
Edited by: Sonia Roman
More infoNon-alcoholic fatty liver disease (NAFLD) affects one-third of the world's adult population and is linked to metabolic syndrome. It can progress to steatohepatitis, cirrhosis and hepatocellular carcinoma. During the last four decades, it has been the subject of exhaustive research in multiple aspects to define its epidemiology, pathophysiological mechanisms and therapy.
In 2020, a group of international experts proposed the change of name to metabolic-associated fatty liver disease (MAFLD) with the main objective of making it an inclusive diagnosis prioritizing metabolic abnormalities. However, the change in terminology included the modification of the diagnostic criteria allowing the non-exclusion of other concomitant liver diseases such as alcohol liver disease, and chronic hepatitis B or C.
The proposal precipitated a wave of debates among experts based on theoretical opinions on the desirability of the rapid adoption of the new terminology. But it also precipitated a wave of epidemiological and clinical studies which, two years later, have provided clinical evidence on the differences and similarities of the two entities, specially, those that could be considered for future refinements of the diagnostic criteria of MAFLD. Likewise, this evidence may contribute to deciding the time of adoption of this terminology.
In this text, we discuss, in general terms, important aspects of the clinical evidence that has been generated to date in cross-sectional and longitudinal studies focusing on clinical characteristics and outcomes, mainly on all-cause and specific mortality of MAFLD.
Non-alcoholic fatty liver disease (NAFLD) is currently the most common cause of liver disease worldwide. This disease affects a third of the adult population and 12% of children [1–3]. It is one of the most common causes of liver cirrhosis and hepatocellular carcinoma in Western countries, particularly in those patients with non-alcoholic steatohepatitis (NASH) and advanced fibrosis [4–8]. The causes of this condition are strongly linked pathogenically to metabolic syndrome (MS), which is a constellation of metabolic abnormalities such as overweight or obesity, type 2 diabetes mellitus (T2DM), dyslipidemia and arterial hypertension, of which the first two seem to be the strongest [9–13]. The increase in its incidence at a global level has been driven by the global obesity epidemic that affects a great percentage of the world population [14,15].
NAFLD is a relatively new condition. In 1980 Ludwig J coined the term for patients who had fatty liver disease (FLD) without any obvious secondary cause, particularly alcohol [16]. During the last four decades to date, there has been intense research on this condition which has significantly increased knowledge in many aspects of this disease.
In 2020 a group of international experts proposed the change name of this disease to metabolic- or metabolic dysfunction- associated fatty liver disease (MAFLD) [17,18]. The main purpose of the change in nomenclature was to highlight the importance of metabolic abnormalities by making this disease an inclusive instead of an exclusive entity such as NAFLD. The proposal seemed logical if one considers the close pathophysiological link that this disease has with MS. However, the change in terminology brought with it also the modification of the diagnostic criteria. NAFLD is defined as the presence of FLD in the absence of known causes of steatosis, with an emphasis on alcohol, as this is the second cause of FLD [19]. Conversely, MAFLD is defined inclusively as the presence of FLD concomitantly with the presence of overweight or obesity and/or T2DM. In the absence of these clinical parameters, the presence of at least two of 7 metabolic risk abnormalities is required [17,18] (Fig. 1). This definition does not exclude concomitant diseases such as alcohol liver disease (ALD), chronic hepatitis B or C, or autoimmunity.
Immediately, several associations for the study of the liver from different regions of the world published guidelines on the management of the new entity [20–24]. However, more than two years after this proposal, the use of the new nomenclature has not become widespread in the different countries or among the different scientific working groups that carry out research on this disease. Conversely, some opinion texts and editorials have been published that fuel the debate about its adoption [25–31].
A group of experts in the United States of America recently gave the opinion that the adoption of the new nomenclature is premature with the following arguments: first, the change in nomenclature may be confusing and jeopardize existing efforts to promote disease awareness among patients, policymakers and non-hepatologists physicians; second, the change in terminology "is justified when a more scientific, complete understanding of its pathogenesis, and/or risk stratification, and/or molecular phenotyping, and/or novel precision medicine-based therapeutic approaches are elucidated" and third, there are concerns that the change in terminology and definition will affect both patient recruitment and endpoint assessment, particularly resolution of NASH with no worsening of liver fibrosis, which is currently a key histological endpoint for conditional drug approval [26].
2Current clinical evidenceTo date, several cross-sectional and longitudinal studies have been carried out in different regions of the world in which the new nomenclature and diagnostic criteria are applied to patients with FLD [32–40]. Some of these studies have used archived data obtained through the Third National Health and Nutrition Examination Survey (NHANES III), which has made it possible to expeditiously study a cohort with a large number of individuals with a follow-up greater than 25 years [34,37,38,40]. The NHANES is a population-based program of surveys conducted by the National Center for Health Statistics (NCHS), whose purpose is to monitor the health and nutritional status of civilian, noninstitutionalized individuals in the U.S. population using a complex, multistage design with data currently released in 2-year cycles.
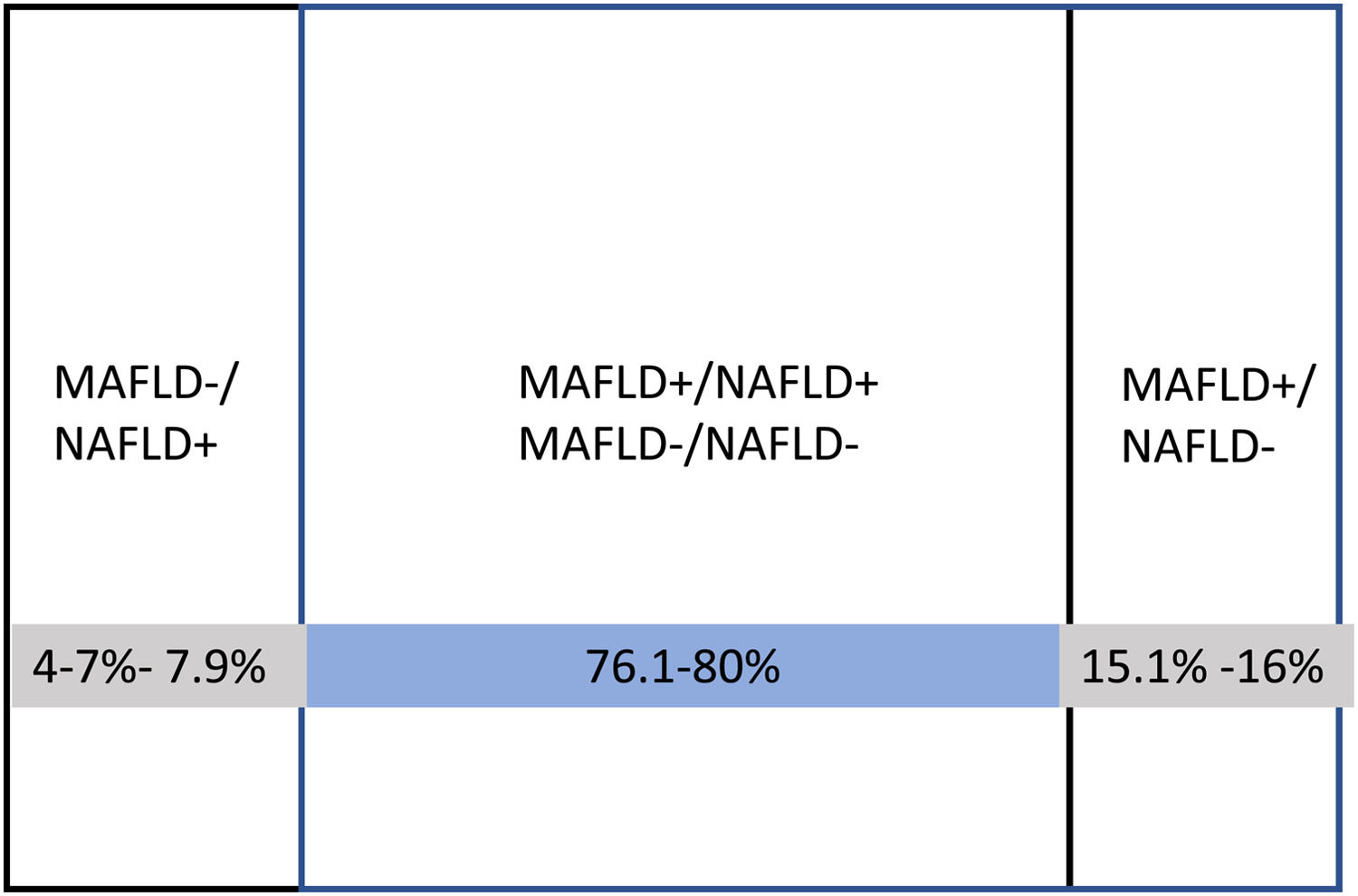
2.1Clinical and epidemiological characteristics of MAFLDThe results of these studies indicate that the prevalence of MAFLD is about similar to the prevalence of NAFLD (24.2%−36% vs. 15.3%−34%, respectively). The clinical characteristics of patients with both entities, in general, are similar, particularly when adjusting the metabolic parameters that define MAFLD [38,40]. In some studies, patients with NAFLD were significantly younger [34,38]). The percentage of overlap between both entities is around 80%, with a high correlation (Kappa, 0.83–0.94). In the remaining 20%, there is either MAFLD without NAFLD or NAFLD without MAFLD. The definition of MAFLD+/NAFLD- includes about 8–11% more patients than the NAFLD+/MAFLD- definition [40,41] (Fig. 2). Two studies have found a higher prevalence of advanced fibrosis in patients with MAFLD+ compared to NAFLD+ by liver biopsy [38] and transitional elastography [33]. At this point, a metanalysis with 17 studies published by Ayada I et al. in 2022 with more than 9 million individuals showed that notably, MAFLD-only group was at significantly increased risk for fibrosis (RR 4.2) and had higher alanine aminotransferase (mean difference: 8.0 U/L) and aspartate aminotransferase (mean difference: 6.4 U/L), compared to NAFLD-only group [41].
This indicates that both entities can be considered approximately equivalent in clinical and epidemiological terms, which could be explained by the high proportion of overlapping. However, the most important differences have been observed in relation to outcomes, especially in non-combined forms such as MAFLD+/NAFLD- and MAFLD-/NAFLD+.
2.2Outcomes2.2.1Risks factors associated with all-cause mortality and cause-specific mortalityIn a study published by Huang Q et al. [37] with 12,480 individuals, it was observed that MAFLD increased the overall risk for total mortality to a greater magnitude than NAFLD [Hazard ratio (HR) 2.07 vs. 1.47]. However, the difference was non-significant after metabolic parameters were adjusted. Risks for cardiovascular, neoplasm and diabetes-related mortality were similar between MAFLD and NAFLD. Referring to individuals without both NAFLD and MAFLD, individuals with only MAFLD independently increased the risk for total mortality [adjusted HR (aHR) 1.47] and neoplasm mortality (aHR 1.58).
In a study published by Kim D et al. [38] with 7761 individuals, it was observed that during a median follow-up of 23 years, individuals with MAFLD had a 17% higher risk of all-cause mortality (HR 1.17). Furthermore, MAFLD was associated with a higher risk of cardiovascular mortality. NAFLD per se did not increase the risk of all-cause mortality. Individuals who met both definitions had higher risk of all-cause mortality (HR 1.13), while individuals who met the definition for MAFLD but not NAFLD had a 1.7-fold higher risk of all-cause mortality (HR 1.66). Estimates for all-cause mortality were higher for those with advanced fibrosis and MAFLD than for those with advanced fibrosis and NAFLD.
In a very recently published study by Younossi ZM et al. [40] with 12,878 individuals followed for a median of 23 years, this group investigated a wide range of clinically relevant predictors of mortality. It was observed that insulin resistance (IR) and high-risk fibrosis were significantly increased risks for cardiac specific mortality among the NAFLD+ (HR 1.83 and 1.91, respectively) but not among the MAFLD+ (HR 1.36 95% CI 0.99–1.86 and HR 1.43 95% CI 0.93–2.19, respectively).
Regarding liver-specific mortality, the top three risk factors significantly associated among the MAFLD+ were high-risk fibrosis (HR 17.15), ALD (HR 4.50), and chronic kidney disease (CKD) (HR 2.92). In contrast, among NAFLD+, the top three risk factors were high-risk fibrosis (HR 9.26), high C-reactive protein (HR 4.47), and IR (HR 3.57). In fact, IR, a hallmark of metabolic abnormality, was not a predictor of liver mortality among MAFLD patients.
Mortality combining MAFLD and NAFLD definitions yielded the following results: the MAFLD+ had a 97% higher increased risk for all-cause mortality than the MAFLD-, whereas the NAFLD+ had a 67% higher risk for all-cause mortality than the NAFLD-. However, when ALD was added to the multivariable model, the MAFLD+ had no longer a significant risk for all-cause mortality, suggesting that the association between MAFLD and mortality was mediated by ALD (defined as ALT levels >40 U/L or AST levels >37 U/L in males, ALT or AST >31 U/L in females or fatty liver disease and excessive alcohol consumption). Of 70 deaths related to liver disease, 23 occurred in individuals with ALD (MAFLD+/NAFLD-) and the remaining 47 occurred in patients with (MAFLD+/NAFLD+).
The above discussed studies suggest that: a) the definition of MAFLD is associated with higher mortality than NAFLD when both are compared with individuals without either of these definitions. However, when both conditions are compared to each other, MAFLD is associated with higher mortality; b). In both MAFLD and NAFLD conditions, fibrosis is a risk factor for all-cause mortality and specific mortality to a greater degree in MAFLD. However, it is important to note that metabolic dysregulation parameters (such as IR) were associated with cardiovascular disease mortality in NAFLD but not in MAFLD. Conversely, in relation to mortality associated with liver disease, metabolic parameters were more relevant in NAFLD, while in MAFLD alcohol explained a large percentage of the deaths (approximately 32%) [40]. The potential negative impact of alcohol on MAFLD outcomes may explain the increase in the percentages of death from all causes and from specific causes, particularly hepatic. Therefore, metabolic abnormalities that should be the main incentive to change the name from NAFLD to MAFLD were not independently associated with mortality among patients with MAFLD. In support of this, a recently published study by van Kleef LA et al.[42] with 12,656 patients showed that both MAFLD and alcohol abuse (defined as an intake >10 g/d for women and >20 g/d for men) were independently and simultaneously associated with increased risk of death in models with full adjustment (aHR 1.21, 95% CI 1.13–1.30 and aHR 1.14, 95% CI 1.04–1.26, respectively). Similarly, MAFLD was associated with an increased risk of death in patients with and without excessive alcohol consumption. Participants with both conditions (MAFLD and excessive alcohol consumption), which accounted for 4% of all individuals, had the highest risk of death. This indicates that excessive alcohol intake may exert a synergistic effect on the risk of mortality in patients with MAFLD.
It is important to emphasize that the clinical evidence known so far previously discussed in this text, has been generated in studies with different methodological designs, patients' characteristics, clinical settings, methods for detecting FLD (ultrasonography, computed tomography scan, magnetic resonance imaging and liver biopsy) and markers of liver fibrosis (non-invasive methods and liver biopsy) which may explain some differences in results among them.
3PerspectivesThis clinical information generated so far could be useful for trigger evidence-based debates among true international experts supported by international scientific liver societies and non-scientific societies, providers and possibly stakeholders for assessing whether the current moment is appropriate for the change of nomenclature.
The history of medicine is full of emerging diseases whose original diagnostic criteria have been modified over time as new evidence about the pathological entity arises [43]. Based on this evidence, it might be appropriate to modify the diagnostic criteria of MAFLD, perhaps by establishing realistic thresholds in alcohol consumption and emphatically pointing out the presence or absence of comorbidities (ALD, chronic hepatitis B or C, autoimmunity, etc.), classifying the disease according to these data as a "pure" entity or a dual-etiology entity. In this context, MAFLD in patients with chronic hepatitis B has already been shown to be an independent risk factor for adverse outcomes [44]
On the other side, we believe that prospective studies (preferably multicenter) are still required, conducted in various regions of the world with different prevalence of ALD and B virus infection and with a large number of patients who are stratified into subgroups according to the type and number of comorbidities. These studies could make it possible the definition of phenotypes, the dominant pathogenic mechanisms in each phenotype and the impact of the comorbidities on outcomes. This will allow the categorization of patients with differences in severity of the disease enabling the selection of a specific treatment either based on a change in lifestyle and/or pharmacological treatment alone or in combination directed to selective targets.
The idea of changing the nomenclature of NAFLD to MAFLD to prioritize metabolic abnormalities in an inclusive diagnosis was a great idea. However, the key factor that induced the debate does not lie in why but in how to concretize this change in the most appropriate way and it is there that it will be necessary to search for effective modifications that improve the defining instrument.
With the increase in the incidence and prevalence of fatty liver (MAFLD or NAFLD) worldwide, given the epidemic of obesity and T2DM, the secondary and tertiary levels of care of these patients have been exceeded and are increasingly attended by the first contact non-hepatologist physician. In this spirit, we must offer these physicians a simple, reliable and pragmatic view for the diagnosis and effective management of these patients.





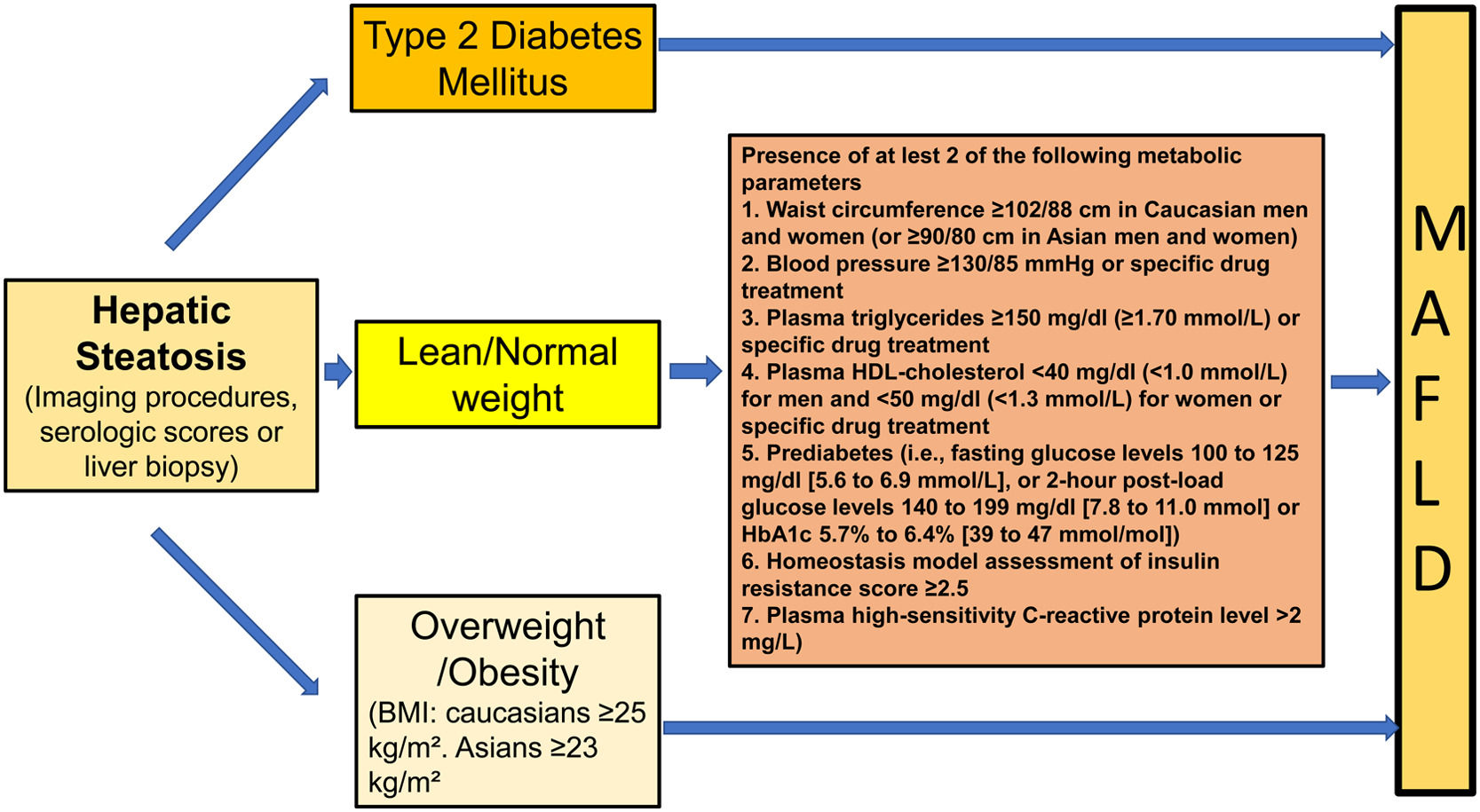
![Diagnostic criteria of MAFLD [17,18]. Diagnostic criteria of MAFLD [17,18].](https://static.elsevier.es/multimedia/16652681/0000002700000006/v3_202307180533/S1665268122001077/v3_202307180533/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Percentages of distribution of isolated and combined forms of MAFLD and NAFLD observed in clinical studies. [32- 41]. Percentages of distribution of isolated and combined forms of MAFLD and NAFLD observed in clinical studies. [32- 41].](https://static.elsevier.es/multimedia/16652681/0000002700000006/v3_202307180533/S1665268122001077/v3_202307180533/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




