Introduction and aim. Thrombosis is a vascular disorder of the liver often associated with significant morbidity and mortality. Cirrhosis is a predisposing factor for portal venous system thrombosis. The aim of this study is to determine differences between cirrhotics and non-cirrhotics that develop thrombosis in portal venous system and to evaluate if cirrhosis severity is related to the development of portal venous system thrombosis.
Material and methods. We studied patients diagnosed with portal venous system thrombosis using contrast-enhanced computed tomography scan and doppler ultrasound at Medica Sur Hospital from 2012 to 2017. They were categorized into two groups; cirrhotics and non-cirrhotics. We assessed the hepatic function by Child-Pugh score and model for end-stage liver disease.
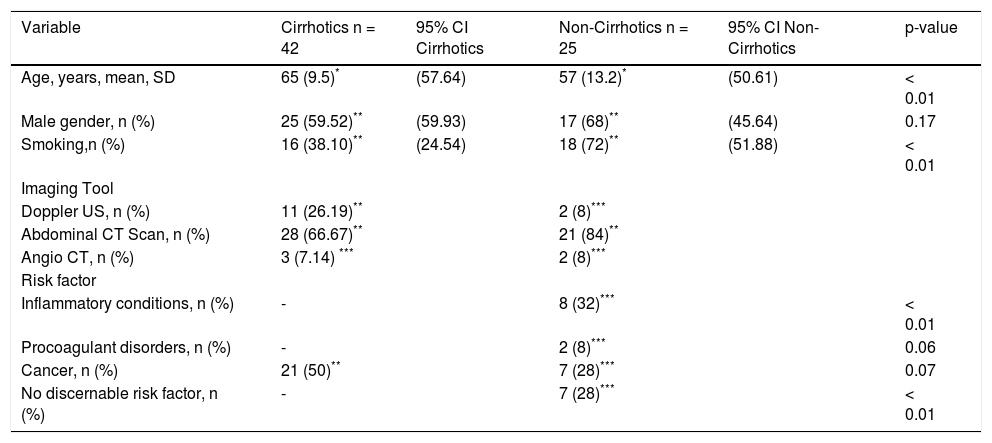
Results. 67 patients with portal venous system thrombosis (25 with non-cirrhotic liver and 42 with cirrhosis) were included. The mean age (± SD) was 65 ± 9.5 years in cirrhotic group and 57 ± 13.2 years (p = 0.009) in noncirrhotic group. Comparing non-cirrhotics and cirrhotics, 8 non-cirrhotic patients showed evidence of extra-hepatic inflammatory conditions, while in the cirrhotic group no inflammatory conditions were found (p < 0.001). 27 (64.29%) cirrhotic patients had thrombosis in the portal vein, while only 9 cases (36%) were found in non-cirrhotics (p = 0.02).
Conclusions. In cirrhotic patients, hepatocellular carcinoma and cirrhosis were the strongest risk factors to develop portal venous system thrombosis. In contrast, extrahepatic inflammatory conditions were main risk factors associated in non-cirrhotics. Moreover, the portal vein was the most frequent site of thrombosis in both groups.
Cirrhosis is a condition in which the hemostatic system is altered. Hepatic coagulation factors are decreased following reduction in hepatic synthesis and platelet count.1,2 However, there are increased levels of non-hepatic procoagulant factors such as factor VIII and Von Willebrand factor and a decrease of anticoagulant factors,1 whereby in certain situations there is a procoagulant tendency.3,4 This explains why epidemiological studies have demonstrated an increased incidence of thrombotic phenomena in patients with chronic liver diseases, especially those with cirrhosis.5,6 Portal venous system thrombosis (PVST) may occur either in non-cirrhotic and cirrhotic patients. In the general population, it is a rare event and the frequency is about 1%7 while in cirrhotic patients occurs relatively frequently (0.6 to 26%),8 depending on the imaging tools used for diagnosis and the clinical characteristics of the evaluated patients. PVST refers to blood clots in the portal vein, splenic and superior mesenteric veins or intrahepatic portal vein branches.9 PVST is a known complication of cirrhosis, mainly influenced by the reduced flow velocity of blood in the portal vein to less than 15 cm/s and perhaps a tendency to thrombophilia in patients with advanced liver disease. At present, few studies have assessed the prevalence of thrombosis in every branch of portal vein system in patients with cirrhosis. Therefore, we sought to investigate whether severity of cirrhosis was linked to the risk of developing PVST as well as to the site and extent of the obstruction in the portal venous system. Furthermore, we compared characteristics of PVST between cirrhotics and non-cirrhotics.
Material and MethodsStudy designWe conducted a cross-sectional retrospective study, from January 1, 2012 to May 31, 2017 at Medica Sur Hospital, Mexico City. The local research ethics committee approved this study (#2017-EXT-216).
Patients and data collectionTo identify patients with PVST diagnosis, we reviewed all imaging records (abdominal computed tomography (CT) scan with contrast, doppler ultrasound (US) and angiography CT) from our institution during the study period. We originally found 83 patients with PVST, but we decided to exclude 16 patients due to incomplete personal health record. Thereafter, patients were categorized into two groups; cirrhotics and non-cirrhotics.
For all cases, we retrospectively obtained medical records, demographic data, comorbidities, biochemical test results, risk factors of PVST development and the thrombus location in the portal vein system as well as its extension to splenic or mesenteric vein. To identify the location of thrombus, we used doppler US or contrastenhanced CT scan. Thrombosis cases in portal and mesenteric vein were stratified according to Yerdel classification.10
In relation to the cirrhotic group, we focused on the severity of cirrhosis, presence of hepatocellular carcinoma (HCC) and the PVST development in order to determine a relationship between them. The severity of liver disease was estimated according to Child-Pugh class and model for end-stage liver disease (MELD) score.
Statistical analysesDescriptive statistics and 95% confidence intervals (CI) were used for the patients’ characteristics. Continuous variables were analyzed using the Student’s t test or the Wilcoxon rank-sum Mann-Whitney test. For categorical variables, we used the Chi-Squared (χ2) test or Fisher’s exact test. A p value < 0.05 was considered statistically significant. Analyses were performed using Stata version 12 (Stata Corp LT, Texas, USA).
ResultsThe study comprised 67 patients with PVST where the overall mean age was 62.2 ± 11.4 years and 63% were men. In particular, in our analysis between cirrhotic (n = 42) and non-cirrhotic (n = 25) patients (Table 1), the cirrhotic group was older with a mean age of 65 ± 9.5 years while in non-cirrhotic group was 57 ± 13.2 years (p = 0.009). The principal risk factors to develop PVST in the cirrhotic population were cirrhosis per se and HCC, whereas in non-cirrhotic group the primary risk factors were inflammatory conditions such as diverticular disease and pancreatitis, smoking, and cancer (pancreatic cancer, malignant tumor of the duodenum, cholangiocarcinoma and sarcomatoid mesothelioma). The thrombus location was diagnosed in most of patients by CT-scan (81%), while in the remaining patients, doppler US was the diagnostic test (19%).
Characteristics of cirrhotic and non-cirrhotic patients.
| Variable | Cirrhotics n = 42 | 95% CI Cirrhotics | Non-Cirrhotics n = 25 | 95% CI Non-Cirrhotics | p-value |
|---|---|---|---|---|---|
| Age, years, mean, SD | 65 (9.5)* | (57.64) | 57 (13.2)* | (50.61) | < 0.01 |
| Male gender, n (%) | 25 (59.52)** | (59.93) | 17 (68)** | (45.64) | 0.17 |
| Smoking,n (%) | 16 (38.10)** | (24.54) | 18 (72)** | (51.88) | < 0.01 |
| Imaging Tool | |||||
| Doppler US, n (%) | 11 (26.19)** | 2 (8)*** | |||
| Abdominal CT Scan, n (%) | 28 (66.67)** | 21 (84)** | |||
| Angio CT, n (%) | 3 (7.14) *** | 2 (8)*** | |||
| Risk factor | |||||
| Inflammatory conditions, n (%) | - | 8 (32)*** | < 0.01 | ||
| Procoagulant disorders, n (%) | - | 2 (8)*** | 0.06 | ||
| Cancer, n (%) | 21 (50)** | 7 (28)*** | 0.07 | ||
| No discernable risk factor, n (%) | - | 7 (28)*** | < 0.01 |
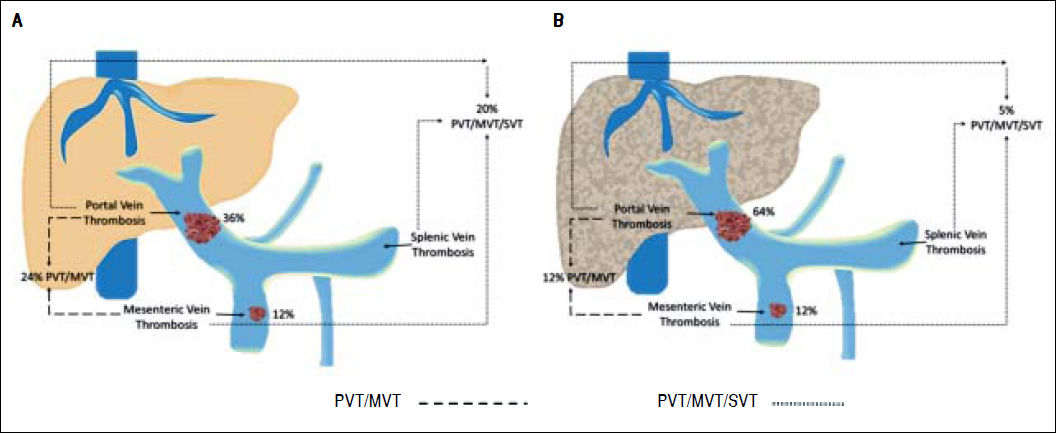
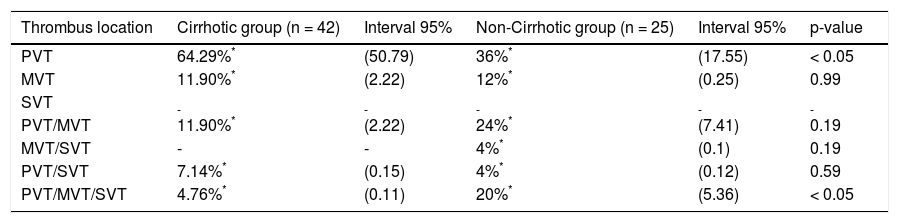
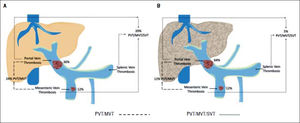
Differences between cirrhotic and non-cirrhotic were also related to thrombus extension (Figure 1 and Table 2). Moreover, the most affected vessel, in both groups, was mainly the portal vein (64.29%; 95% CI: 50.79; and 36%; 95% CI: 7.55; respectively; p ≤ 0.05) (Figure 2). Nevertheless, non-cirrhotic patients had higher prevalence of thrombosis extended to the whole portal vein system than cirrhotic group (20%; 95% CI: 5.36 vs. 4.76%; 95% CI: 0.11; p ≤ 0.05).
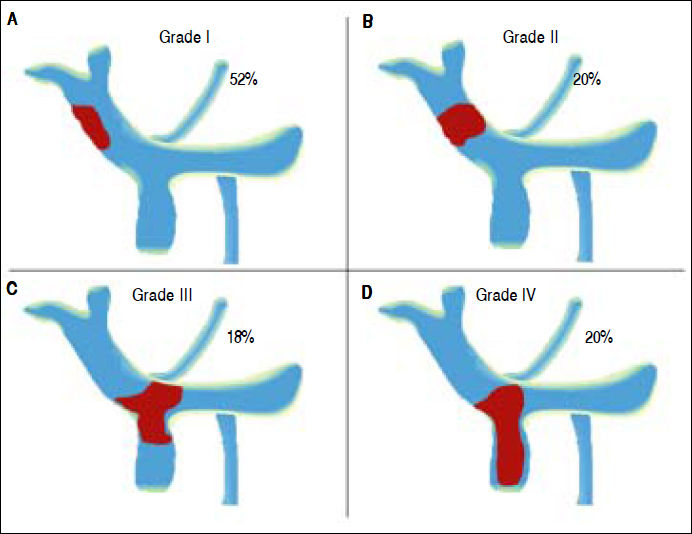
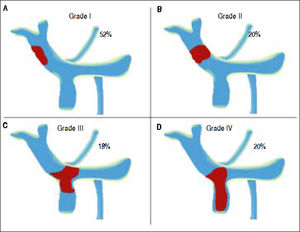
Yerdel Classification of Portal Vein Thrombosis. According to our results, fifty-cases had a thrombus in the portal vein or thrombosis in portal vein with invasion of mesenteric vein. The classification of Yerdel divides the portal vein thrombosis into 4 grades.10 Grade I: The thrombosis of portal vein affects < 50% of the vessel lumen. Grade II: Thrombosis affects more than 50% of the vessel lumen. Grades I and II could have minimal or no invasion of the superior mesenteric vein. Grade III: There is a complete obstruction of the portal vein and proximal invasion of superior mesenteric vein. Grade IV: The obstruction progressed to distal superior mesenteric vein.
Differences of thrombus location between cirrhotic and non- cirrhotic patients.
| Thrombus location | Cirrhotic group (n = 42) | Interval 95% | Non-Cirrhotic group (n = 25) | Interval 95% | p-value |
|---|---|---|---|---|---|
| PVT | 64.29%* | (50.79) | 36%* | (17.55) | < 0.05 |
| MVT | 11.90%* | (2.22) | 12%* | (0.25) | 0.99 |
| SVT | - | - | - | - | - |
| PVT/MVT | 11.90%* | (2.22) | 24%* | (7.41) | 0.19 |
| MVT/SVT | - | - | 4%* | (0.1) | 0.19 |
| PVT/SVT | 7.14%* | (0.15) | 4%* | (0.12) | 0.59 |
| PVT/MVT/SVT | 4.76%* | (0.11) | 20%* | (5.36) | < 0.05 |
Location of the affected vessels in the cirrhotic and non-cirrhotic group. We compared the location of affected vessels between non-cirrhotic and cirrhotic group. Consequently, we observed that porta vein was the most occluded vessel in both groups. PVT: portal vein thrombosis. MVT: mesenteric vein thrombosis. SVT: splenic vein thrombosis.
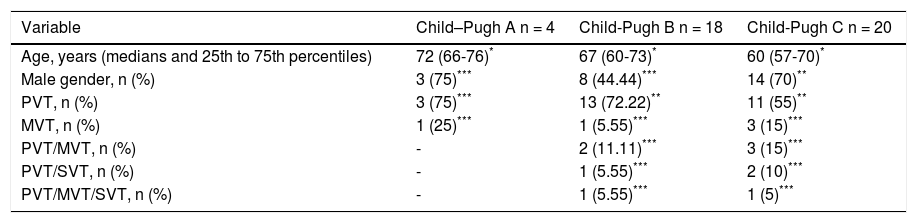
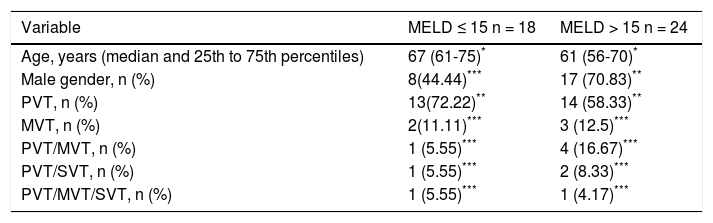
Additionally, we observed that the main causes of cirrhosis among our population were non-alcoholic steatohepatitis (NASH) 17 (40.48%), followed by hepatitis C virus 10 (23.81%) and autoimmune origin 8 (19.05%). With respect to relationship between severity of cirrhosis and PVST occurrence, we found that 90.48% of cirrhotics had higher Child-Pugh class (B/C) whereas 57.14% had MELD score ≥ 15 (Tables 3 and 4).
Demographic variables, sites of thrombotic events and Child-Pugh scores of patients with cirrhosis.
| Variable | Child–Pugh A n = 4 | Child-Pugh B n = 18 | Child-Pugh C n = 20 |
|---|---|---|---|
| Age, years (medians and 25th to 75th percentiles) | 72 (66-76)* | 67 (60-73)* | 60 (57-70)* |
| Male gender, n (%) | 3 (75)*** | 8 (44.44)*** | 14 (70)** |
| PVT, n (%) | 3 (75)*** | 13 (72.22)** | 11 (55)** |
| MVT, n (%) | 1 (25)*** | 1 (5.55)*** | 3 (15)*** |
| PVT/MVT, n (%) | - | 2 (11.11)*** | 3 (15)*** |
| PVT/SVT, n (%) | - | 1 (5.55)*** | 2 (10)*** |
| PVT/MVT/SVT, n (%) | - | 1 (5.55)*** | 1 (5)*** |
MVT: mesenteric vein thrombosis. PVT: portal vein thrombosis. SVT: splenic vein thrombosis._: any subject presented this characteristic. p-value = Not significant.
Demographic variables, sites of thrombotic events and MELD scores of patients with cirrhosis.
| Variable | MELD ≤ 15 n = 18 | MELD > 15 n = 24 |
|---|---|---|
| Age, years (median and 25th to 75th percentiles) | 67 (61-75)* | 61 (56-70)* |
| Male gender, n (%) | 8(44.44)*** | 17 (70.83)** |
| PVT, n (%) | 13(72.22)** | 14 (58.33)** |
| MVT, n (%) | 2(11.11)*** | 3 (12.5)*** |
| PVT/MVT, n (%) | 1 (5.55)*** | 4 (16.67)*** |
| PVT/SVT, n (%) | 1 (5.55)*** | 2 (8.33)*** |
| PVT/MVT/SVT, n (%) | 1 (5.55)*** | 1 (4.17)*** |
MELD: model of end-stage liver disease. MVT: mesenteric vein thrombosis. PVT: portal vein thrombosis. SVT: splenic vein thrombosis. p-value = Not significant.
In this paper, we have shown that the demographic characteristics, risk factors and distribution for thrombosis in the portal system differ between cirrhotic and noncirrhotic patients with PVST. One particularly interesting finding was related to the mean ages between cirrhotic and non-cirrhotic patients. Our non-cirrhotic group has shown a mean age above 57 years old while cirrhotic patients have a mean age above 65 years old. Concerning this, Rajani R, et al.11 had observed that non-cirrhotics with PVST were younger at the time of diagnosis compared to cirrhotics, 54 vs. 60 years old.
On the other hand, several studies have demonstrated that the main risk factors for developing PVST are prothrombotic states, cirrhosis per se, inflammatory conditions, cancer especially HCC, surgical interventions and abdominal infections.12–14 The main risk factor of PVST development in our non-cirrhotic patients was inflammatory conditions such as pancreatitis and diverticular disease. In addition, cigarette smoking could be a possible associated factor due to its high prevalence in this population.15–17 Similarly, in a recent study by Dell’Era, et al.,18 it had been observed that the principal factors to trigger PVST in non-cirrhotics are myeloproliferative disorders, neoplasms and abdominal inflammation such as pancreatitis, diverticulitis, and cholecystitis.
In our cirrhotic population, the main risk factors were HCC and cirrhosis per se, both having equal prevalence in this group. In this respect, Rajani, et al.11 study has demonstrated well-documented relationship between HCC and cirrhosis to develop PVST, establishing that in cases with cirrhosis and HCC, the occurrence of PVST has reached 44% while the risk of PVST in patients only with cirrhosis has reached 26%.11,19,20 According to our results, it is evident that neoplasms, especially HCC, represent a higher risk of PVST development in cirrhotic patients in comparison to non-cirrhotic patients.
NASH was the most common cause of cirrhosis in this cohort and probably it is related to PVST events. In fact, several studies have suggested that NASH contributes to atherogenic risk by the systemic release of proinflammatory mediators such as plasma concentrations of high-sensitivity C-reactive protein (hs-CRP), fibrinogen and plasminogen activator inhibitor-1 (PAI-1).21–25 In this connection, Targher, et al. performed a study, in which they compared the levels of these markers between overweight patients with NASH, overweight individuals without liver steatosis and healthy patients.21 They found that NASH patients had higher levels of hs-CRP, fibrinogen, and PAI-1 activity as well lower adiponectin concentrations compared to patients without liver steatosis. Moreover, they observed that these proinflammatory biomarkers were correlated with increasing severity of NASH. Consequently, they suggested that NASH could predispose a more atherogenic risk profile, regardless of the degree of visceral adiposity. Similarly, Stine, et al. supported the idea of NASH as a prothrombotic state independently of other risk factor.26 They studied the prevalence of PVST in 33,368 patients underwent liver transplantation, finding that 6.3% of their population has developed PVST and 12% of these patients had NASH. Therefore, they compared NASH patients to patients with other causes of cirrhosis, observing that NASH patients have higher prevalence of PVST than patients without NASH-related cirrhosis (10.1 vs. 6%; p < 0.001).
On the other hand, when we analyzed the cirrhotic group, most of the patients had a higher MELD score or Child-Pugh class; however, we did not find a correlation between their scores and the extension of thrombus.
Our study has some limitations including the small sample size and a short follow-up. However, the strength of this paper is that it’s one of the few to analyze the common site of thrombus in PVST events and to use the gold standard tool to diagnose PVST (Abdominal CT Scan with contrast). Nevertheless, most of the patients diagnosed by doppler US were not corroborated by CT scan or magnetic resonance imaging.
In conclusion, PVST is recognized with or without underlying liver disease but the populations are distinctly different. According to our findings, HCC and cirrhosis per se in older patients could be strong risk factors to develop PVST while inflammatory conditions probably are the main predisposing factors in non-cirrhotic, usually younger patients. Further studies are necessary to confirm the main risk factors to develop PVST in cirrhotics and non-cirrhotics as well as to evaluate if cirrhosis severity is linked with PVST development. This could be helpful to prevent PVST events in population at risk, improving its prognosis.
Abbreviations- •
CT: computed tomography.
- •
HCC: hepatocellular carcinoma.
- •
MELD: Model for End-Stage Liver Disease.
- •
NASH: non-alcoholic steatohepatitis.
- •
PVST: portal venous system thrombosis.
- •
US: ultrasound.
None of the listed authors have a conflict of interest. Our institution acts in accordance with the ethical standards of international and institutional research committees, and with the 1964 Helsinki Declaration and its later amendments or similar ethical standards. No human intervention was involved in this study. This study was supported in part by Medica Sur Clinic & Foundation.
Ethical ApprovalThis retrospective study was approved by the local research ethics committee (#2017-EXT-216).
AcknowledgmentsNot applicable.