Although splenic vein embolization (SVE) has been performed for the management of patients with hepatic encephalopathy (HE) related to large spontaneous splenorenal shunts (SSRS) in recent years, its role remains poorly defined. In this study, we aimed to explore the safety and efficacy of SVE for HE patients with large SSRS.
Materials and methodsData from cirrhotic patients who were confirmed to have recurrent or persistent HE related to large SSRS and underwent SVE from January 2017 to April 2021 were retrospectively collected and analyzed at our center. The primary endpoints were the change of HE severity at 1 week after embolization and the recurrence of HE during the follow-up period. The secondary endpoints were procedure-related complications and changes in laboratory indicators and hepatic function (Child-Pugh score/grade and model for end-stage liver disease score).
ResultsOf the eight cirrhotic patients included in the study, six were diagnosed with recurrent HE, and the others were diagnosed with persistent HE. Embolization success was achieved for all patients (100%), and no immediate procedure-related complications, de novo occurrence, or aggravation of symptoms related to portal hypertension were observed during the long-term follow-up. HE status was assessed at 1 week after embolization. The results demonstrated that the symptoms were mitigated in three patients and resolved completely in five patients. During the follow-up period, all patients were free of HE within 1 month after embolization, but one patient experienced the recurrence of HE within 6 months and another one experienced the recurrence of HE within 1 year. Compared with the preoperative parameters, the Child-Pugh score and grade were significantly improved at 1 week and 1 month after embolization (all P<0.05), and the serum ammonia level was significantly lower at 1 month after embolization (P<0.05).
ConclusionsSVE could be considered as a feasible treatment for patients with HE related to large SSRS, but further validation is required.
Portal hypertension (PHT) is the predominant cause of death for patients with decompensated cirrhosis [1, 2]. As the portal pressure increases, various collateral vessels are gradually established [3]. In addition to esophageal and gastric varices, common spontaneous portosystemic shunts (SPSS) include the gastrorenal shunt, splenorenal shunt, and recanalized umbilical or paraumbilical veins [3, 4]. Few attentions have been paid on spontaneous splenorenal shunt (SSRS), although it is a major component of SPSS [5, 6]. SSRS is defined as an abnormal collateral blood vessel that connects the splenic and left renal veins and develops due to a combination of increased portal vein (PV) pressure and decreased shunt resistance [7, 8]. SSRS connects the PV system with the systemic circulation and acts as a “release valve” to relieve the portal pressure to some extent. In turn, as the bypass of normal hepatic hemodynamics, SSRS reduces the perfusion of hepatocytes, deteriorates liver function, and accelerates the progression of chronic liver failure [3, 9]. Qi et al. [10] found that SSRS might be involved with higher incidence of HE (18.2% vs 4.3%), higher Child-Pugh scores (9.50±1.65 vs. 7.43±2.02) and MELD scores (11.26±7.29 vs. 5.67±6.83) than those without SSRS. Guilaume et al. [11] performed a comment and suggested that presence of SSRS should be added in MELD score to better predict the outcome of patients with cirrhosis.
Hepatic encephalopathy (HE) is one of the most common complications of cirrhosis and often results in longer hospital stay, poorer quality of life, and higher mortality rates [12, 13]. Standard medical therapies have been still the mainstay treatments for patients with HE, but the long-term efficacy remains discouraging with the 1-year recurrence rate as high as of 57%[14, 15]. In the past few decades, some studies revealed that large SSRS was significantly associated with HE, particularly persistent or recurrent HE [16, 17]. Therefore, several clinicians have explored the value of shunt embolization for HE patients, with some promising results [18–20]. Related studies suggested that embolization of large portosystemic shunts for patients with HE was effective, but the procedure is often followed by the retention of ascites and aggravation of esophageal and gastric varices because of the extreme elevation in PV pressure after embolization [21, 22].
Splenic vein embolization (SVE), the representative shunt-preserving disconnection of the portal and systemic circulation [23], was first attempted for the management of HE patients with large SSRS at the beginning of the current decade in Japan. Initial results indicated that the procedure could reduce the recurrence of HE and result in mild elevation of the PV pressure with no increase in postoperative complications [23, 24], which is markedly different from the effects observed following the embolization of shunts. However, due to the most of initial studies were reported in case reports or technical notes [24–27], the role of SVE remains poorly defined. Hence, we performed a series investigation to explore the clinical significance of SVE for HE patients with large SSRS and provide an alternative approach for clinical decision-making.
2Materials and methods2.1Patient selectionAll cirrhotic patients (diagnosed via clinical, radiologic, or histologic techniques) who were confirmed to have HE related to large SSRS and received SVE from January 2017 to April 2021 in our center were recruited. The exclusion criteria were as follows: (i) Patients who received hepatectomy, splenic artery embolization, transjugular intrahepatic portosystemic shunt, or surgical shunts; (ii) Patients with hepatocellular carcinoma (HCC) beyond Milan's criteria; (iii) Patients with neurologic or psychiatric disorders; and (iv) Presence of other large SPSS. All eligible patients received standard medical therapies including elimination of the cause of HE and administration of lactulose and/or rifaximin. Of note, once the patient was diagnosed with the recurrence of HE during follow-up, standard medical therapies would be initiated.
Data on gender, age, etiology, HE grade, history of HCC, albumin, total bilirubin, creatinine, international standardization rate (INR), prothrombin time (PT), serum ammonia level, portal vein thrombus (PVT), ascites, esophageal-gastric variceal bleeding (EGVB), embolic materials, procedure-related complications, HE status after embolization, and imaging measurement parameters such as the maximum cross- sectional diameter of shunts, length of the splenic vein (SV) from the splenorenal shunt to PV, diameter of the PV main trunk and primary branch, SV, superior mesenteric vein (SMV), liver volume, and spleen volume were collected from the medical records. The grade of HE was evaluated using the West Haven criteria [15]. The model for end-stage liver disease (MELD) score and the Child-Pugh score and grade were calculated using the reported equations [28, 29].
2.2Splenic vein embolization techniqueAll eligible patients were placed in a supine position under local anesthesia. An 18-gage needle (Cook Incorporated, Indiana, USA) was used to puncture the first or second branch of the intrahepatic PV guided by ultrasound, and a 6.5-F introducer sheath (Terumo, Tokyo, Japan) and 6-F catheter (Terumo, Tokyo, Japan) were inserted into the portal system sequentially. Splenic venography was performed to confirm the presence of large SSRS, superior mesenteric venography was performed to determine the direction of blood flow in the main PV, and inferior mesenteric venography was performed to identify the junction of inferior mesenteric vein (IMV) and PV. SV was occluded with coils (Cook Incorporated, Indiana, USA), Amplatzer plugs (Abbott Medical, MN, USA), or a combination of the two. After embolization, superior mesenteric venography was conducted again, which was used to validate the complete embolization of SV and the increase of PV blood flow. Finally, the transhepatic PV puncture access was occluded with GLUBRAN 2 (GEM, Viareggio, Italy).
2.3DefinitionsLarge shunts were defined as shunts with a maximum cross-sectional diameter exceeding 8 mm [30].
Recurrent HE was defined as HE that occurred repeatedly within 6 months or less. Persistent HE was defined as a pattern of behavioral changes that was consistently present and interacted with the recurrence of overt HE [12].
Embolization success was defined as the complete occlusion of SV based on superior mesenteric venography.
Procedure-related complications were documented on the basis of the Society of Interventional Radiology (SIR) clinical practice guidelines [31].
2.4Follow-up and endpointsAll patients were evaluated at 1 week, 1 month, and 6 months after the embolization procedure, and they were followed up once a year after 6 months. Follow-up items included albumin, total bilirubin, creatinine, INR, PT, serum ammonia level, abdominal ultrasound, and contrast-enhanced CT or MRI. The follow-up endpoints were defined as the death of patients or the last follow-up time. The primary endpoints were the change of HE severity at 1 week after embolization and the recurrence of overt HE during the follow-up period. The secondary endpoints were changes in the laboratory indicators (albumin, total bilirubin, creatinine, INR, PT, serum ammonia level), hepatic function (Child-Pugh score and grade, Meld score), vessel diameter of the PV system, and liver and spleen volumes. The safety endpoints included the immediate procedure-related complications and de novo occurrence or aggravation of symptoms related to PHT during the long-term follow-up.
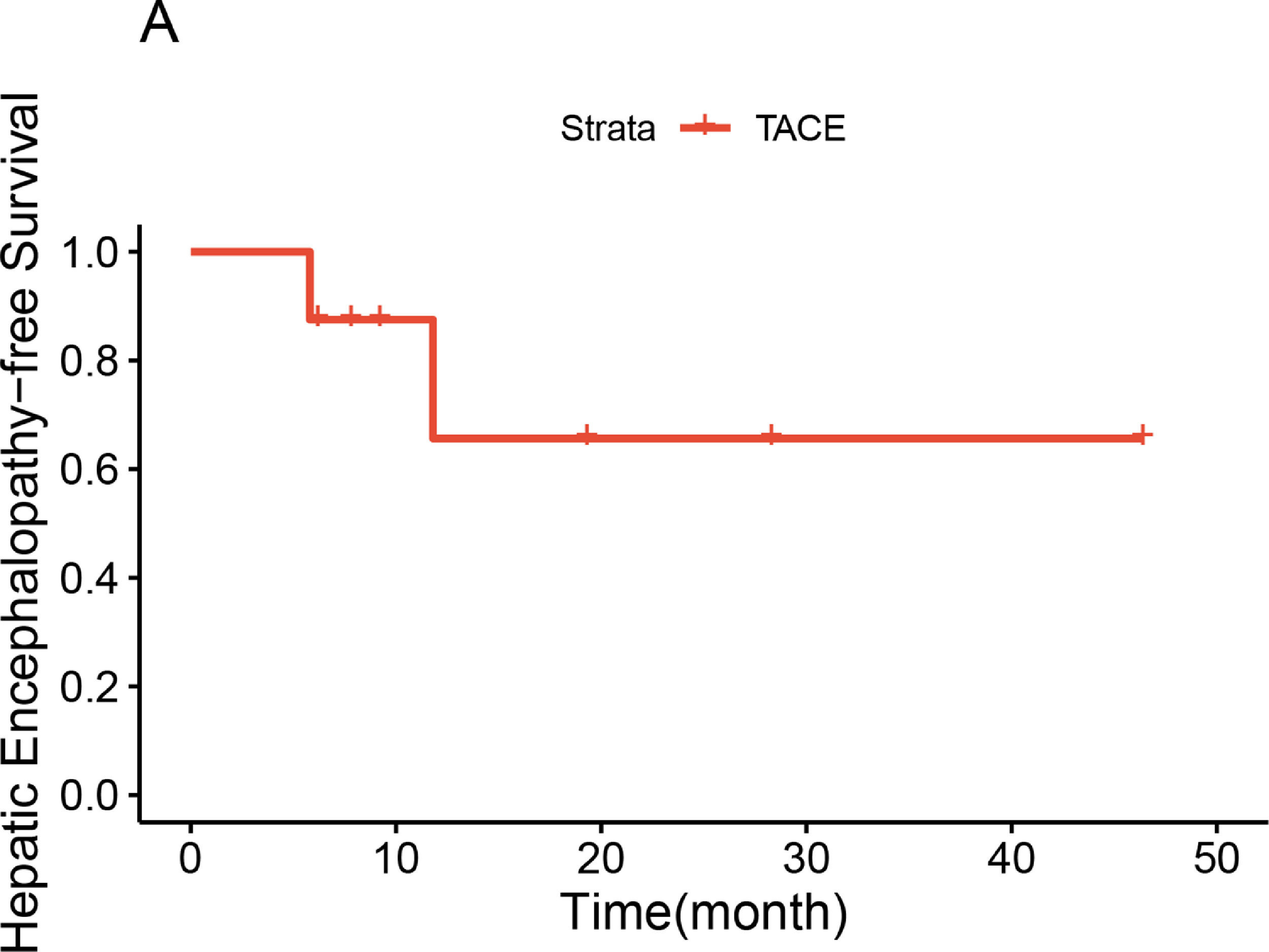
2.5Statistical analysisCategorical variables were expressed as numbers, and differences were compared with the Chi-square test or Fisher's exact test. Continuous variables were expressed as the mean value (standard deviation) or median (quartile) based on the normal distribution test, and differences were compared with the paired or unpaired Student's t-test. The HE-free survival curve was depicted by Kaplan-Meier method. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were conducted using R studio and R (4.1.2).
2.6Ethical statementThis retrospective research was performed under the guidance of Declaration of Helsinki and obtained an approval of institutional review board (Number: 2021_079_01). All the eligible participants were signed the informed consent.
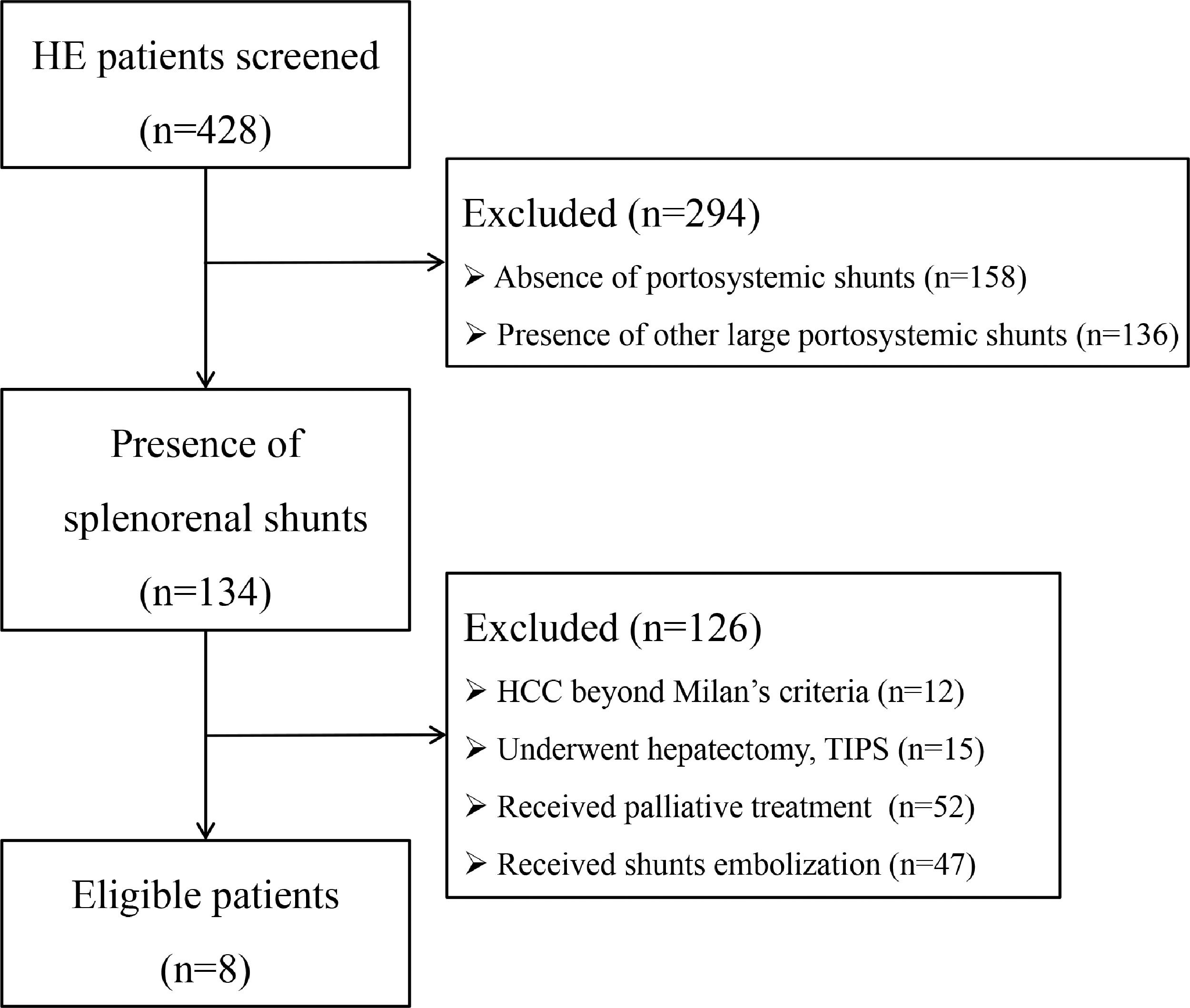
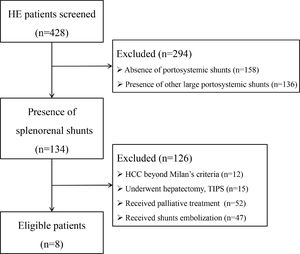
3Results3.1Patient selection, baseline characteristics and follow-upInitially, 428 HE patients were screened (Fig. 1), 294 patients were excluded due to absence of SPSS (n = 158), combined with other large SPSS (n = 136), and 134 patients were identified with splenorenal shunt. Then, 126 patients were excluded with the following reasons: i) HCC beyond Milan's criteria (n = 12); ii) underwent hepatectomy and TIPS (n = 15); iii) received palliative treatment (n = 52); iv) received shunts embolization (n = 47). Finally, eight patients were remained to be analyzed, all of whom were male, with an age range of 41–69 years old. Six patients were diagnosed with recurrent HE and two patients were diagnosed with persistent HE. Before embolization, one patient was diagnosed with HCC according to Milan's criteria and underwent treatment with radiofrequency ablation. One patient developed early HCC 1 year after embolization and was cured by radiofrequency ablation. The maximum SRSS cross-sectional diameter ranged from 11.9 to 23.9 mm, with an average diameter of 19.2 mm. The IMV pooled into SV in four patients, into SMV in three patients, and into the confluence point of SV and SMV in the remaining patient. The proximal length of SV between the splenorenal shunt and PV ranged from 37.5 to 85.4 mm, with an average length of 56.56 mm. The last follow-up time was Oct, 2021, and the median follow-up time was 14.6 months. During the follow-up period, one patient died of pneumonia, one died of HCC, one died of a traffic accident, and the remaining five are still alive with a good quality of life (Table 1).
Baselines characteristics and follow-up of each patient.
* the length of splenic vein from splenorenal shunt to portal vein; HBV, hepatitis B virus; HE, hepatic encephalopathy; HCC, hepatocellular carcinoma; SRSS, spontaneous splenorenal shunt.
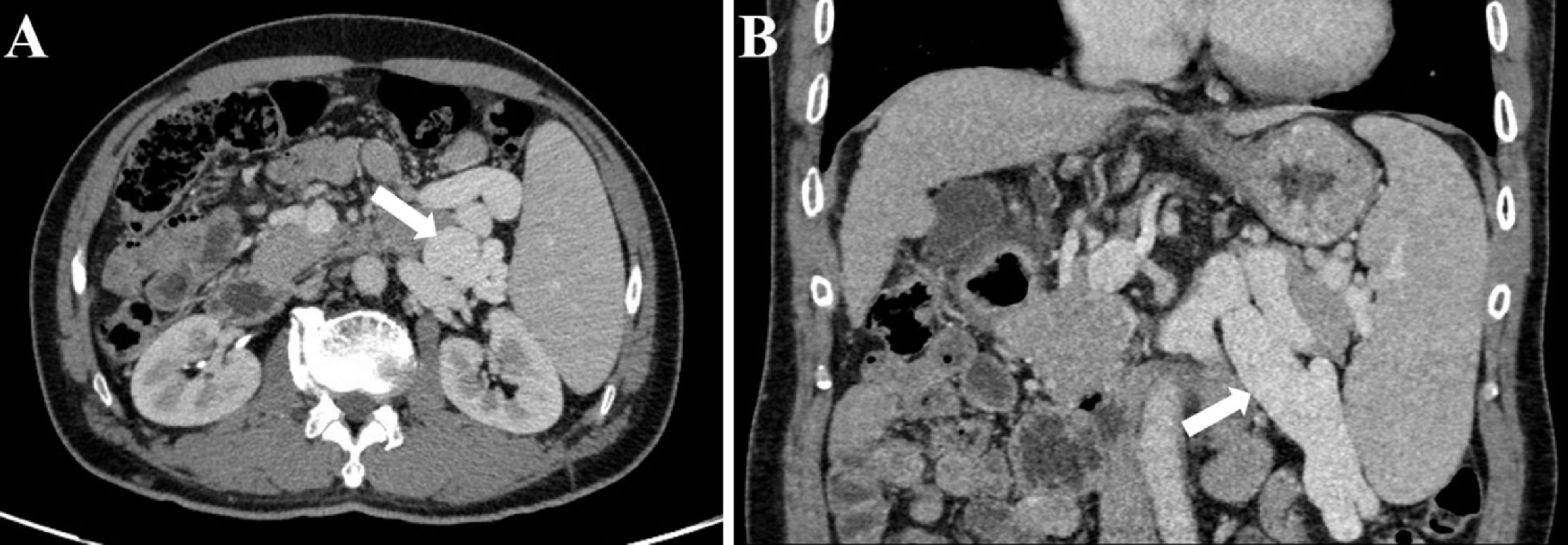
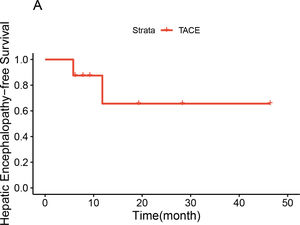
Preoperative contrast-enhanced CT, intraoperative fluoroscopy, and digital subtraction images are shown in Fig. 2 and 3. A schematic illustration of the hemodynamics after embolization is depicted in Fig. 4. Superior mesenteric venography confirmed that four patients had hepatofugal portal flow, and another four had hepatopetal portal flow. Embolization success was achieved in all patients (100%). The embolic materials consisted of coils (n = 2), Amplatzer plugs (n = 5), and a combination of coils and Amplatzer plugs (n = 1). No immediate procedure-related complications were observed, and no de novo occurrences or aggravation of symptoms related to PHT were observed during the long term follow-up. HE status was assessed at 1 week after embolization, and the results demonstrated that the symptoms had been relieved in three patients and resolved completely in five patients. During the follow-up period, no de novo SPSS was observed in imaging examinations. All patients were free of HE within 1 month after embolization, but one patient experienced a recurrence of HE at grade 2 within 6 months due to the progression of HCC. Another patient experienced a recurrence of HE at grade 1 within 1 year due to the progression of cirrhosis (Table 2). The HE-free survival curve was depicted in Fig. 5, but the median HE-free survival was not available.
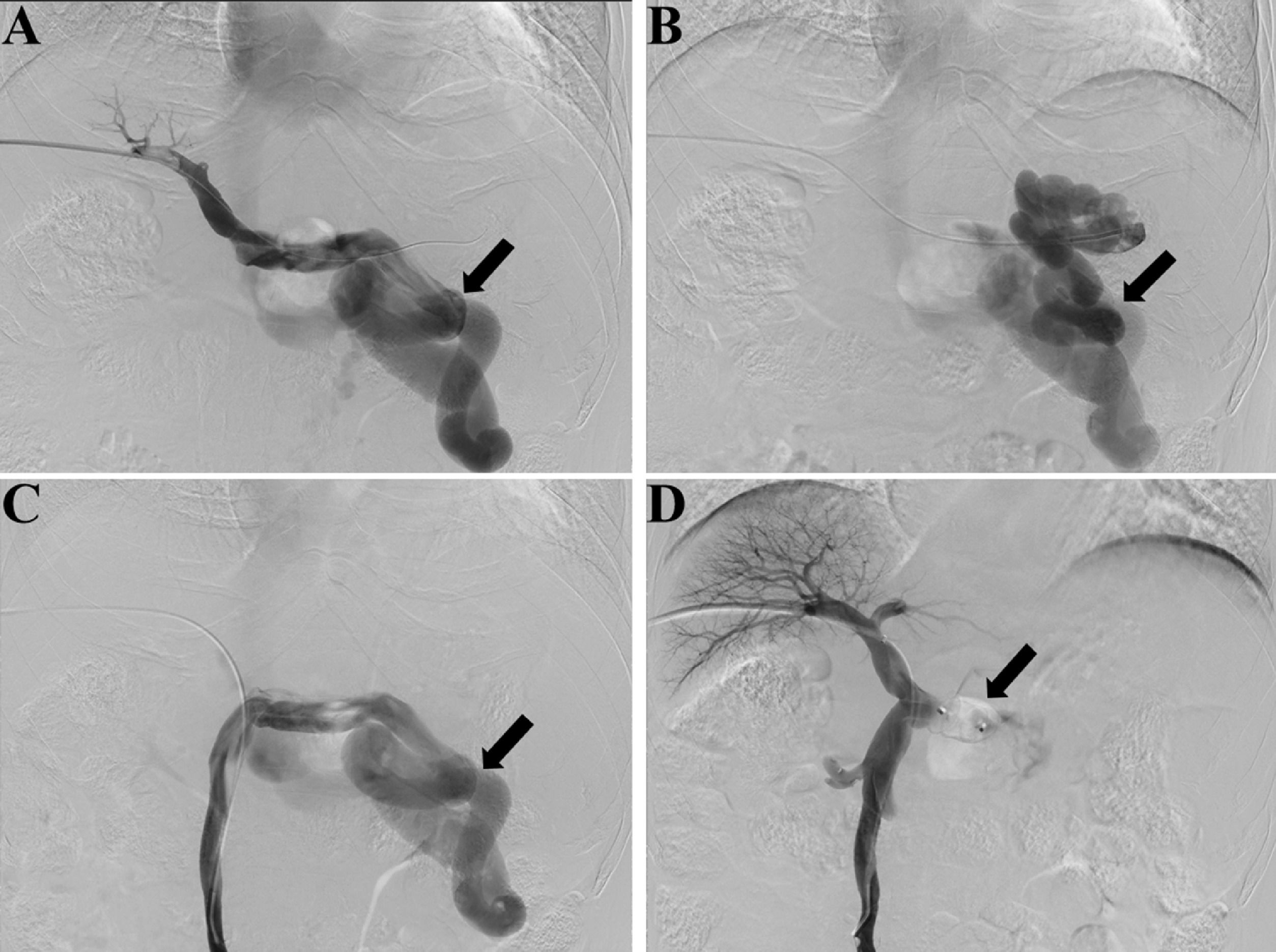
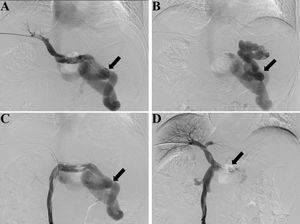
Fluoroscopy and digital subtraction images (A, before embolization, splenic venography shows the hepatofugal flow in the splenic vein and a large splenorenal shunt, black arrow shows the large shunt; B, before embolization, shunt venography shows the blood flow in shunt enters systemic circulation, black arrow shows the large shunt; C, before embolization, superior mesenteric venography shows hepatofugal flow in the splenorenal shunt, black arrow shows the large shunt; D, after embolization, superior mesenteric venography shows increased hepatopetal flow in the portal vein and no flow in splenic vein, black arrow shows the embolic site).
Procedures detail and HE recurrence.
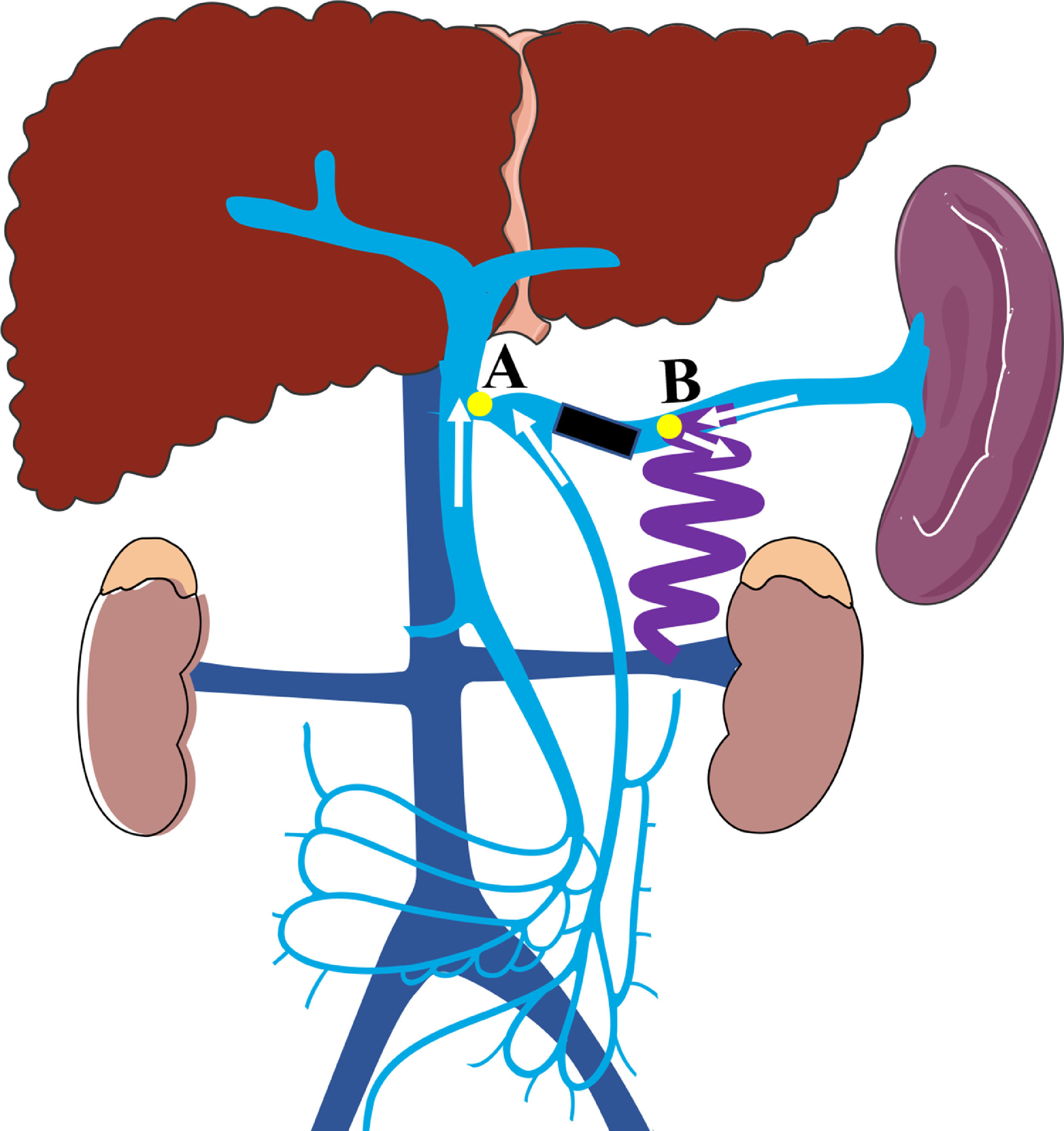
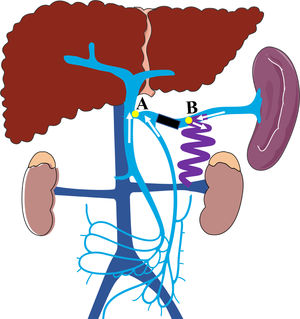
A schematic illustration of the hemodynamics after embolization (Point A: the site of splenic vein drainage into the portal vein; Point B: the site of the large splenorenal shunt. Black rectangular block shows the embolic site, white arrow shows the direction of blood flow in the splenic vein, superior mesenteric vein, and inferior mesenteric vein; After embolization, the blood flow in splenic vein into systemic circulation through splenorenal shunt, the blood flow in superior and inferior mesenteric veins into portal vein).
Compared with the preoperative parameters, the Child-Pugh score and grade were significantly improved at 1 week and 1 month after embolization (all P<0.05), and the serum ammonia level was significantly decreased at 1 month after embolization (P<0.05). However, no significant differences were observed in albumin, total bilirubin, creatinine, INR, PT, MELD score, ascites, PVT, and EGVB before and after embolization (all P>0.05, Table 3). Notably, there was a small possibility that the procedure would enlarge the diameter of the main PV and its first branch, increase the volume of the liver, and shrink the volume of the spleen, although the difference was not statistically significant (all P>0.05, Table 3).
Changes of parameters before and after embolization.
INR, prothrombin time; PT, international standardization rate; MELD, the model for end-stage liver disease; PVT, portal vein thrombus; EGVB, esophageal-gastric variceal bleeding; MPV, main portal vein; LPV, left branch of portal vein; RPV, right branch of portal vein; SV, splenic vein; SMV, superior mesenteric vein; NA, not available.
A comprehensive literature search were preformed in databases (PubMed, Embase, the Cochrane library, Medline, Web of Science) to identify the eligible studies which evaluating the safety and efficacy of SVE for the treatment of HE patients related to SSRS. Finally, A total of 6 studies with 19 patients were enrolled in this literature review [23-27, 32]. The embolic accesses, materials, procedural efficacy and safety were detailedly shown in Table 4. Among the included 19 patients receiving SVE, two patients (10.5%) experienced the recurrence of HE after embolization, and one patient (5.3%) formed a new collateral circulation. What's more, major complications including the worsening of ascites, esophagogastric varices and liver function after embolization were not reported in any of the included studies.
Previous reports regarding splenic vein embolization as the treatment for HE patients related to spontaneous splenorenal shunt.
| Studies | Design | Patients | Classification of HE | Embolization (access/material) | Efficacy/Safety |
|---|---|---|---|---|---|
| Zamora 2004[23] | Case report | 1 | HE | Assess: transhepaticMatetials: metallic coils | -HE was controlled 6 days after operation and recurred at 3 months and 5months after operation-New collateral vessel was observed 3months after embolization |
| Mezawa 2004[21] | Retrospective | 6 | Recurrent HE | Assess: transhepatic(n = 5)+ transjugular(n = 1)Matetials: interlocking detachable coils+N‑butyl cyanoacrylate | -HE has not been observed in four patients, but was observed in the remained two-Neither retention of ascites nor worsening of esophageal varices was observed in any patient.-There were no episodes of variceal bleeding in any patients after the procedure |
| Ikeda 2012[22] | Case report | 2 | Recurrent HE | Assess: transhepatic (n = 2)Matetials:patient 1: metallic coils+interlocking detachable coilspatient 2: 3D guglielmi detachable coil+interlocking detachable coils | -The patients’ encephalopathy resolved immediately and permanently and in the course of 30-month follow-up-There was no evidence of ascites or esophageal varices |
| Inoue 2013[24] | Case report | 1 | HE | Assess: transhepaticMatetials: metallic coils | -HE had not been observed for 25 months after the procedure- Neither retention of ascites nor worsening of esophageal varices and liver functionwas observed |
| Ikeda 2018[30] | Retrospective | 8 | Recurrent HE | Assess: transhepatic (n = 7) + transileocolic (n = 1)Matetials: metallic coils | -All patients improved with respect to HE severity: seven were recorded as grade 0 and the other as grade 1-There were no apparent major complications |
| Haraguchi 2020[25] | Case report | 1 | Refractory HE | Assess: percutaneous transsplenicMatetials: amplatzer vascular plug II+NBCA | -HE did not recur during 12 months of follow-up-There were no apparent major complications |
HE, Hepatic Encephalopathy; NBCA, N‑butyl cyanoacrylate.
HE is a neuropsychiatric syndrome caused by acute and chronic liver failure or portal systemic shunt, and its incidence ranges from 16 to 21% in cirrhotic patients [33, 34]. The relationship between the occurrence of HE and presence of SPSS is well-defined, and previous studies have demonstrated that 46%−71% of patients with persistent or recurrent HE have large SPSS [16, 35]. Because of the high recurrence rate after standard medical therapies for HE patients related to SSPS, the other modalities include liver transplantation, surgical shunt ligation, and interventional therapies have been attempted [36, 37]. However, owing to the organ shortage and apparent surgery-related complications, interventional therapies play an important role in the clinical decision-making process. Currently, shunt embolization is the most popular interventional therapy [36, 38], and the procedure includes percutaneous transhepatic obliteration, balloon-occluded retrograde transvenous obliteration, coil-assisted retrograde transvenous obliteration, and plug-assisted retrograde transvenous obliteration. In addition, SVE has been attempted to treat HE patients with large SPSS, although relevant studies have been reported for individual cases [23, 27].
There is still a debate regarding the selection of interventional therapies to treat patients with HE caused by large SSRS. As the most popular procedure, shunt embolization has been demonstrated to significantly relieve the symptoms of HE related to large SSRS and reduce the risk of rehospitalization [19, 20, 39]. However, due to the extreme elevation of portal pressure after embolization, this procedure often involves a high incidence of complications, including ascites retention and the aggravation of esophageal and gastric varices [21, 22]. An et al. [19] reported that all severe complications occurred in patients with MELD scores >15 and/or HCC. Similarly, the study by Philips et al. [40] revealed that patients with Child-Pugh scores >11 would not benefit from shunt embolization and should be candidates for liver transplantation. SVE leads to a mild elevation of PV pressure, which is different from the portal pressure gradient changes following shunt embolization according to previous studies (not exceeding 4 mmHg with SVE vs. over 8 mmHg with shunt embolization) [25, 26]. In addition, the incidence of serious complications with SVE was lower than that with shunt embolization [27]. However, the disadvantage of SVE should also be noted. Previous studies have demonstrated that the recurrence of HE was the major shortcoming of SVE, and the potential mechanism might be incomplete embolization and formation of new collateral vessel[25, 32].
In the current study, we found that patients who underwent SVE experienced increased portal blood flow, improved hepatic function, and a lower risk of HE recurrence with no worsening of PHT symptoms. Hence, SVE could be considered as an feasible option for the management of HE caused by large SSRS, but the procedure-related complications should not be ignored. Theoretically, the potential immediate complications related to SVE were skin infection at puncture site, fever and the bleeding of puncture pathway; while the long-term complications were the retention of ascites and the worsening symptoms of left-sided portal hypertension including splenomegaly, aggravation of esophagogastric varices and liver function. But in the current study, no patients experienced the immediate complication, as well as the long-term complications, the latter mainly because the splenorenal shunt was reserved and the splenopancreatic venous blood flow could be effectively drained into the systemic circulation with lower risk of left-sided portal hypertension. Of note, this procedure also has corresponding indications. Firstly, an adequate SV length from the splenorenal shunt to PV is necessary to perform the embolization (Fig. 3, the distance from point A to point B). In this study, the length of SV from the splenorenal shunt to PV ranged from 37.5 to 85.4 mm, with an average of 56.56 mm. In our opinion, if IMV drains into SMV or the confluence point of SMV and SV, the minimum length of SV from the splenorenal shunt to PV should be at least 4 cm. Moreover, if IMV drains into SV, the length of SV between IMV and the splenorenal shunt should be kept to at least 4 cm, which is in accordance with previous reports [24, 27]. Secondly, although multiple collaterals connect the renal vein, only SSRS is considered as an indication for SVE.
Of note, the timing of embolization is worthy of further discussion. Recently, Philips et al. [41] performed a retrospective study to compare the efficacy of early embolization (conducted at the first occurrence of HE) and late embolization (conducted for recurrent or persistent HE) for HE management. The results suggested that early embolization could reduce the incidence of events related to both PHT and PVT and significantly improve the quality of life and long-term survival of patients. Furthermore, patients with advanced liver function who receive shunt embolization might experience a high incidence of complications and even lose the chance of intervention. Significantly, the potential lead-time bias in estimating the benefit of early embolization was hard to avoid; hence, those findings should be interpreted cautiously. Whether patients should receive shunt embolization at the early stage when confirmed to have SSRS via imaging examinations remains a topic of discussion.
There were several limitations in this research. Firstly, the medical records of all eligible patients were collected retrospectively; therefore, selection and recollection biases were difficult to avoid. Secondly, due to the short follow-up period and absence of the control group, long-term observation of the efficacy of this procedure was insufficient. Finally, the sample size in this preliminary study might weaken the stability and reliability of the conclusion.
5ConclusionIn patients with HE related to large SSRS, SVE could be considered as a feasible treatment to relieve the symptoms of HE, decrease the recurrence of HE and improve the liver function, and not experience the de novo occurrence or aggravation of symptoms related to PHT. However, the conclusion needs to be validated by further controlled and prospective studies.
Authors’ contributionZhiyong Wang, Weimin Wu, Linbin Qiu, Yubin Jiao, Yixing Xie: acquisition of data, Xinhui Huang and Ling Li: conception and design of the study. Qiao Ke: analysis, interpretation of data, and drafting of the article. Xiaosong Peng, Jingfeng Liu and Wuhua Guo: critical revision and final approval. All authors contributed to the article and approved the submitted version.
FundingThis work was supported by social development medical project of Fuzhou, Fujian, P.R.C (2018-S-103–5), the Joint Funds for the Innovation of Science and Technology of Fujian province, Fujian, P.R.C (2017Y9117), the Scientific Foundation of Fuzhou Municipal Health commission, Fujian, P.R.C (2021-S-wt4), Fujian provincial medical center of hepatobiliary, the Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (201912002), the Startup Fund for scientific research, Fujian Medical University, Fujian, P.R.C (2020QH1242).
Declaration of interestNone
We thank Medjaden Inc. for scientific editing of this manuscript.