This meta-analysis aims to evaluate the efficacy of stem cell therapy (SCT) for liver failure.
Materials and MethodsThe study adhered to the recommended guidelines of the PRISMA statement. Eligible studies published prior to May 13, 2023, were comprehensively searched in databases including PubMed, Web of Science, and Embase. Quality assessment was conducted using the Cochrane risk-of-bias tool, and the standard mean differences were calculated for the clinical parameters. The hazard ratios were determined by extracting individual patient data from the Kaplan-Meier curve.
ResultsA total of 2,937 articles were retrieved, and eight studies were included in the final analysis. Most of the studies focused on HBV-related liver failure and were randomized controlled trials. All studies utilized mesenchymal stem cells (MSCs), with the majority (62.5%) being allogeneic. The analysis revealed that combining stem cell therapy with standard medical treatment or plasma exchange significantly enhanced patient survival and reduced MELD scores. Specifically, allogeneic stem cells showed superior efficacy in improving survival outcomes compared to autologous stem cells. Furthermore, deep vessel injection plus a single injection demonstrated better effectiveness than peripheral vessel injection plus multiple injections in reducing MELD scores.
ConclusionsThis comprehensive analysis underscores the potential of MSC therapy in significantly improving survival and clinical outcomes in patients with liver failure, highlighting the superior benefits of allogeneic MSCs and deep vessel plus single injection administration.
Liver failure is a severe condition characterized by impaired liver function. It can manifest itself suddenly as acute liver failure or emerge gradually over a period of time as chronic liver failure (CLF). Acute-on-chronic liver failure (ACLF) has a worldwide prevalence of 35% among patients with decompensated cirrhosis, with the highest occurrence in South Asia at 65%. At present, the global 90-day mortality rate stands at 58%, with the highest rate found in South America at 73% [1]. The primary treatment approach for liver failure is centered on addressing the underlying causes, including antiviral therapy for viral hepatitis, discontinuation of hepatotoxic drugs, promoting alcohol cessation and avoiding hepatotoxic substances. Additionally, comprehensive medical management, artificial liver support therapy and liver transplantation (LT) surgery, are employed. LT is currently the only curative approach available for liver failure [2]. In recent years, an improvement in post-LT survival rates has been observed (current 5-year survival rates: 75% in Asia, 74% in Europe and 78.1% in the United States). However, the high cost of the surgery, coupled with the scarcity of liver donors, leads to delayed treatment for numerous patients on LT waiting list [3]. Moreover, the high mortality of acute graft-versus-host disease following LT contributes to treatment failure [4]. Therefore, the development of novel approaches for the treatment of liver failure is of utmost importance in terms of clinical practice.
Human stem cells may be essentially categorized into embryonic stem cells (ESCs), mesenchymal stem cells (MSCs) and somatic stem cells. Among these categories, ESCs represent the most proliferative type of pluripotent stem cells, although ethical issues arise from their usage. Recent times have seen the development of somatic stem cells (also termed induced pluripotent stem cells, or iPSCs), which are obtained by introducing specific genes, such as Myc, Oct3/4 and Klf4, into the somatic cells, thereby converting them into pluripotent stem cells. Although iPSCs show great promise, the current lack of a standardized generation procedure limits their applicability at present [5,6]. MSCs are pluripotent stem cells derived from various tissues, such as bone marrow, adipose tissue, umbilical cord blood, placenta and peripheral blood. They have low immunogenicity, and possess the ability to self-proliferate and differentiate. MSCs, including bone marrow MSCs (BM-MSCs), adipose MSCs (AD-MSCs), umbilical cord MSCs (UC-MSCs) and peripheral blood derived MSCs (PB-MSCs), have shown potential in treating liver diseases via their differentiation into hepatocyte-like cells, with their subsequent participation in immune regulation, cell proliferation and injury repair [7,8]. Due to their abundant sources, easy obtainability, low immunogenicity and compatibility with different delivery methods, MSCs hold promise as an ideal source for stem cell research in liver diseases. The mechanism associated with MSCs function comprises their differentiation into hepatocyte-like cells and the paracrine secretion of numerous signaling pathway factors. The immunomodulatory activities and antifibrotic properties of MSCs are important in terms of the treatment of liver failure. MSCs indirectly regulate the activity of hepatic stellate cells (HSCs) through their influence of immune cells. In an inflammatory environment, MSCs migrate to the liver injury site, where they secrete various factors [e.g., nitric oxide (NO), prostaglandin E2 (PGE2), indoleamine 2,3‐dioxygenase (IDO), interleukin (IL)-6, IL-10 and stromal cell-derived factor 1 (SDF-1)] to inhibit immune cell proliferation and activation, thereby reducing both fibrosis and the accumulation of extracellular matrix (ECM) [9]. Moreover, MSCs exert direct anti-fibrotic effects by inhibiting the proliferation of HSCs and ECM synthesis [10]. They also reduce gene expression of collagen and inhibit the notch pathway [11].
In recent years, numerous studies have reported on the safety of stem cell therapy (SCT) in liver failure [12]; however, the therapeutic effects have been found to be inconsistent. Although systematic reviews and meta-analyses on the use of stem cells in the treatment of liver failure have been published, the majority of them have included studies on liver cirrhosis due to an insufficient number of clinical trials having been performed. It is important to note that liver failure and cirrhosis proceed via distinct pathogenic mechanisms. Additionally, previously published meta-analyses have primarily pooled the overall survival (OS) rate to address the impact of SCT on the prognosis of patients with liver failure [12–15]. Moreover, the inclusion of studies with varying follow-up durations in these meta-analyses may have had the effect of limiting the accuracy and comprehensiveness of the OS rate in reflecting the true survival status of patients [16]. Therefore, the present study aimed to systematically include and analyze the clinical trials associated with SCT for liver failure, with a comprehensive utilization of the reported survival curves, in order to evaluate and summarize the therapeutic efficacy of stem cells in the treatment of liver failure; in addition, we further discuss the limitations present in existing research in this field.
2Patients and methodsThis meta-analysis study was conducted according to the recommended guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17].
2.1Eligibility criteria for selectionPrevious studies of human stem cell-based treatment for liver failure were collected and collated for this meta-analysis. To ensure the inclusion of high-quality clinical investigations, the present study only considered publications in English. Specifically, our focus was on formally published randomized controlled trials (RCTs) that examined the use of stem cell therapy for liver failure. Conference abstracts were excluded in the present study. The selected studies were required to meticulously report detailed information for further comprehensive re-investigation. The details sought after included the number of participants, diagnostic criteria for liver failure, clear definition of groups, treatment methods, durations of follow-up, clinical parameters and survival outcome.
2.2Search strategy using various databasesA thorough investigation was conducted in the PubMed, Web of Science (WOS) and Embase databases to identify relevant studies published prior to May 13th, 2023. Our search queries were constructed in accordance with the retrieval guidelines of these databases, using a combination of keywords, fields and Boolean logical operators. For PubMed, the search terms employed were “{(stem cell transplantation[Title/Abstract]) OR (stem cells transplantation[Title/Abstract]) OR (stem cells infusion[Title/Abstract]) OR (stem cell infusion[Title/Abstract]) OR (stem cells therapy[Title/Abstract]) OR (stem cell therapy[Title/Abstract]) OR (umbilical cord[Title/Abstract]) OR (bone marrow[Title/Abstract]) OR (mesenchymal stromal[Title/Abstract])) AND (liver failure [Title/Abstract])}”. In the case of WOS, our search term was “TI (limit to title) = [(stem cell* transplantation) OR (stem cell* infusion) OR (stem cell* therapy) OR (umbilical cord) OR (mesenchymal stromal) OR (bone marrow)] AND (liver failure) OR AB (abstract) = [(stem cell* transplantation) OR (stem cell* infusion) OR (stem cell* therapy) OR (umbilical cord) OR (mesenchymal stromal) OR (bone marrow)] AND (liver failure)”. Lastly, for Embase, the search terms “(‘stem cell transplantation’:ab,ti OR ‘stem cells transplantation’:ab,ti OR ‘stem cells infusion’:ab,ti OR ‘stem cells infusion’:ab,ti OR ‘stem cells therapy’:ab,ti OR ‘stem cells therapy’:ab,ti OR ‘umbilical cord’:ab,ti OR ‘bone marrow’:ab,ti OR ‘mesenchymal stromal’:ab,ti) AND ‘liver failure’:ab,ti AND ‘human’/de AND (‘article’/it OR ‘review’/it)” were employed.
2.3Study selection and data extractionAfter eliminating redundant references, three researchers (HM, ZL and DZ) scanned the titles and abstracts in order to identify relevant studies. Should any discrepancies arise, the supervisors (JP and YZ) assumed the responsibility of making final judgments. The full texts and supplementary materials of the qualifying studies were subjected to thorough screening by two researchers (SL and HG), who worked independently of each other. YZ subsequently reviewed the final data for any inconsistencies, and facilitated the data correlation. JP reviewed, revised and finalized the manuscript. The following data were collected, when available: i) the study characteristics, i.e., the year of publication, primary author, country and study design; ii) the patient characteristics, i.e., the number of participants in each arm, etiologies, diagnostic criteria and duration of follow-up; iii) stem cells, i.e., the cell type, dosage, treatment frequency and route of administration; and iv) study outcomes, i.e., survival rates/curves, adverse events, model for end-stage liver disease (MELD) scores, the levels of albumin (ALB), total bilirubin (TBIL), alanine aminotransferase (ALT), and the international normalized ratio (INR) at various follow-up time points. Due to the focus on a specific time point within the Kaplan-Meier (K-M) curves and the lack of a consistent follow-up duration across the studies, utilizing the survival rate alone may not adequately reflect variations in patient prognosis among the different intervention groups [16]. On the other hand, using the hazard ratio (HR), which encompasses comprehensive information relating to the entire K-M curve, has been shown to accurately reflect the treatment effects throughout the entire follow-up period [18]. For articles that did not explicitly present HR values, the graphs and extract data points were digitized using the tool IPDfromKM [19].
2.4Quality assessment and statistical analysisThe assessment of publication bias risk for each included study was performed by two researchers (SL and HG) employing the Cochrane risk-of-bias (ROB) tool. The Cochrane ROB tool offers a structured approach to evaluate the potential for bias in studies. It encompasses five domains: i) randomization-associated bias; ii) intervention deviation-associated bias, iii) missing data-associated bias, iv) outcome measurement-associated bias; and v) reported result selection-associated bias. The bias risk within each domain was evaluated and categorized as low, high, or uncertain [20].
The continuous variables were calculated as the standard mean difference (SMD) with 95% confidence intervals (95% CI) utilizing Hedges' g methodology [21]. The estimated logarithm of the HR (logHR) and standard error (SE) obtained from IPDfromKM were employed to compute the estimated HRs and corresponding 95% CIs. To assess heterogeneity among the included studies, the Tau-squared (Tau2) value, Chi-square test and I2 statistics were computed. Notable levels of heterogeneity were indicated by a large value of Tau2, P < 0.05 for the Chi-square test, and I2>50%. In such cases, a random effect model was employed to pool the data; otherwise, a common effect model was utilized. Subsequently, sensitivity analysis was performed by systematically omitting one study at a time. Additionally, publication bias was evaluated using the Funnel plot and Egger's test. In cases where an asymmetric Funnel plot existed alongside a P-value <0.05 from the Egger's test, a trim-and-fill analysis was implemented while constructing an adjusted Funnel plot. Furthermore, subgroup analyses were performed encompassing different follow-up time points, distinct cell sources (autologous or allogeneic), injection route variations (deep vessel or peripheral vessel) and differing injection frequencies (single or multiple). The entire analysis was conducted utilizing the open-source R statistical language (R-project, version 4.1.2) and relevant packages, including tidyverse [22] for reference management and selection, and meta [23] and metafor [24] for core procedures.
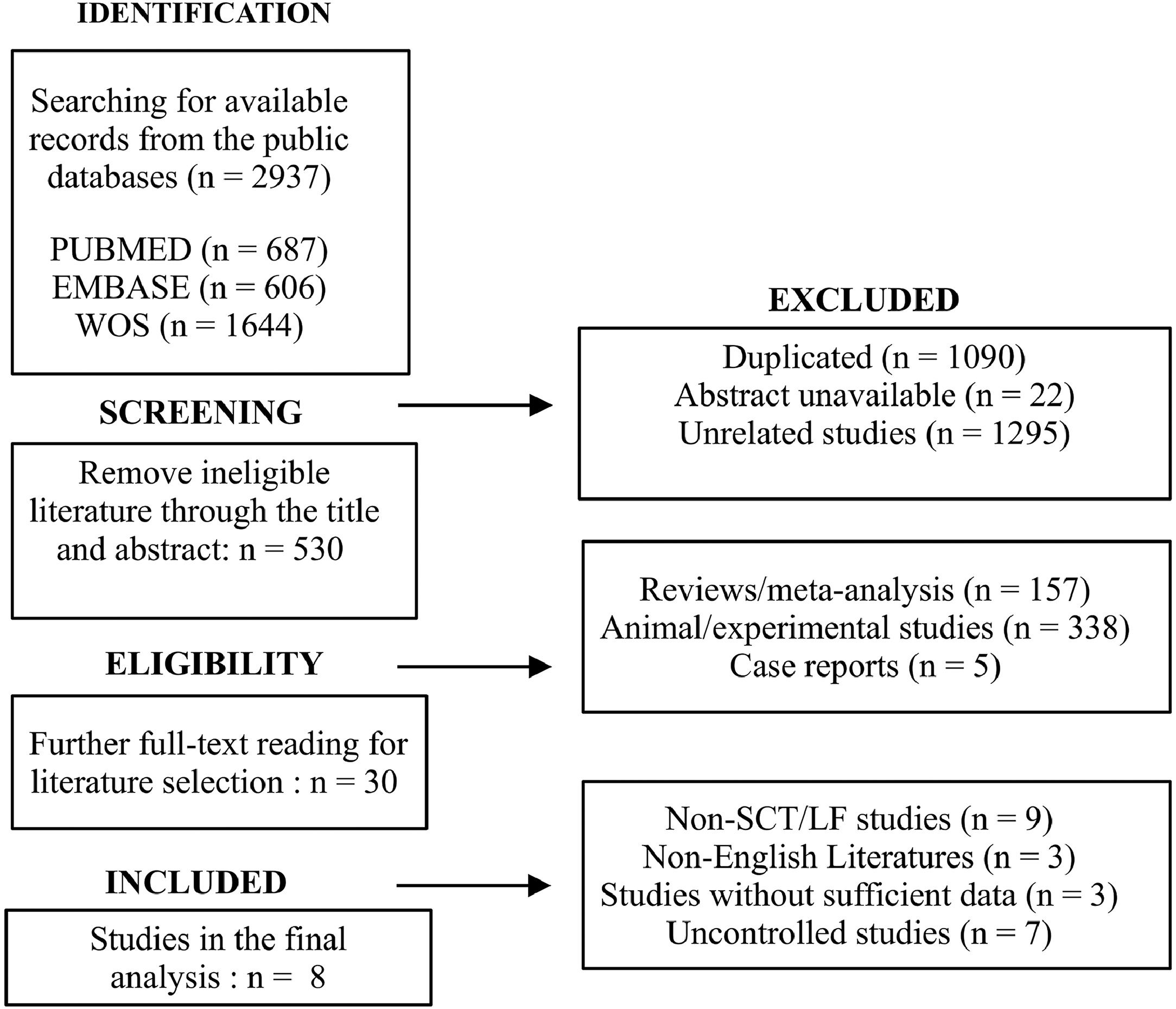
3Results3.1Search resultsThe initial retrieval yielded a total of 2937 articles, encompassing 687 articles from PubMed, 606 from Embase and 1644 from WOS. Following the elimination of duplicates, unavailable and unrelated studies, 530 articles remained for further eligibility evaluation. Furthermore, 157 reviews/meta-analyses, 338 animal/experimental studies and five case reports were excluded. Thirty studies were subsequently retained for meticulous review. Eight studies were included in the final analysis, subsequent to the exclusion of 22 studies, of which nine were not specific for SCT or liver failure, three were non-English, three lacked sufficient data, and seven had uncontrolled designs. The literature retrieval and screening procedures are shown in the flowchart (Fig. 1).
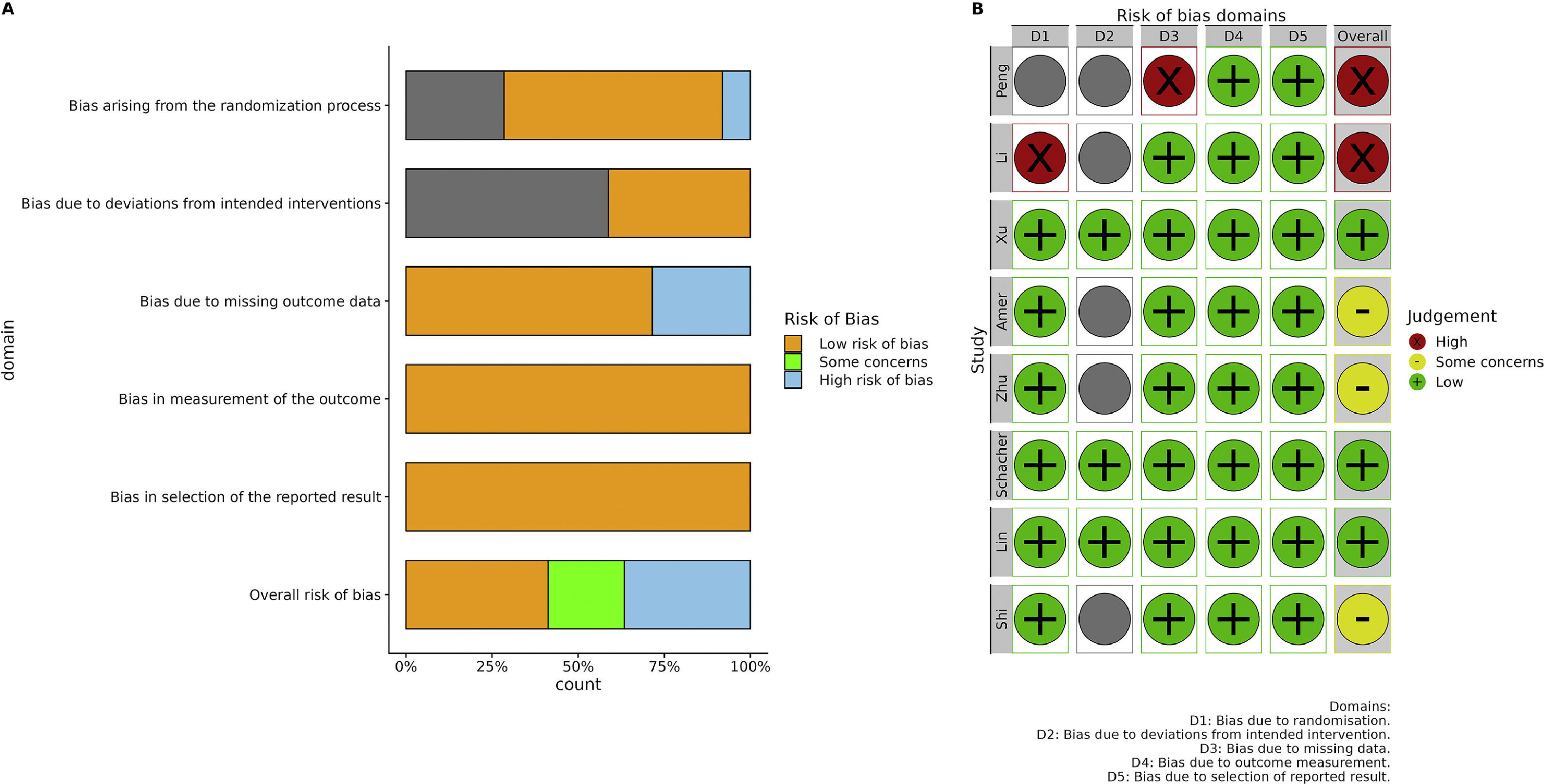
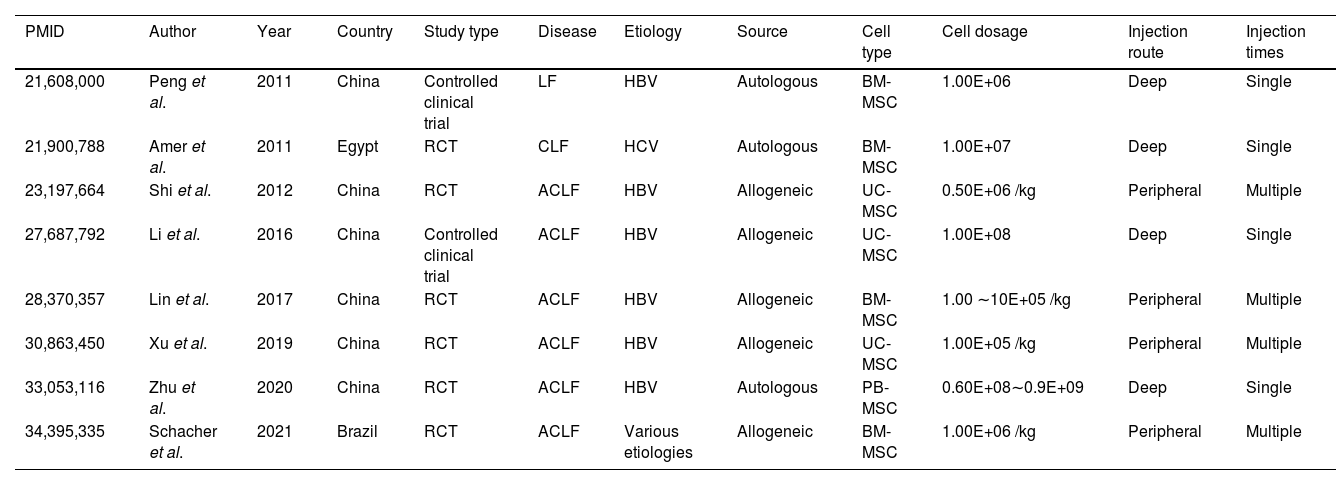
3.2Characteristics and quality of included studiesThe characteristics of the selected eight studies [25–32] are shown in Table 1. These studies were published between 2017 and 2021. Out of the eight included studies, the majority were conducted by Chinese researchers (n = 6), whereas the remaining pair of articles originated from Egypt and Brazil. In terms of study design, 75% of the studies were RCTs, whereas the remaining 25% were controlled clinical trials. Regarding the etiology of the disease, six studies focused on HBV-associated ACLF, one study was on hepatitis C virus (HCV)-associated CLF, and one study investigated ALCF with various etiologies, of which 56% of cases were attributed to viral hepatitis. As for the source of stem cells, 62.5% (5/8 studies) were allogeneic, whereas the remaining 37.5% (3/8 studies) were autologous. In terms of stem cell type, four studies utilized BM-MSCs, three studies employed UC-MSCs and one study utilized PB-MSCs. The administration routes (deep or peripheral vessel) and the frequency of injections (single or multiple) were evenly distributed. The majority of the included studies exhibited a low overall risk of bias (Fig. 2A). The main reason for potential bias was the lack of a full description of the randomization process. Two studies were found to have a high risk of bias due to concerns regarding the randomization process, deviation from the intended intervention, and missing data (Fig. 2B).
Characteristics of the included studies.
| PMID | Author | Year | Country | Study type | Disease | Etiology | Source | Cell type | Cell dosage | Injection route | Injection times |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 21,608,000 | Peng et al. | 2011 | China | Controlled clinical trial | LF | HBV | Autologous | BM-MSC | 1.00E+06 | Deep | Single |
| 21,900,788 | Amer et al. | 2011 | Egypt | RCT | CLF | HCV | Autologous | BM-MSC | 1.00E+07 | Deep | Single |
| 23,197,664 | Shi et al. | 2012 | China | RCT | ACLF | HBV | Allogeneic | UC-MSC | 0.50E+06 /kg | Peripheral | Multiple |
| 27,687,792 | Li et al. | 2016 | China | Controlled clinical trial | ACLF | HBV | Allogeneic | UC-MSC | 1.00E+08 | Deep | Single |
| 28,370,357 | Lin et al. | 2017 | China | RCT | ACLF | HBV | Allogeneic | BM-MSC | 1.00 ∼10E+05 /kg | Peripheral | Multiple |
| 30,863,450 | Xu et al. | 2019 | China | RCT | ACLF | HBV | Allogeneic | UC-MSC | 1.00E+05 /kg | Peripheral | Multiple |
| 33,053,116 | Zhu et al. | 2020 | China | RCT | ACLF | HBV | Autologous | PB-MSC | 0.60E+08∼0.9E+09 | Deep | Single |
| 34,395,335 | Schacher et al. | 2021 | Brazil | RCT | ACLF | Various etiologies | Allogeneic | BM-MSC | 1.00E+06 /kg | Peripheral | Multiple |
hepatitis B virus, HBV; hepatitis C virus, HCV; bone marrow derived mesenchymal stem cells, BM-MSC; umbilical cord derived mesenchymal stem cells, UC-MSC; peripheral blood derived mesenchymal stem cells, PB-MSC; liver failure, LF; chronic liver failure, CLF; acute-on-chronic liver failure, ACLF.
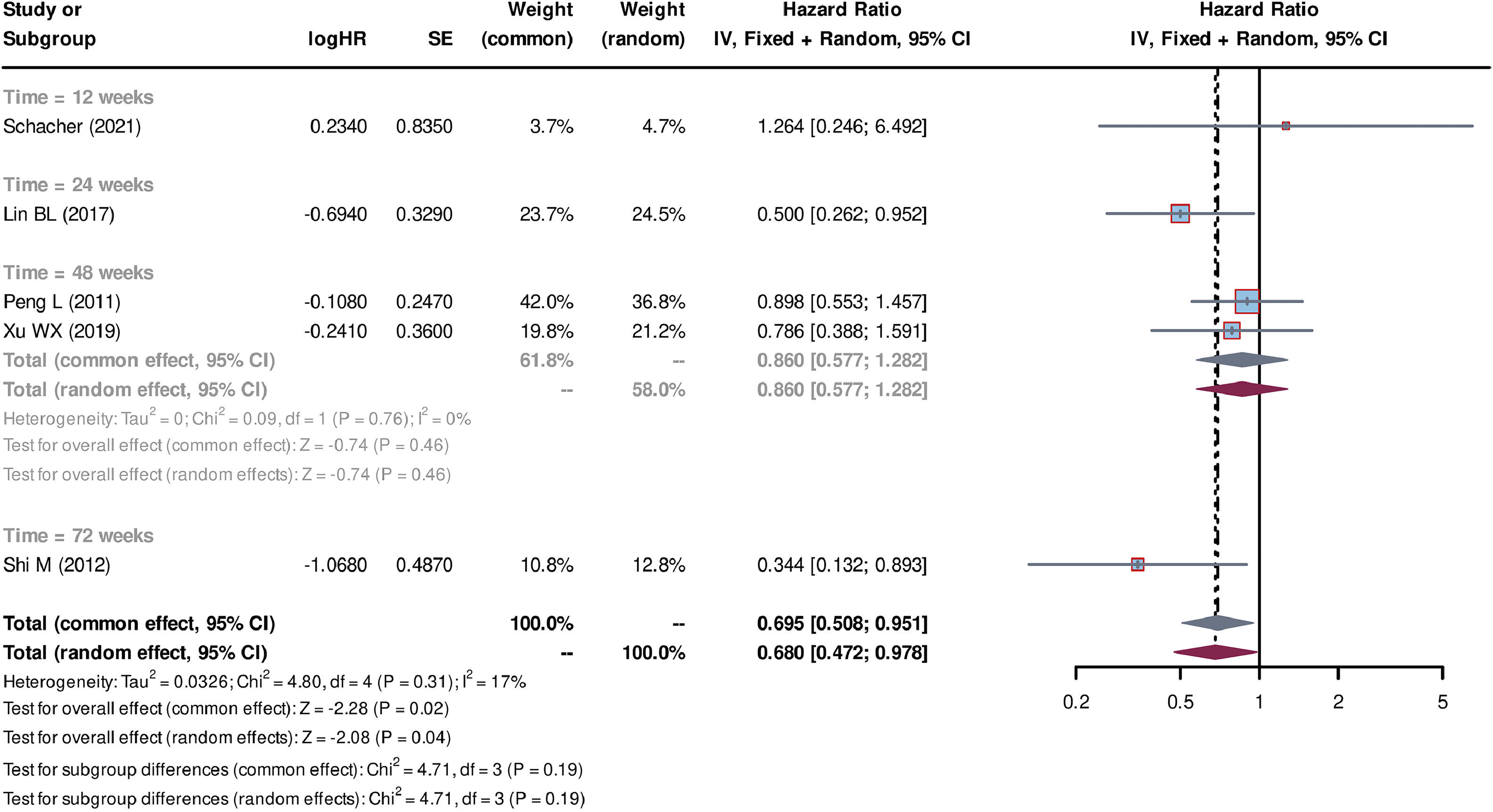
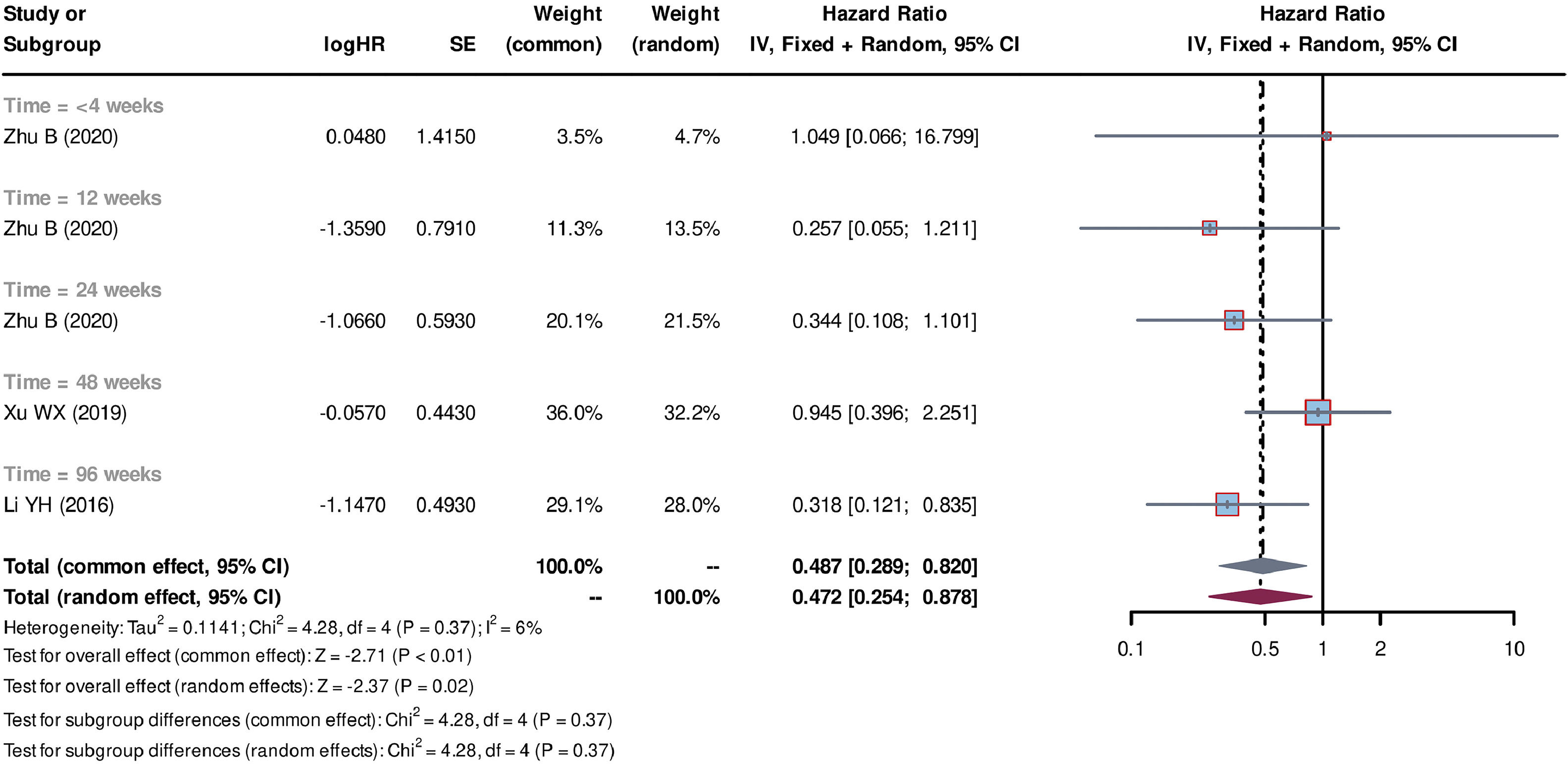
Individual patient data from seven studies [26–32] were extracted from the K-M curves. logHR and its corresponding standard error (SE) were estimated using these data. These values were subsequently used to calculate the HR and their corresponding 95% CI for each study. Out of the seven studies, five studies [27–30,32] compared SCT plus standard medicine treatment (SMT) with SMT alone. The SCT+SMT group consisted of 167 patients, whereas the SMT group comprised 213 patients. The follow-up time varied among these studies (follow-up times of 8, 12, 24, 48 and 72 weeks were reported), with each duration subgroup having only one available study. Heterogeneity analysis showed a low level of heterogeneity among the studies, with Tau2=0.033, Chi-square test P = 0.31, and I2=17%, leading to the use of a common effect model. The pooled HR was 0.695 (95% CI=0.508-0.951, P = 0.02), indicating that stem cell-based treatment had a significant effect on improving the survival of liver failure patients compared with SMT alone (Fig. 3). Three studies [26,31,32] compared the treatment strategy of plasma exchange (PE) plus SCT (SCT+PE) with the treatment of PE alone. The SCT+PE group included 51 patients, whereas the PE group comprised 84 patients. Similar to the previous comparison, only one study was available for each follow-up duration, with low heterogeneity among the studies (Tau2=0.114, Chi-square test P = 0.37, and I2=6%). The common effect model showed a pooled HR of 0.487 (95% CI=0.289-0.820, P < 0.01), indicating that the SCT+PE treatment led to an improvement in the survival rate of patients with liver failure compared with PE treatment alone (Fig. 4).
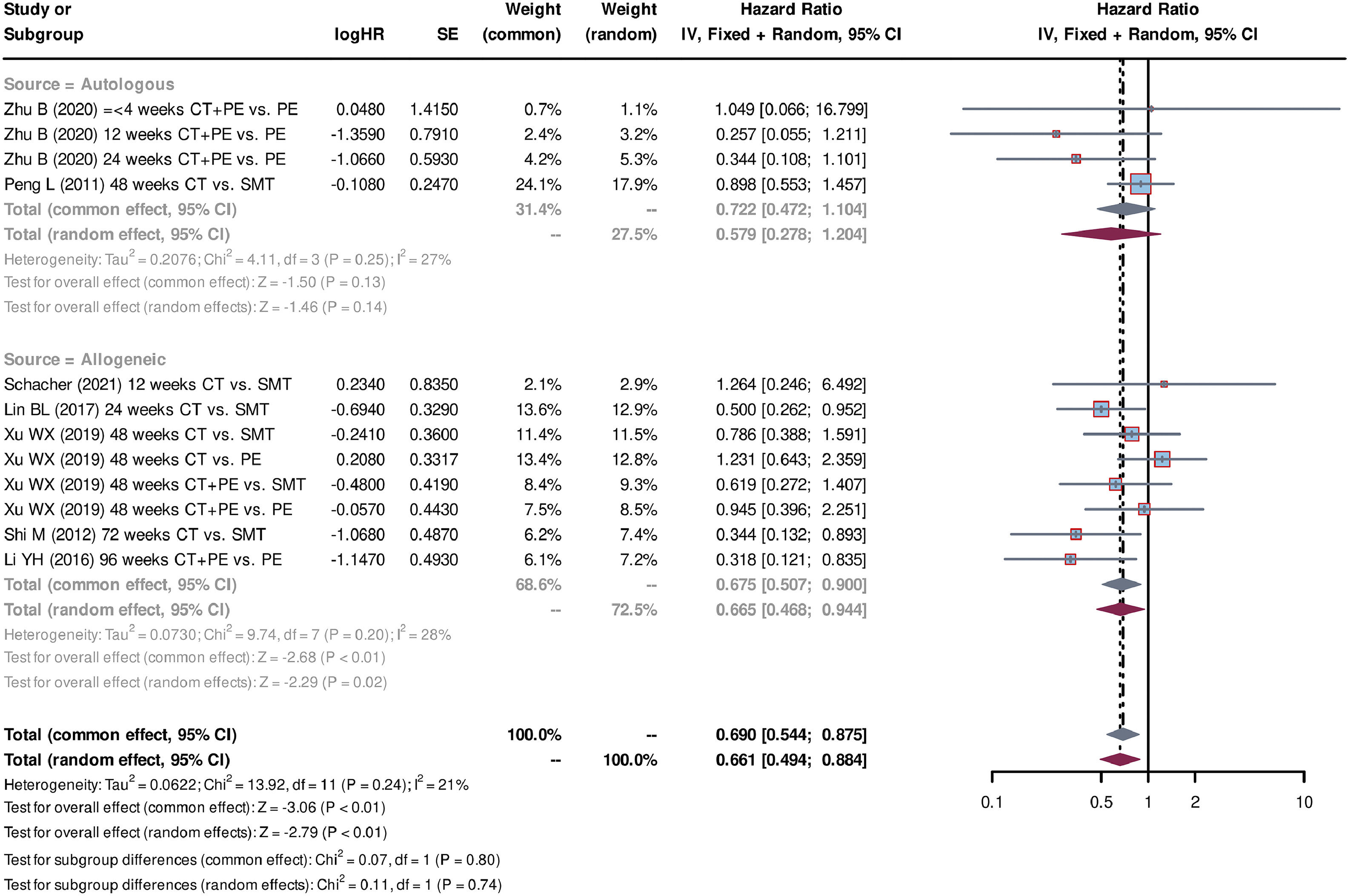
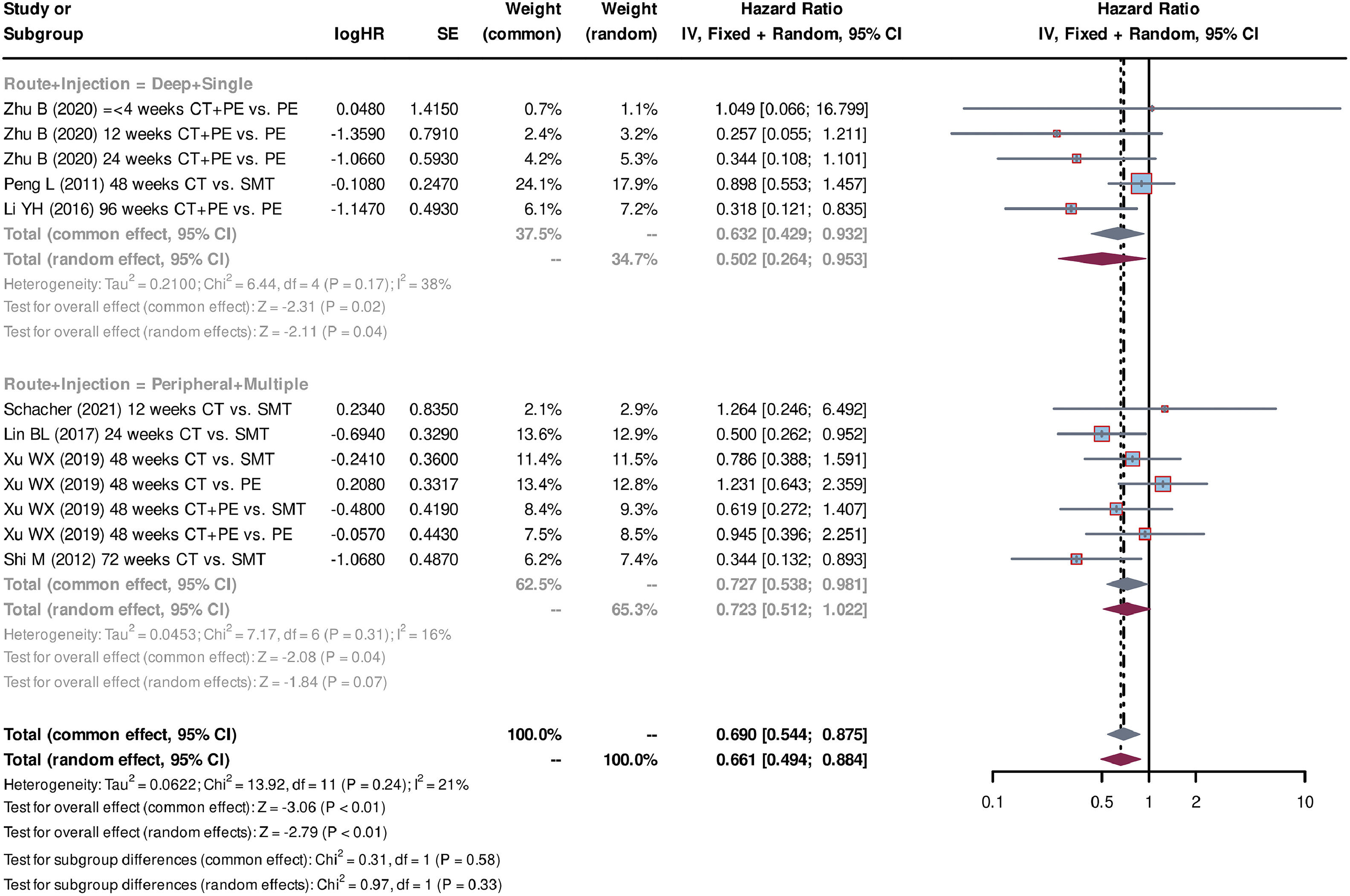
Additionally, subgroup analyses were conducted for cell sources (autologous or allogeneic), injection route variations (deep vessel or peripheral vessel), and differing injection frequencies (single or multiple). Two studies [28,31] used autologous stem cells, whereas five studies [26,27,29,30,32] used allogeneic stem cells. The pooled HR for allogeneic stem cells was 0.675 (95% CI=0.507-0.900, P < 0.01, standard effect model), indicating improved survival outcomes compared with autologous stem cell treatment with a pooled HR of 0.722 (95% CI=0.472-1.104, P = 0.13, standard effect model) (Fig. 5). Regarding the injection route and injection frequencies, three studies [26,28,31] used the deep vessel route and single injection, whereas four studies [27,29,30,32] used the peripheral vessel route and multiple injections. Both administration groups were associated with a significant improvement in survival (for the deep vessel + single injection group: pooled HR=0.632, 95% CI=0.429-0.932, P = 0.02, common effect model; for the peripheral vessel + multiple injections group: pooled HR=0.727, 95% CI=0.538-0.981, P = 0.04, common effect model) (Fig. 6). The analyses of sensitivity confirmed the reliability of the above results (Fig. S1). Additionally, the symmetry funnel plots and negative Egger's test (P > 0.05) indicated a low risk of publication bias among the studies (Fig. S2).
Five studies [27–30,32] compared SCT+SMT treatment with SMT treatment alone to observe the changes in MELD scores over various follow-up durations. The SCT+SMT group comprised 183 patients, whereas the control group consisted of 228 patients. The pooled SMD of MELD scores was -0.251 (95% CI=-0.386 to -0.115, P < 0.01, the common effect model). Subgroup analyses for each follow-up duration also demonstrated a decrease in the MELD scores in the SCT+SMT group compared with the control; for follow-up durations of ≤4 weeks (five studies, pooled SMD=-0.229, 95% CI=-0.428 to -0.030, P = 0.02, the common effect model), 8 weeks (one study, SMD=-0.369, 95% CI=-0.880 to -0.141), 12 weeks (two studies, pooled SMD=-0.177, 95% CI=-0.479 to 0.125, P = 0.25, the common effect model), 24 weeks (four studies, pooled SMD=-0.499, 95% CI=-1.034 to 0.035, P = 0.07, the random effect model), and 48 weeks (one study, SMD=-0.228, 95% CI=-1.178 to 0.721) (Fig. S3). Two studies [26,32] that compared SCT+PE with PE alone treatments reported on the changes of MELD scores: The pooled SMD of MELD scores was found to be -0.400 (95% CI=-0.626 to -0.173, P < 0.01, the common effect model) (Fig. S4). These results indicated that SCTs could reduce the MELD scores of patients. In terms of stem cell sources, two studies [25,28] used autologous stem cells, resulting in a pooled SMD of MELD scores of -0.523 (95% CI=-0.774 to -0.271, P < 0.01, the common effect model). On the other hand, four studies [26,27,30,32] employed allogeneic stem cells and exhibited a pooled SMD of MELD scores of -0.201 (95% CI=-0.313 to -0.088, P < 0.01, the common effect model) (Fig. S5). Both approaches were found to result in decreased MELD scores. With regard to injection route and frequency, three studies [25,26,28] utilized the deep vessel route with a single injection, whereas two studies [27,30] used the peripheral vessel route with multiple injections. The deep vessel + single injection approach showed a significant reduction in MELD scores (pooled SMD=-0.607, 95% CI=-803 to -0.411, P < 0.01, the common effect model), whereas the peripheral vessel + multiple injections administration route did not (pooled SMD=-0.121, 95% CI=-0.242 to 0.000, P = 0.05, the common effect model) (Fig. S6).
Sensitivity analyses were subsequently performed to confirm the reliability of the aforementioned results. However, the Egger's test (P < 0.05) revealed asymmetry in the funnel plots, suggesting the presence of publication bias among the included studies. As a result, random effect models were constructed using the trim-and-fill method to calculate the adjusted pooled SMD for each analysis, which helped to reduce the effect of publication bias (Fig. S7).
3.5Effect of stem cell therapy on laboratory indicatorsALT, TBIL and ALB are commonly used indices of liver function in a clinical setting. Neither the levels of ALT or TBIL were found to be significantly changed following treatment in studies comparing SCT+SMT treatment with SMT or SCT+PE with PE alone (Figs. S8-11). By contrast, a significant increase in ALB was observed in the SCT+SMT treatment group compared with SMT (pooled SMD=0.475, 95% CI=0.092-0.856, P = 0.01, the random-effect model). Although no significant differences in ALB levels were noted between the SCT+PE and PE groups, a trend of increased levels was observed (Figs. S12 and 13). The INR is an important indicator of coagulation function. No significant differences in INR were observed between the SCT+SMT and SMT groups; however, only two studies were included (Fig. S14). On the other hand, in studies comparing SCT+PE with PE alone, a significant decrease in INR was observed in the SCT+PE group (pooled SMD=-0.909, 95% CI=-1.375 to -0.443, P < 0.01, the random-effect model) (Fig. S15). Subsequent analyses revealed asymmetry in the funnel plot, indicating the presence of publication bias among the included studies (Egger's test,E P < 0.05). The trim-and-fill method was therefore employed to calculate the adjusted pooled SMD of INR (-0.913; 95% CI=-1.382 to -0.444) (Fig. S16).
4DiscussionThis study holds significant potential in advancing the understanding and application of SCT for liver failure, particularly highlighting the effectiveness of MSCs. Notably, no significant side effects were reported in the included clinical trials. By systematically evaluating and quantifying the benefits of SCT in improving patient survival and clinical outcomes, the results indicated that both SCT+SMT and SCT+PE combination therapies resulted in significantly improved survival rates for the patients. This study also revealed a decrease in the MELD score following SCT, a finding that was consistent with previous studies [13,15]. Allogeneic stem cells demonstrated superior therapeutic efficacy in terms of improving patient survival compared with autologous stem cells. Both deep vessel + single and peripheral vessel + multiple injections led to significant improvements in survival. However, the deep vessel + a single injection administration route appeared to be more effective than peripheral vessel + multiple injections in terms of reducing patients’ MELD scores. Consistent with previous studies [13,15], the employment of SCT may not have had a significant effect on patients’ INR levels. ALB levels were found to be increased following SCT+SMT treatment, but were not significantly increased after SCT+PE treatment. The present study did not identify significant differences in TBIL and ALT levels comparing between SCTs and their respective controls, which was in contradiction with previous meta-analyses [33,15]. This discrepancy, however, may be attributed to the differences in inclusion criteria of studies, as mentioned earlier in this article.
Although researchers have made great progress in terms of SCT in liver failure, there are still some concerns that require clarification. MSCs have a unique ability to migrate to, and home in on, the injury to the liver. The homing mechanism is a crucial aspect of their therapeutic potential in liver failure. In addition, the homing process may also occur in healthy liver. In a previous study, BM-MSCs were cultured and labeled with radioactive tracer (111In-oxine) before being infused into healthy rats via deep vessel injection. The distribution of the infused MSCs was monitored using real-time imaging, and the radioactivity in various organs was measured after 48 h. The results obtained showed that MSCs were primarily detected in the lungs, followed by the liver and other organs, suggesting that MSCs have multiple homing sites [34]. Subsequently, in a study of liver injury, a thioacetamide-induced acute liver injury rat model was established. After infusion of UC-MSCs via the tail vein, the MSCs were also found to be mainly distributed in the lung and liver [35]. This phenomenon has also been observed in patients with liver cirrhosis. Following injection with 111In-oxine-labeled BM-MSCs via the peripheral vein, the uptake of radioactivity was initially observed in the lungs, and this was gradually increased in the liver and spleen over a time period of hours to days. The spleen uptake was found to exceed that in the liver in all patients. The study also showed that the percentage of cells homing to the liver increased from 0-2.8% immediately after infusion to 13.0-17.4% on day 10. The homing process of MSCs to the liver has been demonstrated to comprise a complex interplay of various factors [36]. One key factor is the release of chemotactic signals from the damaged liver tissue. These signals include cytokines, growth factors and chemokines, which act as attractants for MSCs. The SDF-1/CXCR4 signaling pathway is one of the well-studied pathways that have been found to regulate the migration and homing of MSCs. In the rat model of acute liver injury, the expression level of SDF-1 was found to increase over time following MSC infusion, whereas the blockage of CXCR4 led to an inhibition of the migration of MSCs to the liver [37]. However, it is also important to note that the homing mechanism of MSCs to the liver is not yet fully understood, and this remains an active area of research. Both understanding and harnessing this mechanism should be able to potentially enhance the therapeutic efficacy of MSCs-based therapies for liver diseases.
Few previously published clinical studies have investigated the dose-response relationship of SCT for liver failure, and therefore the optimal dosages for treatment remain uncertain. Two RCTs evaluated bone marrow-derived mononuclear cells (BM-MNCs) in liver treatment: In one, 1 × 109 cells were administered intraportally [38], and in the other, 5 × 107 cells per kg were administered through the hepatic artery [39]. Neither trial revealed any benefit compared with the control group. However, enriching BM-MNCs for CD133 (approximately 5 × 106 cells) or CD34/CD133 (5 × 107 cells) resulted in partial improvements, suggesting that reducing cell numbers through enrichment appeared to be beneficial [38,40]. A recent study in rat models has demonstrated dose-dependent effects of MSCs in terms of reducing mortality and liver inflammation in acetaminophen-induced acute liver failure. The researcher infused UC-MSCs into rats via the tail vein at doses ranging from 5 × 105 to 2 × 106 cells. The result showed a gradual increase in the survival rate of mice as the dose was raised from 5 × 105 to 1 × 106 cells; however, no further improvements were observed at higher doses. Furthermore, the serum transaminase levels in the surviving mice exhibited a significant dose-dependent decrease [41]. Future clinical studies on SCT should include treatment protocols with varying dose gradients to establish the optimal dosage for liver failure treatment using stem cells, thereby promoting standardization in subsequent associated studies.
There is currently no consensus on the optimal route for infusing stem cells. It is known that a significant proportion of the cells initially reside in the lungs following administration, a phenomenon referred to as the ‘pulmonary-first’ phenomenon [42]. Therefore, the peripheral vascular infusion of stem cells may not result in a sufficient number of cells homing to the liver. On the other hand, there are various options available for deep vascular infusion, including the hepatic artery, hepatic vein, splenic artery, femoral artery and the femoral vein. To the best of our knowledge, previous clinical studies have not compared different infusion routes. It is generally understood that using the portal vessel system can effectively bypass the pulmonary-first phenomenon, allowing an adequate number of stem cells to reach the liver. In an animal model study, researchers compared the femoral vein, femoral artery and intraperitoneal routes of infusion, and found no significant difference between the femoral vein and femoral artery administration routes in terms of the number of stem cells homing to the liver, whereas only a small fraction of cells migrated to the liver with intraperitoneal infusion [34]. In a prospective study by Amer et al., the hepatic artery and splenic artery injection routes were compared, and no significant differences in any of the clinical parameters were observed [25]. Moreover, the results of the present study indicated that both the deep vascular infusion and peripheral vascular infusion routes led to a significant improvement in patients’ survival. However, combining deep vascular infusion with single injections could lead to a marked reduction in patients’ MELD scores. This may be related to the deep vascular route, which can reduce the retention time of stem cells in the lung and spleen, thereby increasing the number of stem cells that reach the liver. Although deep vascular infusion carries a risk of increased bleeding due to the requirement of deep vascular puncture, which, in terms of bleeding, is of particular concern in liver failure patients with impaired coagulation function, a previous study demonstrated the safety of the usage of deep vascular injection by experienced practitioners during SCT [31].
The administration method, whether through a single or multiple infusions, is an important consideration in the clinical application of stem cells. The key consideration is to minimize the number of infusions while ensuring a sufficient therapeutic cell dose to reduce the risk of treatment-associated complications. Additionally, it is necessary to determine the optimal source of stem cells for therapy. The use of autologous stem cells is known to minimize the risk of host immune rejection. However, due to the limitations of cell harvesting, a large quantity of autologous stem cells (typically obtained from bone marrow) cannot be obtained in a single collection. A large number of bone marrow samples (typically ranging from 100-200 ml per procedure) are usually required, which inevitably places a psychological burden on the patients. Moreover, the prolonged cultivation time (typically one week) may delay the treatment of patients with liver failure [25,28]. On the other hand, allogeneic stem cells (typically obtained from umbilical cord blood) can be prepared in sufficient quantities in a single collection, and these have good immunocompatibility [5]. Our results suggested that allogeneic stem cells had similar therapeutic effects to those of autologous stem cells, and were even superior in terms of improving the survival outcomes of patients. However, due to the limited number of included studies, this meta-analysis was unable to compare stem cells from different tissue origins.
Artificial liver support therapy is one of the treatment options for patients with end-stage liver disease, apart from LT. PE is a commonly used method in artificial liver treatment, which comprises replacement of the patient's plasma to effectively remove metabolic toxins, improve the internal environment, and promote the repair and regeneration of the liver [43,44]. Although there is currently a lack of solid evidence from evidence-based medicine to support the efficacy, for certain patients who are unable to undergo LT for various reasons, artificial liver treatment has been demonstrated to alleviate the patient's condition, even avoiding the requirement for LT eventually [45]. The present study suggested that the combination of PE with stem cell transplantation leads to an enhancement in the survival rate of patients with liver failure, as well as reducing their MELD scores and INR values.
Stem cells are highly valued in other chronic liver diseases, such as nonalcoholic fatty liver disease and autoimmune liver disease, due to their self-renewal and multipotency [8]. However, these same characteristics pose significant tumorigenic risks, restricting their use in liver cancer. ESCs can form teratomas when injected into immunodeficient mice [46,47], while iPSCs have been associated with a 20% tumor incidence in mice due to the expression of specific transcription factors [48]. In hepatocellular carcinoma (HCC) treatment, immunological approaches, particularly immune checkpoint inhibitors (ICIs), have shown promise, though clinical outcomes have yet to meet expectations [49]. MSCs, known for their "homing" effect, can migrate to sites of inflammation or tumors, making them potential vehicles for delivering pro-apoptotic drugs to multiple tumor sites. Additionally, iPSC-derived natural killer (NK) cells that inhibit the TGF-β signaling pathway have demonstrated enhanced anti-tumor activity in HCC cell line and mouse models [50]. Ongoing research is crucial to fully realize the potential of stem cell therapies in liver cancer.
However, this study has several limitations. The included studies had small sample sizes and limited populations, which may affect the generalizability of the findings. Additionally, restricting the analysis to English-language publications introduces potential publication bias. The heterogeneity in stem cell sources and administration routes also impacted the study's reliability, despite efforts to mitigate these issues using subgroup analysis, random effects models and trim-and-fill methods. As with any meta-analysis, controlling for study's subjects selection criteria was challenging, and the inclusion of both ACLF and CLF, along with varying diagnostic criteria, may have introduced bias. The predominance of studies in viral hepatitis-related liver failure and conducted in China further limits the generalizability of our results. Variations in follow-up durations could have led to inaccuracies in pooled survival outcomes. Therefore, the HRs of the survival curves were estimated to refine the accuracy of the results.
While this study provides valuable insights, it also highlights significant knowledge gaps. A deeper understanding of the long-term effects and safety of SCT in liver failure is needed. Although numerous clinical trials have confirmed its safety, long-term observations are necessary to evaluate the risk of tumorigenicity [51]. The variability in treatment protocols, including differences in MSCs sources (allogeneic vs. autologous) and administration methods, underscores the importance of developing standardized guidelines. Future research should prioritize long-term follow-up studies and transplantation free survival to address these issues, as well as larger, multicenter trials with standardized protocols to validate findings and establish best practices for SCT in liver failure. Despite the substantial resources required for stem cell research, the promising potential it has demonstrated in treating chronic and degenerative diseases has prompted some developing countries to invest in this field [52,53]. However, the significant resources needed for stem cell therapy can present challenges in low-resource settings. To make stem cell therapy more accessible in these areas, it will be essential to explore strategies such as developing cost-effective methods for cell production and storage, utilizing locally available resources, and simplifying administration techniques.
5ConclusionsIn conclusion, the present systematic meta-analysis has shown that the combinations of SCT+SMT and SCT+PE as treatment methods led to a significant improvement in patient survival, with reduced MELD scores in patients with liver failure. Allogeneic MSCs exhibited greater therapeutic effectiveness when compared with autologous MSCs. Furthermore, both the deep vessel + single injection and peripheral vessel + multiple injections administration routes were associated with improved outcomes. However, the deep vessel + single injection route appeared to be more effective.
Authors' contributionsSL, HG and YZ designed the study; HM, ZL, and DZ conducted the literature search and data collection; JP and YZ evaluated and selected the final articles. SL and HG performed the literature screening, data analysis and wrote the manuscript jointly; YZ reviewed the final data and manuscript; SL and HG contributed equally to this study and should be considered as the co-first authors.