negative CHB and a relatively low HBsAg level. Additionally, metformin did not affect the following metabolism indicators: weight, BMI, fasting glucose, triglycerides, and total cholesterol.
These findings raise the question of why those impactful antiviral and immunomodulatory effects of metformin confirmed in preclinical studies do not translate into a clinical benefit. Firstly, the environment in the human body is more complex than that in vitro or in rodents [22,23]. It is difficult to ensure that the drugs that play a role in vitro or in rodents play the same role in the human body environment. Secondly, controversy has arisen concerning the use of relatively high concentrations of metformin in vitro studies (250 to 2000 µmol/L) and in rodents (200–400 mg/kg per day) when compared with the concentrations achieved in plasma (40–70 µmol/L) from diabetics treated with metformin (25–30 mg/kg per day) [24]. Thirdly, publication bias might exist in preclinical studies as only a few researchers report negative findings and only a few journals accept them, resulting in the selective publication of positive findings [25]. Finally, metformin dose, course, or both in this clinical trial might have been insufficient. As the study population comprised patients with CHB and without diabetes, we chose a relatively safe dose (12–20 mg/kg per day) and a short course (six months) after literature review and expert consultation.
Regardless of its efficacy, metformin add-on therapy is safe in patients with CHB and without diabetes. There are many clinical trials dedicated to expanding the indications of metformin, and its safety is favourable in these more serious conditions. In a clinical trial using metformin to improve the cardiovascular risk profile in patients with ST segment elevation myocardial infarction without diabetes, metformin (1000 mg per day for 4 months) did not affect the levels of fasting glucose, post-challenge glucose, insulin, and high-density lipoprotein cholesterol [26]. Two clinical trials using metformin (3000 mg per day for 22–28 weeks or 2000 mg per day for 38 weeks) to reduce maternal weight gain in obese pregnant women without diabetes or to prevent late miscarriage in pregnant women with polycystic ovary syndrome reported no substantial between-group differences in SAEs in either mothers or offspring and none of the SAEs were deemed to be drug-related [27,28]. Another clinical trial using metformin (1500 mg per day for 12 months) to reduce disease flares in systemic lupus erythematosus patients without diabetes showed a favourable safety profile [29]. All these findings are consistent with our data.
Our study had some limitations. First, as the dose and course of metformin were limited in this trial and the study population was relatively small and from one hospital, the generalisability of our findings is uncertain. Second, HBsAg clearance is considerably difficult to achieve. The mean HBsAg reduction in CHB patients with five years of entecavir therapy was only 1.33 IU/mL per year [20]. Hence, 24 weeks of metformin add-on therapy in this trial may have been insufficient to observe its effect on HBsAg clearance. Considering the safety profile of metformin, future studies on this topic may use a larger dose, a longer course, or both. Third, specific secondary outcomes, such as weight, BMI, triglycerides, and total cholesterol, might have been affected by factors other than metformin (e.g., dietary factors and exercise habits), which were not considered in this trial [30]. However, these factors are unlikely to have affected our results because randomisation would have ensured that they evenly affected both groups.
5ConclusionsIn summary, our findings illustrated that the impactful antiviral and immunomodulatory effects of metformin noted in preclinical studies did not translate into a clinical benefit in patients with HBeAg-negative CHB and a relatively low HBsAg level. Nevertheless, the generalisability of our findings requires more clinical trials to confirm due to the limited dose and course of metformin in just one trial.
FundingThis study was supported by the Innovative Research Group Project of the National Natural Science Foundation of China [grant number 81721002].
Accessibility of data and materialsThis randomised controlled trial was reported according to the CONSORT guidelines. The CONSORT checklist (Supplementary material 1) and flow diagram (Supplementary material 2) accompanied by a detailed description of randomisation procedure (Supplementary material 3) are available in the supplementary materials online. The full study protocol and the datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributionsFSW and JYZ contributed to study conception. WZ, YYL, QHS, JXL, WPJ, ZAS, HBX, QMG, LT, and XWW contributed to study design. FSW and YMM contributed to study supervision. WZ, YYL, QHS, LQ, MMS, GC, and YA contributed to literature search. LQ, MMS, GC, and YA contributed to data collection. WZ, YYL, and QHS contributed to data verification. JXL, WPJ contributed to statistical analysis. WZ, YYL, QHS, JXL, and WPJ contributed to results interpretation. WZ, YYL, and QHS contributed to original draft writing. LQ, MMS, GC, YA, JXL, WPJ, ZAS, HBX, QMG, LT, XWW, JYZ, FSW, and YMM contributed to review and editing. All authors approved the final version of the article, including the authorship list.





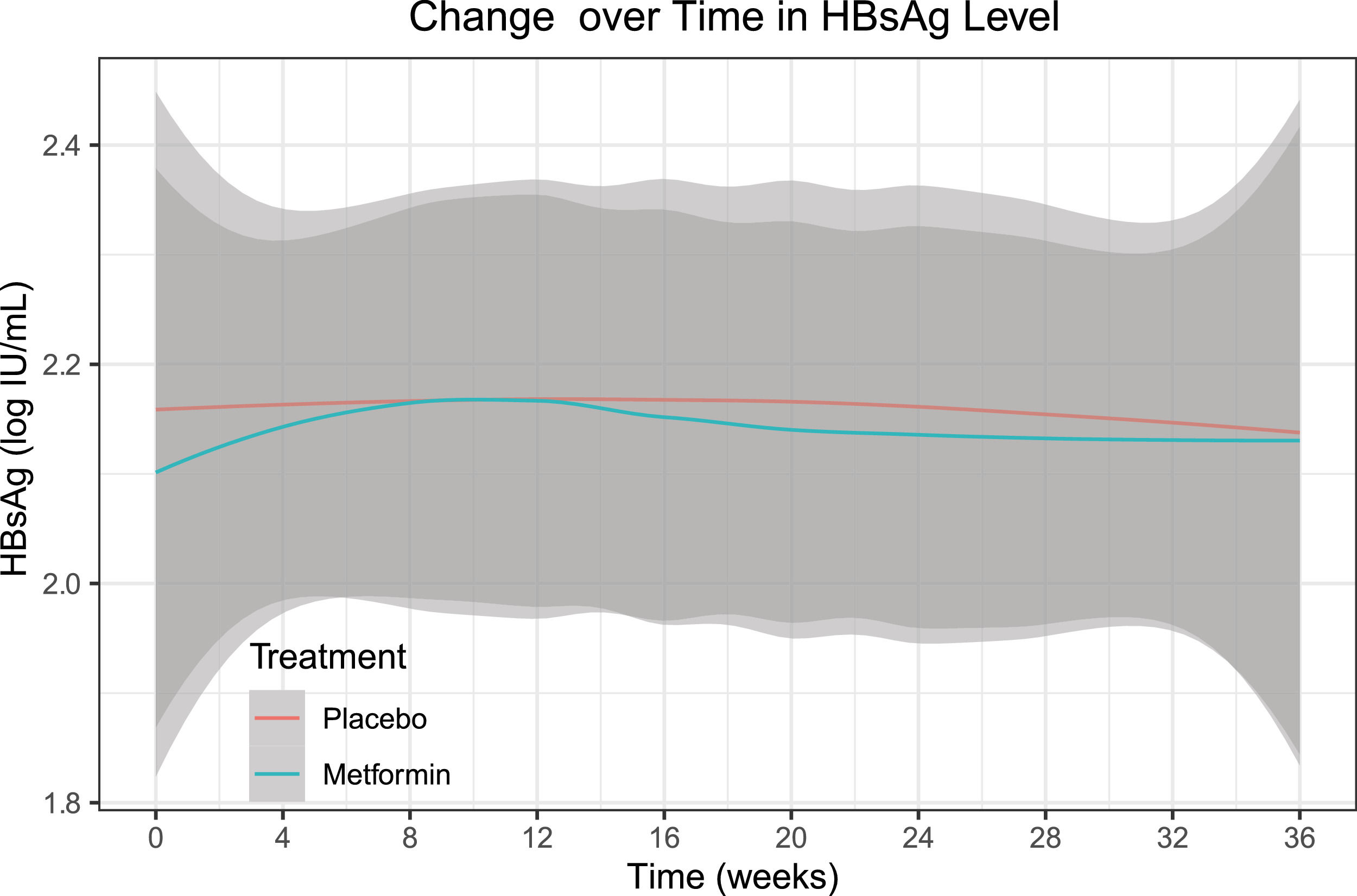
![Change over time (smooth curve [95% CI]) in HBsAg level of the intention-to-treat set. There was no significant effect of group-by-time interaction on the HBsAg level (p = 0.814) throughout the trial. Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen. Change over time (smooth curve [95% CI]) in HBsAg level of the intention-to-treat set. There was no significant effect of group-by-time interaction on the HBsAg level (p = 0.814) throughout the trial. Abbreviations: CI, confidence interval; HBsAg, hepatitis B surface antigen.](https://static.elsevier.es/multimedia/16652681/0000002700000006/v3_202307180533/S1665268122000874/v3_202307180533/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Changes over time (smooth curve [95% CI]) in secondary outcomes of the intention-to-treat set. A, weight. B, BMI. C, fasting glucose. D, HbA1c. E, triglycerides. F, total cholesterol. There was no significant effect of group-by-time interaction on the weight (p = 0.830), BMI (p = 0.821), fasting glucose (p = 0.687), triglycerides (p = 0.082), or total cholesterol (p = 0.768) throughout the trial, except for a significant effect of group-by-time interaction on the HbA1c (p = 0.008). Abbreviations: BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin. Changes over time (smooth curve [95% CI]) in secondary outcomes of the intention-to-treat set. A, weight. B, BMI. C, fasting glucose. D, HbA1c. E, triglycerides. F, total cholesterol. There was no significant effect of group-by-time interaction on the weight (p = 0.830), BMI (p = 0.821), fasting glucose (p = 0.687), triglycerides (p = 0.082), or total cholesterol (p = 0.768) throughout the trial, except for a significant effect of group-by-time interaction on the HbA1c (p = 0.008). Abbreviations: BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin.](https://static.elsevier.es/multimedia/16652681/0000002700000006/v3_202307180533/S1665268122000874/v3_202307180533/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




