Introduction and aim. Acute-on-chronic liver failure (ACLF) is defined by the development of acute deterioration of liver function associated with failure of other organs and high short-term mortality in patients with chronic liver disease (CLD). There is no consensus on the diagnostic criteria, and its independence from ordinary decompensation of CLD has frequently been questioned. This study aimed to identify and characterize this condition and to test the CLIF-C OF score comparing it to the 2016-MELD (with sodium) and the Child-Pugh.
Material and methods. 18-month prospective observational study with systematic inclusion of admitted patients with CLD decompensation.
Results. 39 patients had ACLF (33.1%). These patients experienced higher 28-day and 90-day mortality, when compared to patients without ACLF (43.6% and 64.1% vs. 2.5% and 7.6% respectively, p < 0.0001). ACLF was linked with a higher acute infection rate (74.4%). For all patients (N = 118), the scores 2016-MELD, CLIF-C OF and Child-Pugh showed an area under the curve (AUC) for 28-day mortality of 0.908, 0.844, 0.753 and for 90-day of 0.902, 0.814, 0.724 respectively, p < 0.0001 for all scores. The 90-day mortality 2016-MELD AUC was greater than the CLIF-C OF AUC, p = 0.021. Within ACLF patients, the 2016-MELD, CLIF-C ACLF and Child-Pugh scores showed an AUC of 0.774, 0.734, 0.584 (28-day) and 0.880, 0.771, 0.603 (90-day); for 2016-MELD p = 0.004 (28-day) and p < 0.0001 (90-day).
Conclusion. ACLF is a frequent and relevant condition, associated with high mortality. The CLIF-C OF score revealed good accuracy and diagnoses ACLF when it is present. However, the 2016-MELD performed better for 90-day mortality prediction.
Acute-on-chronic liver failure (ACLF) is a clinical entity related to cirrhosis decompensation that has been the focus of research over the last years. The concept is defined by the development of acute deterioration of liver function associated with other organ failure (OF), usually after an acute event, in a patient with chronic liver disease (CLD).1 It is distinct from an ordinary decompensation of CLD and leads to elevated short-term mortality, generally assumed to be at least > 15% on the 28th day. However, there is no consensus on the definition since different societies have provided distinct criteria.2,3 In recent years, the clinical relevance and even its existence as an autonomous nosological entity has been questioned and challenged.4 In 2013, a multi-center trial denominated CANONIC conducted by a European group, the Chronic Liver Failure Consortium (CLIF-C), generated new scientific evidence to support the concept that ACLF is an independent condition.5 This landmark study led to the development of a new score, the CLIF-SOFA. This score is based on the, already existing, sepsis-related organ failure assessment (SOFA) score6 with the proper adjustments for liver failure. It congregates all the criteria that define each possible OF. Those events of organ failure, when present, lead to the ACLF syndrome. The CLIF-SOFA was later simplified into the CLIF-C OF to allow the direct diagnosis of the syndrome by determining the specific organ dysfunction or failure. Additionally, the same CLIF consortium developed two new scores. The CLIF-C ACLF (CLIF-C acute-on-chronic liver failure) score, that is used to determine prognosis when ACLF is present, and the CLIF-C AD (CLIF-C acute decompensation) score, for acute decompensation (AD) without ACLF.7 In their study, the CLIF consortium suggests that the CLIF-C OF is a better score for the assessment of prognosis compared to the model for end-stage liver disease (MELD). When used appropriately, both the CLIF-C ACLF and the CLIF-C AD also performed better than other scores such as the MELD and Child-Pugh.7,8
The MELD score was developed in the early 2000s. It was intended to anticipate the survival probability of patients undergoing the transjugular intrahepatic portosystemic shunt procedure.9 After validation, the score was extended to the general prognosis in the field of chronic liver disease, and, more importantly, it was applied to organ allocation in liver transplantation programs all over the world.10
Initially, this score integrated the values of bilirubin, creatinine, international normalized ratio (evaluating prothrombin time) and the etiology of the liver disease that was posteriorly eliminated.9 Some years later, new evidence showed the importance of hyponatremia as a marker of advanced decompensated cirrhosis, and, therefore, warranted its inclusion in the MELD score.11,12 This led to the expansion of scores incorporating sodium value, with the model for end-stage liver disease-sodium (MELD-Na) being the most notable.13,14 In 2016, the Organ Procurement and Transplantation Network changed the official MELD formula used for organ allocation in the USA, adding the sodium value to the original MELD.15 To avoid confusion, we refer to this later iteration of the MELD score as the 2016 MELD throughout this study. While both 2016 MELD and MELD-Na incorporate sodium, these two scores are distinct as they have different calculation formulae.
ObjectiveThis study aimed to identify the incidence of ACLF (major outcome) and characterize this condition in a Gastroenterology department including its associated intensive care unit (ICU). Secondly, we wanted to evaluate the prognostic accuracy in determining short-term (28-day) and 90-day mortality of the scores CLIF-C OF, “new” 2016 MELD and Child-Pugh. Thirdly, we sought to test the scores CLIF-C ACLF and CLIF-C AD in their respective groups.
Material and MethodsStudy designWe designed a prospective, single-center observational study with systematic inclusion of every patient admitted to the Gastroenterology ward or ICU diagnosed with decompensation of CLD motivated by encephalopathy, refractory or new onset ascites, jaundice, gastrointestinal bleeding or acute infection.
CLD was defined as cirrhosis assessed before or at the admission by biopsy or by a combination of clinical signs, laboratory and imaging alterations.
Infection included spontaneous bacterial peritonitis (SBP) or acute bacterial assumed infection with other primary location (urinary tract, respiratory tract, a different site or bacteremia with no apparent origin) identified by clinical, laboratory and imaging typical changes, justifying admission.
Exclusion criteria considered were: any stage of hepatocellular carcinoma, acute or chronic extra-hepatic severe medical condition that might have contributed to the acute decompensation and organ failure, particularly, chronic kidney disease, chronic decompensated heart failure, human immunodeficiency virus infection, any oncologic active condition, and trauma. Patients without at least three months of follow-up after study recruitment were also excluded.
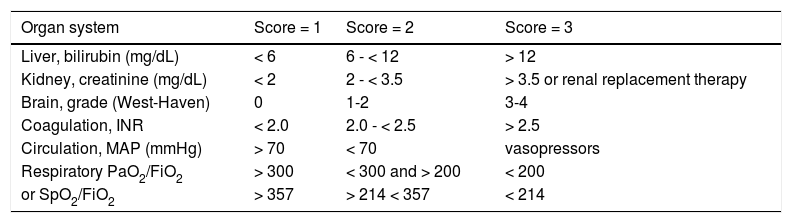
ACLF definition and scores calculationFor ACLF diagnosis the criteria produced by the CLIF-C group in the CANONIC study5 were followed because it was considered to better represent the concept supporting this clinical condition. Consequently, ACLF was assumed whenever one of the three following circumstances was present: renal failure alone defined by acute, de novo elevation of creatinine > 2 mg/dL; renal dysfunction (creatinine 1.5-1.9 mg/dL) and/or cerebral dysfunction (hepatic encephalopathy grade 1-2 by West Haven classification) together with any other OF; two to three any OFs. The criteria for organ failure are defined in table 1. ACLF grade 1 was assumed if there was only one OF, grade 2 for 2 OF and 3 OF defined grade 3.
Specific organ parameters allowing determination of organ failures (OF) and the calculation of the CLIF-C OF score.
| Organ system | Score = 1 | Score = 2 | Score = 3 |
|---|---|---|---|
| Liver, bilirubin (mg/dL) | < 6 | 6 - < 12 | > 12 |
| Kidney, creatinine (mg/dL) | < 2 | 2 - < 3.5 | > 3.5 or renal replacement therapy |
| Brain, grade (West-Haven) | 0 | 1-2 | 3-4 |
| Coagulation, INR | < 2.0 | 2.0 - < 2.5 | > 2.5 |
| Circulation, MAP (mmHg) | > 70 | < 70 | vasopressors |
| Respiratory PaO2/FiO2 | > 300 | < 300 and > 200 | < 200 |
| or SpO2/FiO2 | > 357 | > 214 < 357 | < 214 |
Patients were included only once, at their first admission during the study period, either having ACLF or CLD decompensation without ACLF. To ensure that the ACLF trigger was present before or just after admission, the study allowed only the diagnosis of ACLF from admission to the hospital to the 7th day of hospitalization. As a clinical trigger, we considered, as in the CANONIC study, the main factors that could originate the syndrome: infection, current alcohol drinking, acute reactivation of chronic viral hepatitis or a recent medical procedure like paracentesis or transjugular intrahepatic portosystemic shunt positioning. None of the causes of CLD decompensation were considered as potential triggers (gastrointestinal bleeding was considered in CANONIC) to select and determine the potential specific triggers. Infection was defined by the criteria expressed above. Active alcohol consumption was defined as drinking, at least, 14 drinks per week for women and 21 drinks per week in men within the last three months (based on CANONIC criteria). Data were collected prospectively and analyzed retrospectively. The scores were calculated at the first point of admission (with or without ACLF) or calculated afterward in the cases when the patients developed ACLF in the initial days of hospital admission. Then, they were correlated with the 28-day and 90-day mortality to determine prognostic accuracy.
The 2016 MELD score was announced after the last of the patients had been enrolled in the study. It was decided that the CLIF-C OF should be tested with an equivalent up-to-date quality score. Therefore, the “new” 2016 MELD version was assumed (referred in this article as 2016 MELD), while original score editing was made according to the laboratory data (including natremia) corresponding to the patient’s admission date, consequently preserving the initial study design and solely updating the MELD values.
The 2016 MELD was calculated with the following formula (Figure 1A).
If the MELD(i) was higher than 11, additional MELD was calculated as follows (Figure 1B).
Statistical analysisFor categorical variables, the chi-squared test (x2 test) and the Fisher’s exact test, for groups with less than 20 cases, were used. Normality was assessed with the Kolmogorov-Smirnov and Shapiro-Wilk tests. Continuous variables were expressed as the mean ± standard deviation (SD) or as the median and interquartile range (IQR) whether they were parametric or non-parametric and the Student’s t-test or Mann-Whitney U test were used, respectively. Multivariate analysis was executed with binary logistic regression. Receiver operating characteristic (ROC) curves were calculated, and the area under the curve (AUC) was evaluated do determine the score accuracy (c-statistic). A p < 0.05 was considered for statistical significance. SPSS was used for all tests, ROC curves design and AUC calculations (SPSS v. 20 for OS, Chicago, IL, USA). ROC curves AUC analysis between different scores, using DeLong test, was executed with Medcalc (Medcalc v. 17.9, MedCalc Software bvba, Ostend, Belgium).
Ethical considerationsThis was an observational study with no interference in patient's follow-up or medical decision making. It was validated by the local ethical committee. The patient’s anonymity was kept, and all principles of Helsinki Declaration were respected.
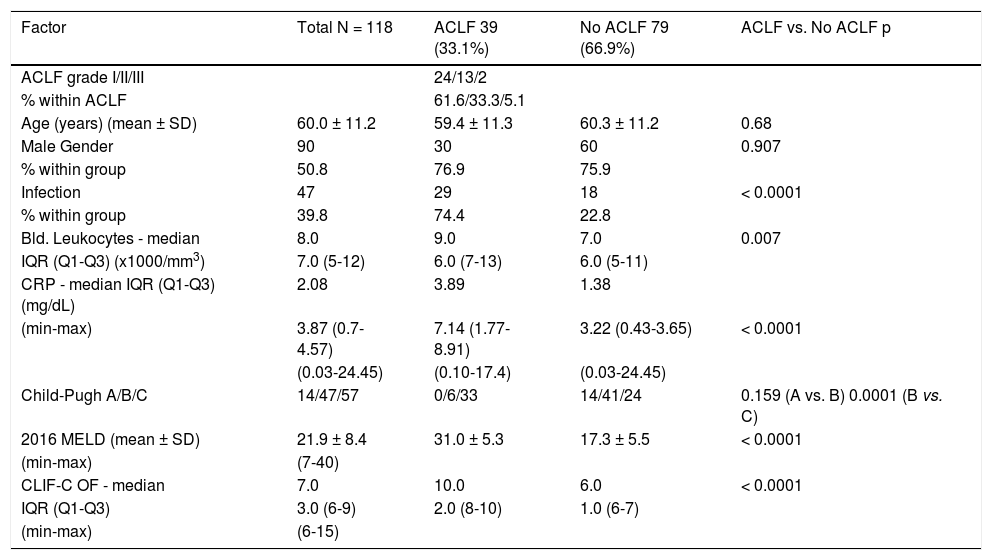
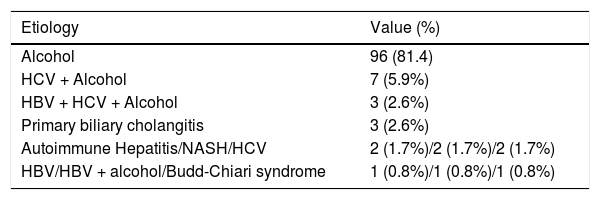
ResultsACLF incidenceDuring the study’s 18 months duration (July 2014 to December 2015) all the patients admitted to the department ward or specific ICU with decompensation of past or recently recognized CLD were systematically considered for study incorporation. After strict use of the exclusion criteria, 118 patients were enrolled. The majority were men: 90 (76.3%), with a mean age of 60.0 ± 11.2 years, limits of 36 and 84. The leukocytes, C-reactive protein (CRP) and CLIF-C OF showed a non-normal distribution: Kolmogorov-Smirnov and Shapiro-Wilk tests < 0.0001 for these variables. Therefore, medians (instead of means) were compared for these three variables (Table 2). The most common etiologies of CLD were alcohol consumption (81.4%), hepatitis C virus (HCV) infection and alcohol consumption (5.9%), hepatitis B virus infection (HBV) plus HCV and alcohol consumption (2.6%) and primary biliary cholangitis (2.6%). The specific CLD etiologies are expressed in table 3. As the major outcome, 39 patients admitted had ACLF (33.1%), the majority, 24 with grade 1 ACLF (61.6%), 13 with grade 2 (33.3%) and 2 with grade 3 (5.1%). The mortality was higher both at 28-day for ACLF grade 2 comparing to grade 1: 76.9% vs. 25%, p = 0.002, and at 90-day: 92.3% vs. 45.8%, p = 0.005. Because grade 3 was represented by only 2 cases, no correlations can be established with this grade.
Patients' data summary for the global, acute-on-chronic liver failure (ACLF) and acute decompensation (AD) groups.
| Factor | Total N = 118 | ACLF 39 (33.1%) | No ACLF 79 (66.9%) | ACLF vs. No ACLF p |
|---|---|---|---|---|
| ACLF grade I/II/III | 24/13/2 | |||
| % within ACLF | 61.6/33.3/5.1 | |||
| Age (years) (mean ± SD) | 60.0 ± 11.2 | 59.4 ± 11.3 | 60.3 ± 11.2 | 0.68 |
| Male Gender | 90 | 30 | 60 | 0.907 |
| % within group | 50.8 | 76.9 | 75.9 | |
| Infection | 47 | 29 | 18 | < 0.0001 |
| % within group | 39.8 | 74.4 | 22.8 | |
| Bld. Leukocytes - median | 8.0 | 9.0 | 7.0 | 0.007 |
| IQR (Q1-Q3) (x1000/mm3) | 7.0 (5-12) | 6.0 (7-13) | 6.0 (5-11) | |
| CRP - median IQR (Q1-Q3) (mg/dL) | 2.08 | 3.89 | 1.38 | |
| (min-max) | 3.87 (0.7-4.57) | 7.14 (1.77-8.91) | 3.22 (0.43-3.65) | < 0.0001 |
| (0.03-24.45) | (0.10-17.4) | (0.03-24.45) | ||
| Child-Pugh A/B/C | 14/47/57 | 0/6/33 | 14/41/24 | 0.159 (A vs. B) 0.0001 (B vs. C) |
| 2016 MELD (mean ± SD) | 21.9 ± 8.4 | 31.0 ± 5.3 | 17.3 ± 5.5 | < 0.0001 |
| (min-max) | (7-40) | |||
| CLIF-C OF - median | 7.0 | 10.0 | 6.0 | < 0.0001 |
| IQR (Q1-Q3) | 3.0 (6-9) | 2.0 (8-10) | 1.0 (6-7) | |
| (min-max) | (6-15) |
Etiologies of chronic liver disease in the patients enrolled.
| Etiology | Value (%) |
|---|---|
| Alcohol | 96 (81.4) |
| HCV + Alcohol | 7 (5.9%) |
| HBV + HCV + Alcohol | 3 (2.6%) |
| Primary biliary cholangitis | 3 (2.6%) |
| Autoimmune Hepatitis/NASH/HCV | 2 (1.7%)/2 (1.7%)/2 (1.7%) |
| HBV/HBV + alcohol/Budd-Chiari syndrome | 1 (0.8%)/1 (0.8%)/1 (0.8%) |
Approximately one-third of the patients (33.9%) were admitted to the ICU. The incidence of ACLF was not significantly different in this group, when compared to those admitted to the ward: 37.5% (15/40) vs. 30.8% (24/78), p = 0.462. All the other clinical and laboratory main patients’ characteristics are summarized in table 2. The development of ACLF when comparing the patients with CLD caused only by active or previous alcohol consumption, with no viral hepatitis as a co-factor, with the patients with other etiologies, was not significantly different, 33.3% vs. 31.8%, p = 0.892.
There were no significant differences in age or gender, but the ACLF group was associated with much higher values of blood leukocytes, CRP and 2016 MELD values.
The ACLF group was characterized by a significant higher short-term (28-day) and 90-day mortality compared to the no-ACLF group: 43.6% and 64.1% vs. 2.5% and 7.6% respectively, p < 0.0001.
Acute infection was the main trigger for ACLF, it was present in 74.4% cases. Active alcoholism also played a role, as it was present in 31.7% cases. In more than half of these (17.9%), active alcohol consumption was present simultaneously with acute infection. In 11.8% cases, a clear trigger could not be identified.
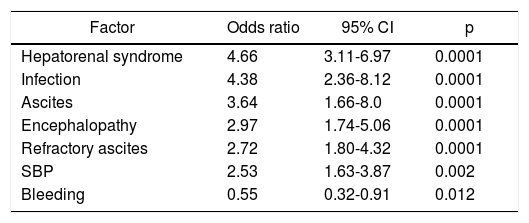
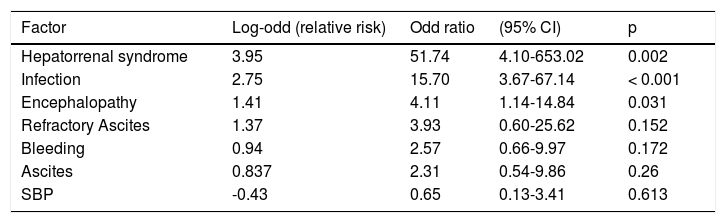
Univariate and multivariate analysisIn the univariate analysis, we looked for the admission causes and other risk factors to test for a potential relationship with ACLF development. Thus, hepatorenal syndrome, infection by SBP or from another site, ascites, encephalopathy, refractory ascites and SBP all correlated with higher ACLF risk. On the other hand, acute upper digestive bleeding (variceal bleeding or not) had a negative correlation for ACLF (odds ratio 0.55) p = 0.012 (Table 4). However, in the global multivariate analysis only the presence of hepatorenal syndrome, infection and encephalopathy showed a statistically significant correlation with ACLF diagnosis (Table 5).
Univariate analysis of risk factors for acute-on-chronic liver failure (ACLF) development.
| Factor | Odds ratio | 95% CI | p |
|---|---|---|---|
| Hepatorenal syndrome | 4.66 | 3.11-6.97 | 0.0001 |
| Infection | 4.38 | 2.36-8.12 | 0.0001 |
| Ascites | 3.64 | 1.66-8.0 | 0.0001 |
| Encephalopathy | 2.97 | 1.74-5.06 | 0.0001 |
| Refractory ascites | 2.72 | 1.80-4.32 | 0.0001 |
| SBP | 2.53 | 1.63-3.87 | 0.002 |
| Bleeding | 0.55 | 0.32-0.91 | 0.012 |
Multivariate analysis of risk factors for acute-on-chronic liver failure (ACLF) development.
| Factor | Log-odd (relative risk) | Odd ratio | (95% CI) | p |
|---|---|---|---|---|
| Hepatorrenal syndrome | 3.95 | 51.74 | 4.10-653.02 | 0.002 |
| Infection | 2.75 | 15.70 | 3.67-67.14 | < 0.001 |
| Encephalopathy | 1.41 | 4.11 | 1.14-14.84 | 0.031 |
| Refractory Ascites | 1.37 | 3.93 | 0.60-25.62 | 0.152 |
| Bleeding | 0.94 | 2.57 | 0.66-9.97 | 0.172 |
| Ascites | 0.837 | 2.31 | 0.54-9.86 | 0.26 |
| SBP | -0.43 | 0.65 | 0.13-3.41 | 0.613 |
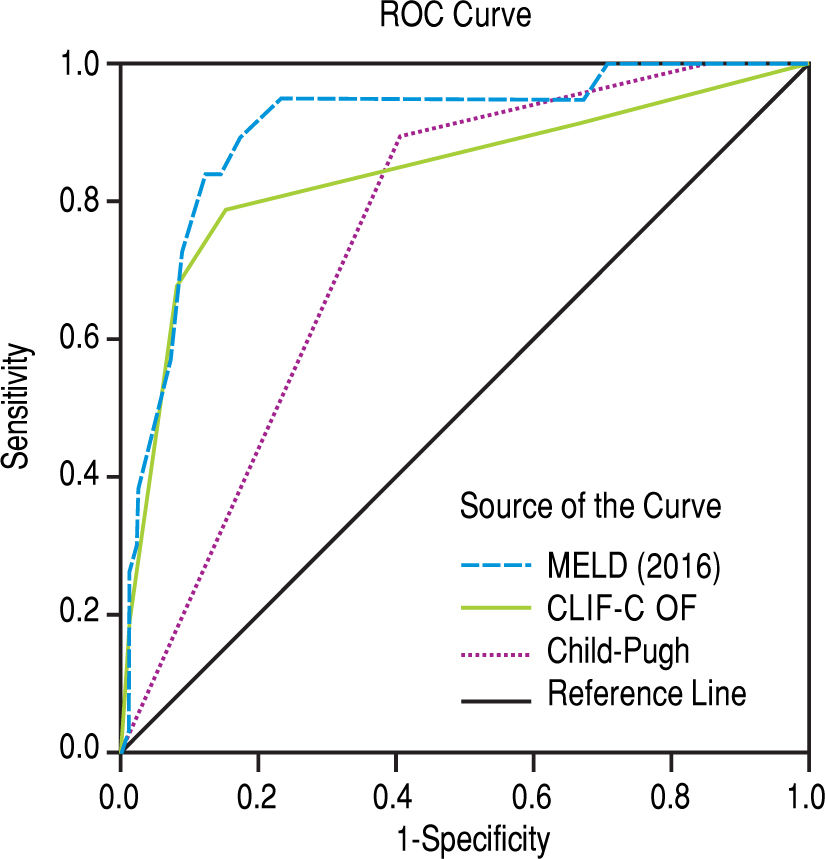
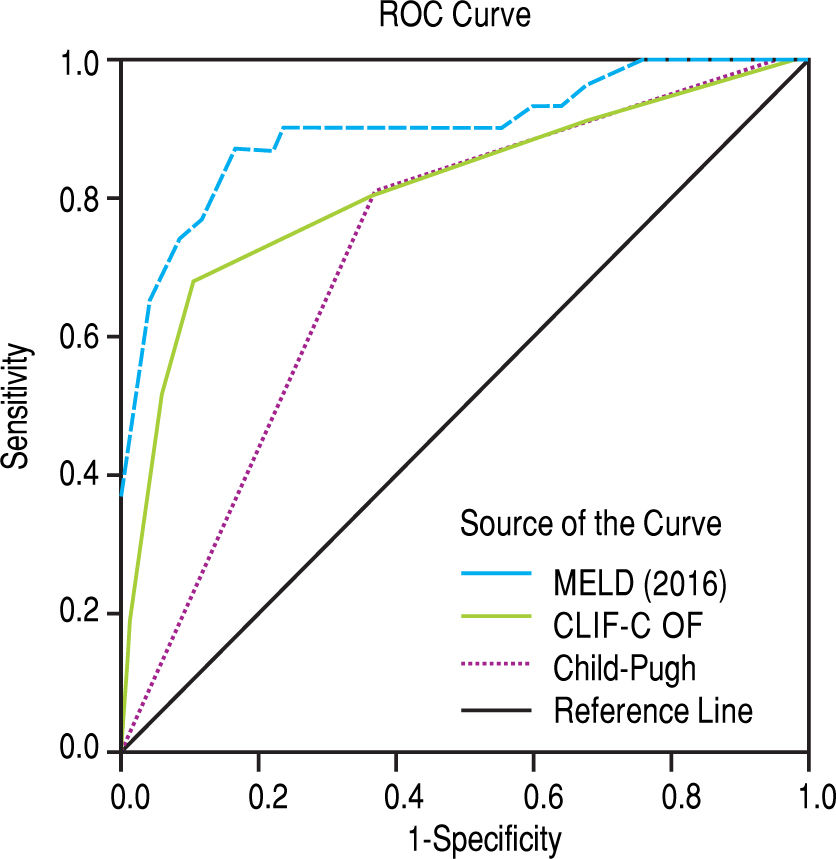
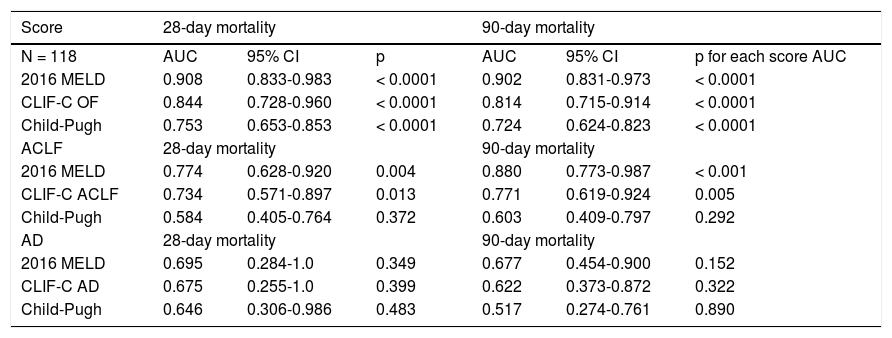
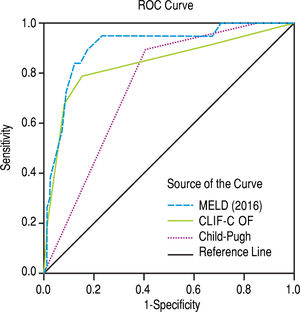
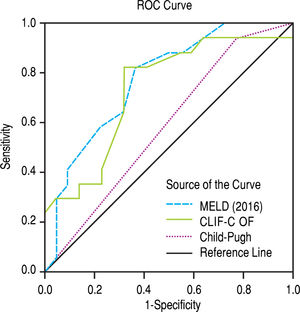
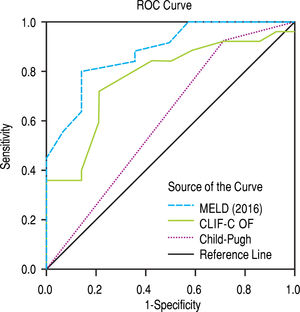
The scores 2016 MELD and CLIF-C OF in the group with ACLF had much higher values, 31.0 ± 5.3 vs. 17.3 ± 5.5 and 10.0, IQR 2.0 vs. 6.0, IQR 1.0, respectively. In the entire study group (N = 118) the AUC for the 28-day mortality determination with the scores 2016 MELD, CLIF-C OF, and Child-Pugh was respectively 0.908, 0.844, 0.753 (Figure 2) and for the 90-day mortality 0.902, 0.814, 0.724 (Figure 3), p < 0.0001 for AUC in all scores (Table 6). Comparing the AUCs with the DeLong test in the 28-day mortality period, only the 2016 MELD vs. Child-Pugh difference was significant, p < 0.0001. Conversely, in the 90-day period, the 2016 MELD vs. CLIF-C OF, 2016 MELD vs. Child-Pugh and CLIF-C OF vs. Child-Pugh differences were statistically significant, respectively, p = 0.021, p < 0.0001, and p = 0.035.
Area under curve (AUC) for the scores 2016 MELD, CLIF-C OF and Child-Pugh anticipating 28-day and 90-day mortality in the global group (N) and the specific scores for the acute-on-chronic liver failure (ACLF) and acute decompensation (AD) subgroups.
| Score | 28-day mortality | 90-day mortality | ||||
|---|---|---|---|---|---|---|
| N = 118 | AUC | 95% CI | p | AUC | 95% CI | p for each score AUC |
| 2016 MELD | 0.908 | 0.833-0.983 | < 0.0001 | 0.902 | 0.831-0.973 | < 0.0001 |
| CLIF-C OF | 0.844 | 0.728-0.960 | < 0.0001 | 0.814 | 0.715-0.914 | < 0.0001 |
| Child-Pugh | 0.753 | 0.653-0.853 | < 0.0001 | 0.724 | 0.624-0.823 | < 0.0001 |
| ACLF | 28-day mortality | 90-day mortality | ||||
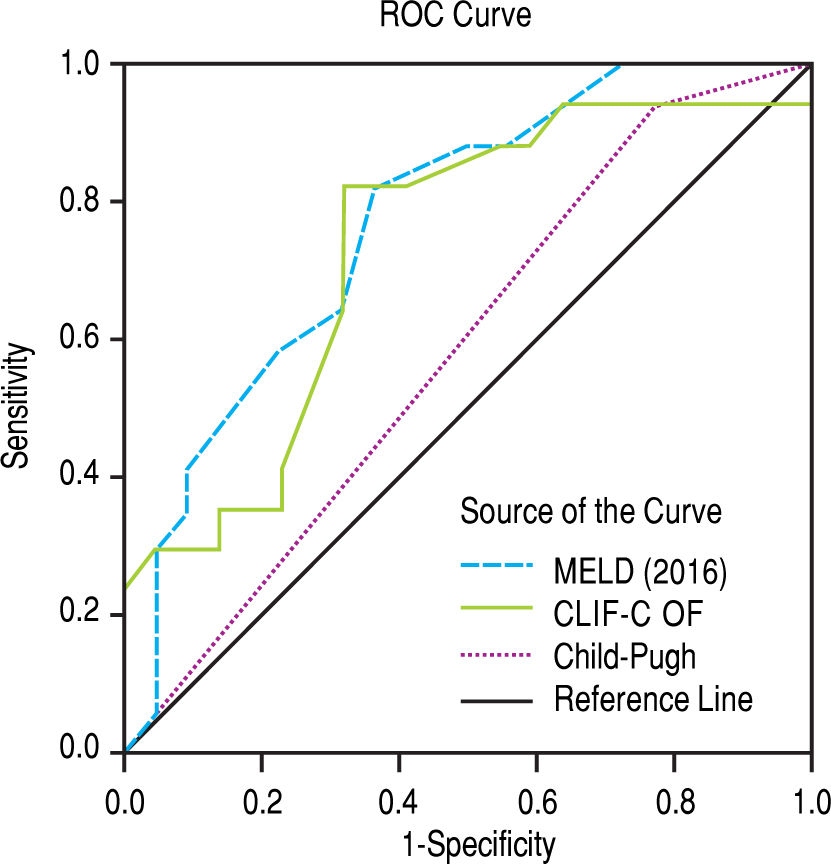
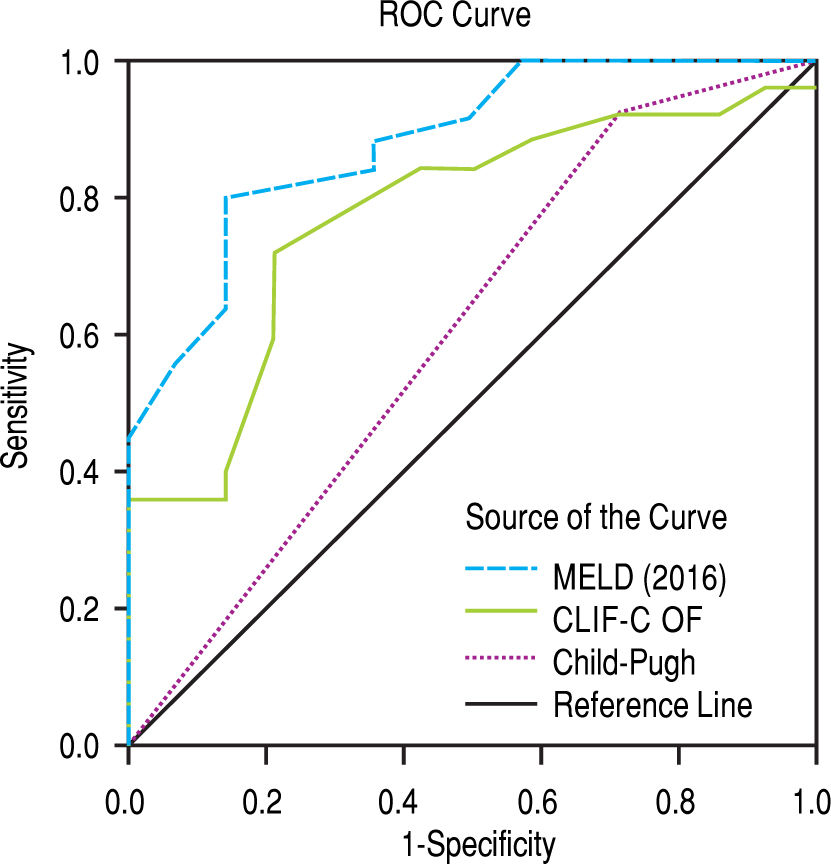
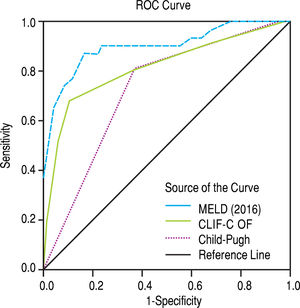
| 2016 MELD | 0.774 | 0.628-0.920 | 0.004 | 0.880 | 0.773-0.987 | < 0.001 |
| CLIF-C ACLF | 0.734 | 0.571-0.897 | 0.013 | 0.771 | 0.619-0.924 | 0.005 |
| Child-Pugh | 0.584 | 0.405-0.764 | 0.372 | 0.603 | 0.409-0.797 | 0.292 |
| AD | 28-day mortality | 90-day mortality | ||||
| 2016 MELD | 0.695 | 0.284-1.0 | 0.349 | 0.677 | 0.454-0.900 | 0.152 |
| CLIF-C AD | 0.675 | 0.255-1.0 | 0.399 | 0.622 | 0.373-0.872 | 0.322 |
| Child-Pugh | 0.646 | 0.306-0.986 | 0.483 | 0.517 | 0.274-0.761 | 0.890 |
After subdivision depending on whether ACLF was present or not, within the group with this condition, the values for assessing 28-day mortality with the scores 2016 MELD, CLIF-C ACLF and Child-Pugh were respectively 0.774, 0.734, and 0.584; p = 0.004 and p = 0.013 for the 2016 MELD and CLIF-C ACLF AUC (Figure 4). For the 90-day mortality, the results were: 0.880, 0.771, and 0.603; p < 0.0001 and p = 0.005 for the 2016 MELD and CLIF-C ACLF AUC (Figure 5, Table 6).
Comparing the AUCs in the ACLF 28-day and 90-day periods, only the 2016 MELD versus Child-Pugh difference was significant, respectively, p = 0.020 and p = 0.0001.
In the group without ACLF, for anticipating the 28-day and 90-day mortality the values for the scores 2016 MELD, CLIF-C AD and Child-Pugh were respectively 0.695, 0.675, 0.646 and 0.677, 0.622, 0.517; no statistical significance (Table 6).
During the period of patients’ follow-up, at least until three months after the last patient was enrolled, only five patients were submitted to liver transplantation, with no differences between groups: 2 within ACLF vs. 3 in the no-ACLF, p = 0.736.
DiscussionACLF and risk factorsACLF is a condition based on a relatively recent concept that has became more clearly defined by new precise criteria supported by good quality medical scientific evidence.5 While the CLIF consortium considers this entity a complete change in the cirrhosis paradigm,16 the real-life clinical utility and relevance of this unique condition has been contested. Addressing this question was the first motivation to conduct this study. Besides assessing its unique behavior and independence, we wanted to test the new score CLIF-C OF and its associated scores that proved to be promising in recent data.16
First, this study confirms that ACLF is an individual entity characterized by high mortality (> 15%) with values reaching 43.6% and 64.1%, respectively for 28 and 90-day, with significant differences from the group without ACLF. This higher mortality, when compared with the CANONIC study (29.6% and 51.1%), can be justified by our very high rate of acute infection in the ACLF group (74.4%) that is known to be a crucial prognosis factor for poor outcome.17-20 Additionally, this study included a considerable percentage of patients with alcohol consumption even when other CLD causes, like chronic HBV or HCV infection, were present. While maintenance of active alcohol habits was identified objectively in only 31.7%, this percentage might be an underestimation. In fact, current alcohol consumption is a known ACLF development trigger and has also been linked to higher mortality, which is known to be connected to the common combination of acute alcoholic hepatitis and ACLF, both developing in association to an intense pro-inflammatory back-ground.5,17
The incidence of ACLF (33.1%) was similar to the one in the CANONIC study (30.9%), suggesting that this condition is quite frequent in the CLD clinical setting. Surprisingly, there was no considerable difference in the presence of ACLF in the ICU versus general ward (37.5% vs. 30.8%). One can say that this is justified because ACLF can develop in a patient that apparently is clinically stable, and, for that reason, is not admitted to the ICU. However, if the patient presents, for example, with acute kidney injury with creatinine rising to values > 2.0 mg/dL in the context of acute infection, ACLF is present even if the patient may seem to be apparently “well”. Conversely, a patient that receives a closer observation in an ICU setting, because his admission was motivated by variceal bleeding, has a lower risk for ACLF development as our analysis and other studies indicate.5,21 This fact strongly emphasizes the possibility that ACLF is not commonly associated with gastrointestinal bleeding in the context of CLD. The systematic use of antibiotic prophylaxis following upper variceal bleeding, such as a 7-day ceftriaxone course used in our cohort, may play a crucial role in this issue. In this context, our study underscores the importance of awareness to slight changes in the cirrhotic patient, such as infection occurrence or acute kidney injury since they can lead to or consist of the first signs of ACLF.
In what concerns the risk factors, the multivariate analysis confirmed the concept that infection is a relevant potential trigger of ACLF. Hepatorenal syndrome and portosystemic encephalopathy also showed high correlation with ACLF, but this must be interpreted with caution because this fact probably reflects the organ dysfunction or failure consequent to the ACLF syndrome.
Scores and their prognostic accuracyThe new score CLIF-C OF performed very well as a prognostic tool to predict mortality at 28-day and 90-day, respectively with an AUC of 0.844 and 0.814 achieving good accuracy. However, the 2016 MELD score performed even better for both periods, although only significantly better for the 90-day mortality period. The values obtained were considerably higher than the ones found by a meta-analysis.22 In that study, the MELD AUC for “inhospital mortality” and 90-day mortality were respectively 0.744 and 0.794. However, it is not possible to establish a precise comparative analysis because we used the 2016 MELD (incorporating sodium) and those values are related to the MELD without sodium (before 2016).
Accordingly, the original MELD with sodium integration (2016 MELD) still has a relevant role in the era of the CLIF-C OF.
As expected, the score Child-Pugh was not as accurate as the others, particularly, comparing to the 2016 MELD that performed significantly better for both time periods.
In the ACLF group, the score CLIF-C ACLF showed an accuracy for 28 and 90-day mortality of0.734 and 0.771, respectively, that can be considered good and close (even higher for 90-day) to the AUC published in the study that created this score (0.790 and 0.760).7 However, the 2016 MELD score showed a, not significantly different, higher value of 0.774 and 0.880.
Thus, the 2016 MELD performed, at least, with the same accuracy as the CLIF-C ACLF and this demonstrates its reliability as a score, even in the presence of ACLF.
When applied in the group without ACLF all the scores showed worse results and, particularly, the CLIF-C AD did not perform well at all. This group was more heterogeneous (comparing to the ACLF) and included patients in different stages of decompensation, motivated by various causes of decompensation. Therefore, the scores might have reflected the dispersion of outcomes that result from that creating some degree of unpredictability. These results also lead to theorizing that the more severe the patient’s condition is, the more accurate the score is likely to be.
Overall, we argue that the score CLIF-C OF is a good predictor of short-term and 90-day mortality allowing direct diagnosis of ACLF. The score 2016 MELD performed even better in the 90-day period defining an excellent accuracy. We hypothesize that the 2016 MELD score acquired a better prognostic accuracy following its recent changes in the formula integrating the sodium value. This theory still needs to be tested in other larger scale cohorts.
Management of ACLF is currently limited because there are no specific effective treatments. Liver transplantation is the only available option that can significantly improve long-term survival, and, consequently, must be systematically considered as a possibility.17 The patient should be admitted to an intensive care unit, and general management should be focused on the treatment of potential triggers such as infection and supporting organ failures. Liver support devices, such as molecular adsorbent recirculating system (MARS) or Prometheus, can be offered if available, but the current evidence did not prove their role in increasing survival.23 New biologic agents, like granulocyte-colony stimulating factor, are being tested and the first trials look promising.24
Although the treatment of ACLF is beyond the scope of this study, we noticed a very low number of patients that were submitted to liver transplantation. This fact can be due to several factors. First, a substantial percentage of patients did not fulfill the transplantation criteria, namely because of acute alcoholic hepatitis. Additionally, the high rate of patients with a severe global condition and acute infection leading to the status of “too sick to transplant”, may have contributed.
This study has some limitations. First, the sample is not extensive enough to allow us to conclude with the same certainty as in other large cohort multi-centric trials. This factor adversely impacted the subdivision of ACLF/no ACLF groups, in specific. While this is a prospective study, the accuracy of some data recording may be compromised because of the subjective nature of some clinical variables. Nevertheless, the principal scores that were tested, CLIF-C OF and 2016 MELD, include mainly objective data (except encephalopathy in CLIF-C OF). The study also incorporates Child-Pugh in the comparative analysis because it is a widely-accepted score that includes subjective variables (encephalopathy and ascites), allowing us to test those factors.
In conclusion, the present study provides evidence that ACLF is an independent syndrome associated with organ failure and to higher short-term mortality (> 15%). Acute infection acts as a strong potential trigger associated with this syndrome.
The score CLIF-C OF has a good prediction accuracy and the advantage of automatically defining ACLF when this condition is present. However, in our data, it did not suggest being better than the 2016 MELD, which showed excellent accuracy.
Abbreviations- •
ACLF: acute-on-chronic liver failure.
- •
OF: organ failure.
- •
CLD: chronic liver disease.
- •
CLIF-C: chronic Liver failure consortium.
- •
SOFA: Sepsis-related organ failure assessment.
- •
CLIF-C ACLF: CLIF-C acute-on-chronic liver failure.
- •
CLIF-C AD: CLIF-C acute decompensation.
- •
AD: acute decompensation.
- •
MELD: model for end-stage liver disease.
- •
MELD-Na: model for end-stage liver disease-sodium.
- •
ICU: ntensive care unit.
- •
SBP: spontaneous bacterial peritonitis.
- •
SD: standard deviation.
- •
IQR: interquartile range.
- •
ROC: receiver operating characteristic.
- •
AUC: rea under curve.
- •
CRP: C -reactive protein.
- •
HCV: hepatitis C virus.
- •
HBV: hepatitis B virus.
- •
MARS: molecular adsorbent recirculating system.
The authors declares that there is no conflict of interest regarding the publication of this article.