Cirrhotic cardiomyopathy (CCM) means cardiac dysfunction in individuals with cirrhosis, in the absence of known heart disease. CCM mainly manifests as altered diastolic relaxation, referred to as ‘diastolic dysfunction’ (DD). DD represents a pivotal and early manifestation of CCM. Unlike systolic dysfunction (SD), the other component of CCM, which typically emerges in more advanced disease stages, DD frequently precedes overt cardiac failure. The hyperdynamic state in cirrhosis, hallmarked by an increased cardiac output and a decreased systemic vascular resistance, underlies the development of DD. DD remains often silent until significant stress occurs, such as insertion of a transjugular intrahepatic portosystemic shunt or a liver transplantation (LT). A detailed review on DD and CCM in cirrhosis can be found in [1].
Until recently, there was no official consensus for the diagnosis of DD. Preliminary diagnostic criteria were proposed in 2005. With significant advancements in echocardiography and the recognition of challenges in interpreting the proposed parameters in cirrhosis, these criteria are now considered outdated. In 2019, new diagnostic criteria were introduced, based on based on the recommendations from The American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines (see Graphical abstract figure) [2,3].
Data regarding the prevalence of DD in patients with end-stage liver disease are scarce since the disease usually remains silent until late stages where the cardiac reservoir is obliterated, or only becomes apparent at the time of LT. We chose DD as an investigative focus in this manuscript to better understand the influence of this specific component of CCM on LT outcomes. Its impact on the pre- and post-LT outcome is not clear yet, and it is not known whether the DD improves after LT in affected patients. This study aimed to determine the prevalence and clinical impact of DD in cirrhotic LT candidates.
2Materials and Methods2.1Patient dataThis retrospective study focused on adult patients (≥18 years old) with cirrhosis listed for LT at the Ghent University Hospital between January 1, 2005, and March 31, 2017 (n = 233). The etiology of cirrhosis was categorized as alcohol-related liver disease, hepatitis B or C viral infection, auto-immune and biliary liver disease, metabolic dysfunction-associated steatotic liver disease (MASLD), or other causes. Patients with insufficient pre-LT echocardiographic data, a known prior heart disease, or portopulmonary hypertension were excluded. For those patients with multiple echocardiograms within the year prior to LT, the most recent and comprehensive one was included for analysis in this study.
Demographic and clinical data, including information regarding age, sex, race, the etiology (including alcohol misuse) and severity of the liver disease (Child-Pugh, MELD), pre-LT decompensation events, smoking and comorbidities (pulmonary: asthma, COPD; cardiovascular: history of ischemic or valvular heart disease, arrythmia, peripheral vascular disease) were obtained from the patient files. Outcome data included transplantation status and pre-LT and post-LT mortality. The presence of DD was evaluated using echocardiography variables and based on the original criteria from 2005 and the revised criteria from 2019. The original criteria from 2005 define DD as deceleration time <200 ms, E/A < 1 and isovolumic relaxation time >80 ms. The revised criteria from 2019 define DD as left atrial volume index >34 mL/m2, septal e’ <7 cm/s or lateral e’ <10 cm/s, tricuspid regurgitation peak velocity >2.8 m/s and E/e’ >=15.
2.2Statistical analysesThe analyses were performed using SPSS Statistics version 29. Normality was assessed by the Shapiro-Wilk test. Results are presented as mean ± standard deviation or median (interquartile range) for quantitative variables and as n (%) for categorical variables. The paired and unpaired Student's t-test, Mann-Whitney-U test, χ2-test and Fisher-Exact-test were used. The association between time-to-event outcomes and covariates was estimated using Cox proportional hazard regression. Survival was evaluated with Kaplan–Meier curves and compared by the log-rank test.
2.3Ethical statementsThe study received approval from our local Ethical Committee (11/2010–2010/582 and 05/2017–2017/0635), conducted in accordance with the principles outlined in the 1975 Declaration of Helsinki. As approved by the Ethical Committee, patients were actively informed about this retrospective study and its aims and were provided with the option to oppose through opting-out.
3Results3.1Patient characteristicsAll LT candidates had sufficient echocardiographic data to adequately define the presence or absence of DD. The median study follow-up was 1290 days. Study population characteristics are provided in Table 1. The cause of cirrhosis was alcohol-related in 49,8 %, MASLD in 9,4 %, and viral hepatitis in 24,1 %.
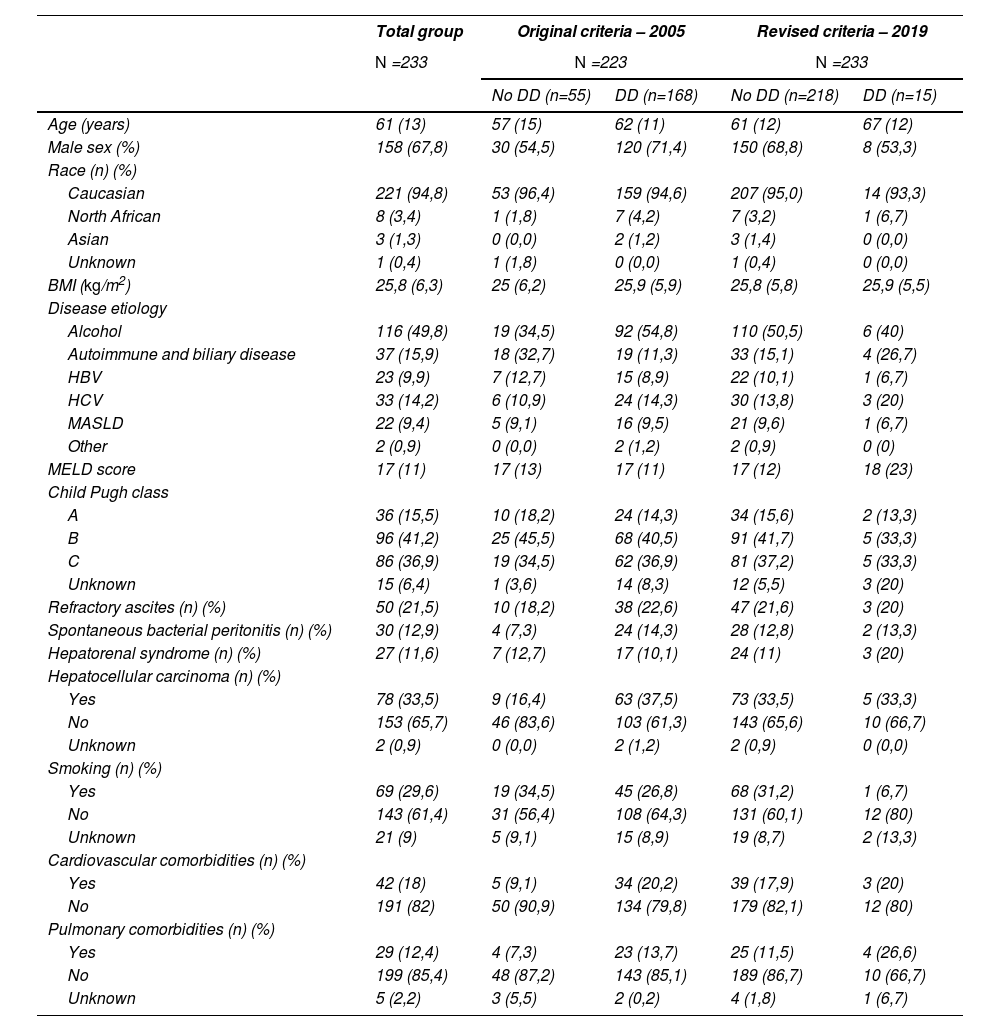
Baseline characteristics of patients with and without DD by the original and revised criteria of 2005 and 2019.
| Total group | Original criteria – 2005 | Revised criteria – 2019 | |||
|---|---|---|---|---|---|
| N =233 | N =223 | N =233 | |||
| No DD (n=55) | DD (n=168) | No DD (n=218) | DD (n=15) | ||
| Age (years) | 61 (13) | 57 (15) | 62 (11) | 61 (12) | 67 (12) |
| Male sex (%) | 158 (67,8) | 30 (54,5) | 120 (71,4) | 150 (68,8) | 8 (53,3) |
| Race (n) (%) | |||||
| Caucasian | 221 (94,8) | 53 (96,4) | 159 (94,6) | 207 (95,0) | 14 (93,3) |
| North African | 8 (3,4) | 1 (1,8) | 7 (4,2) | 7 (3,2) | 1 (6,7) |
| Asian | 3 (1,3) | 0 (0,0) | 2 (1,2) | 3 (1,4) | 0 (0,0) |
| Unknown | 1 (0,4) | 1 (1,8) | 0 (0,0) | 1 (0,4) | 0 (0,0) |
| BMI (kg/m2) | 25,8 (6,3) | 25 (6,2) | 25,9 (5,9) | 25,8 (5,8) | 25,9 (5,5) |
| Disease etiology | |||||
| Alcohol | 116 (49,8) | 19 (34,5) | 92 (54,8) | 110 (50,5) | 6 (40) |
| Autoimmune and biliary disease | 37 (15,9) | 18 (32,7) | 19 (11,3) | 33 (15,1) | 4 (26,7) |
| HBV | 23 (9,9) | 7 (12,7) | 15 (8,9) | 22 (10,1) | 1 (6,7) |
| HCV | 33 (14,2) | 6 (10,9) | 24 (14,3) | 30 (13,8) | 3 (20) |
| MASLD | 22 (9,4) | 5 (9,1) | 16 (9,5) | 21 (9,6) | 1 (6,7) |
| Other | 2 (0,9) | 0 (0,0) | 2 (1,2) | 2 (0,9) | 0 (0) |
| MELD score | 17 (11) | 17 (13) | 17 (11) | 17 (12) | 18 (23) |
| Child Pugh class | |||||
| A | 36 (15,5) | 10 (18,2) | 24 (14,3) | 34 (15,6) | 2 (13,3) |
| B | 96 (41,2) | 25 (45,5) | 68 (40,5) | 91 (41,7) | 5 (33,3) |
| C | 86 (36,9) | 19 (34,5) | 62 (36,9) | 81 (37,2) | 5 (33,3) |
| Unknown | 15 (6,4) | 1 (3,6) | 14 (8,3) | 12 (5,5) | 3 (20) |
| Refractory ascites (n) (%) | 50 (21,5) | 10 (18,2) | 38 (22,6) | 47 (21,6) | 3 (20) |
| Spontaneous bacterial peritonitis (n) (%) | 30 (12,9) | 4 (7,3) | 24 (14,3) | 28 (12,8) | 2 (13,3) |
| Hepatorenal syndrome (n) (%) | 27 (11,6) | 7 (12,7) | 17 (10,1) | 24 (11) | 3 (20) |
| Hepatocellular carcinoma (n) (%) | |||||
| Yes | 78 (33,5) | 9 (16,4) | 63 (37,5) | 73 (33,5) | 5 (33,3) |
| No | 153 (65,7) | 46 (83,6) | 103 (61,3) | 143 (65,6) | 10 (66,7) |
| Unknown | 2 (0,9) | 0 (0,0) | 2 (1,2) | 2 (0,9) | 0 (0,0) |
| Smoking (n) (%) | |||||
| Yes | 69 (29,6) | 19 (34,5) | 45 (26,8) | 68 (31,2) | 1 (6,7) |
| No | 143 (61,4) | 31 (56,4) | 108 (64,3) | 131 (60,1) | 12 (80) |
| Unknown | 21 (9) | 5 (9,1) | 15 (8,9) | 19 (8,7) | 2 (13,3) |
| Cardiovascular comorbidities (n) (%) | |||||
| Yes | 42 (18) | 5 (9,1) | 34 (20,2) | 39 (17,9) | 3 (20) |
| No | 191 (82) | 50 (90,9) | 134 (79,8) | 179 (82,1) | 12 (80) |
| Pulmonary comorbidities (n) (%) | |||||
| Yes | 29 (12,4) | 4 (7,3) | 23 (13,7) | 25 (11,5) | 4 (26,6) |
| No | 199 (85,4) | 48 (87,2) | 143 (85,1) | 189 (86,7) | 10 (66,7) |
| Unknown | 5 (2,2) | 3 (5,5) | 2 (0,2) | 4 (1,8) | 1 (6,7) |
Amount (percentage); median (IQR). Abbreviations: DD, diastolic dysfunction; BMI, body mass index; HBV, hepatitis B; HCV, hepatitis C; MASLD, metabolic dysfunction-associated steatotic liver disease.
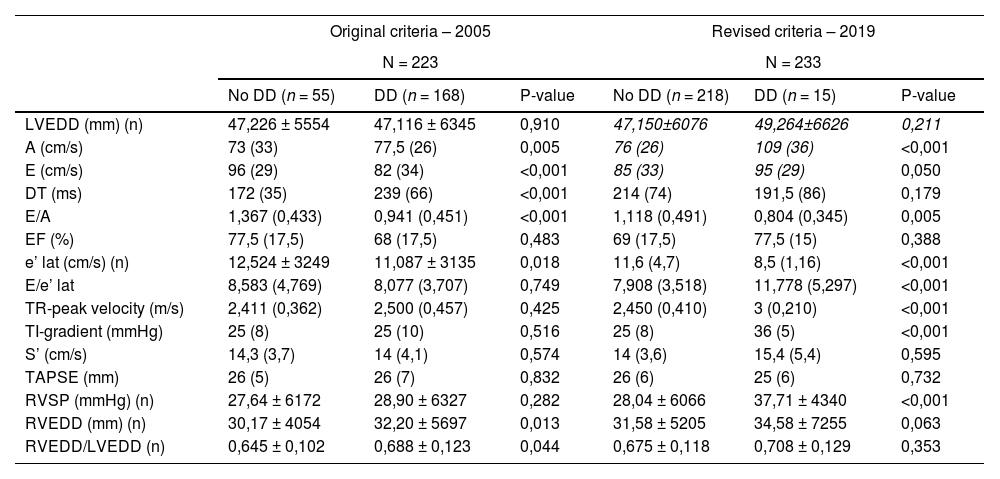
168/233 (72,1 %) patients met the original criteria for DD, while 15/233 (6,4 %) met the revised criteria for DD. Echocardiographic data are shown in Table 2. Patients with DD had increased E and A velocity, lower E/A, decreased lateral e' velocity, higher E/e' lateral, tricuspid regurgitation peak velocity, tricuspid insufficiency gradient and right ventricular systolic pressure (Table 2). Patients with DD by the revised criteria were significantly older (median 67 vs 61 years; P = 0,005). The etiology and severity of the underlying liver disease were comparable between patients with and without DD. The occurrence of refractory ascites, spontaneous bacterial peritonitis and hepatorenal syndrome was comparable in patients with and without DD (Table 1) (20 % vs 21,6 %; 13,3 % vs 12,8 %; 20 % vs 11 %, respectively), according to the revised criteria).
Echocardiographic data in patients with or without DD by the original and revised criteria of 2005 and 2019.
| Original criteria – 2005 | Revised criteria – 2019 | |||||
|---|---|---|---|---|---|---|
| N = 223 | N = 233 | |||||
| No DD (n = 55) | DD (n = 168) | P-value | No DD (n = 218) | DD (n = 15) | P-value | |
| LVEDD (mm) (n) | 47,226 ± 5554 | 47,116 ± 6345 | 0,910 | 47,150±6076 | 49,264±6626 | 0,211 |
| A (cm/s) | 73 (33) | 77,5 (26) | 0,005 | 76 (26) | 109 (36) | <0,001 |
| E (cm/s) | 96 (29) | 82 (34) | <0,001 | 85 (33) | 95 (29) | 0,050 |
| DT (ms) | 172 (35) | 239 (66) | <0,001 | 214 (74) | 191,5 (86) | 0,179 |
| E/A | 1,367 (0,433) | 0,941 (0,451) | <0,001 | 1,118 (0,491) | 0,804 (0,345) | 0,005 |
| EF (%) | 77,5 (17,5) | 68 (17,5) | 0,483 | 69 (17,5) | 77,5 (15) | 0,388 |
| e’ lat (cm/s) (n) | 12,524 ± 3249 | 11,087 ± 3135 | 0,018 | 11,6 (4,7) | 8,5 (1,16) | <0,001 |
| E/e’ lat | 8,583 (4,769) | 8,077 (3,707) | 0,749 | 7,908 (3,518) | 11,778 (5,297) | <0,001 |
| TR-peak velocity (m/s) | 2,411 (0,362) | 2,500 (0,457) | 0,425 | 2,450 (0,410) | 3 (0,210) | <0,001 |
| TI-gradient (mmHg) | 25 (8) | 25 (10) | 0,516 | 25 (8) | 36 (5) | <0,001 |
| S’ (cm/s) | 14,3 (3,7) | 14 (4,1) | 0,574 | 14 (3,6) | 15,4 (5,4) | 0,595 |
| TAPSE (mm) | 26 (5) | 26 (7) | 0,832 | 26 (6) | 25 (6) | 0,732 |
| RVSP (mmHg) (n) | 27,64 ± 6172 | 28,90 ± 6327 | 0,282 | 28,04 ± 6066 | 37,71 ± 4340 | <0,001 |
| RVEDD (mm) (n) | 30,17 ± 4054 | 32,20 ± 5697 | 0,013 | 31,58 ± 5205 | 34,58 ± 7255 | 0,063 |
| RVEDD/LVEDD (n) | 0,645 ± 0,102 | 0,688 ± 0,123 | 0,044 | 0,675 ± 0,118 | 0,708 ± 0,129 | 0,353 |
Mean ± standard deviation; median (IQR). Abbreviations: DD, diastolic dysfunction; LVEDD, left ventricular end-diastolic diameter; A, late diastolic filling; E, early diastolic filling; DT, deceleration time; EF, ejection fraction; e’ lat, lateral mitral annular early diastolic velocity; E/e’, early mitral inflow velocity to mitral annular early diastolic velocity ratio; TR, tricuspid regurgitation; TI, tricuspid insufficiency; S’, lateral tricuspid annulus peak systolic velocity; TAPSE, tricuspid annular plane systolic excursion; RVSP, right ventricular systolic pressure; RVEDD, right ventricular end-diastolic diameter.
194/218 (89 %) patients without DD and 12/15 (80 %) patients with DD according to the 2019 criteria were transplanted (Table 3). The median time from listing to LT was not different between groups (142 vs 114 days, P = 0,382). 13,3 % of patients with DD died on the waiting list compared to 9,2 % of patients without (NS, Graphical abstract figure, Table 3). The most common cause of pre-LT death was multiple organ failure.
Outcome on the waiting list of patients with or without DD by the revised criteria of 2019.
| Revised criteria – 2019 | ||
|---|---|---|
| N = 233 | ||
| No DD (n = 218) | DD (n = 15) | |
| Waiting time (days) | 142 (283) | 114 (192) |
| Outcome on waiting list (n) (%) Transplanted Died before transplantation Too good or recovered Too sick | 194 (89)20 (9,2)1 (0,5)3 (1,4) | 12 (80)2 (13,3)1 (6,7)0 (0) |
Median (IQR).
Post-LT survival was similar in patients who were transplanted with and without DD according to the revised criteria (91,7 % vs 85,6 %, P = 0,391, Graphical abstract figure, Table 4). 8,3 % of patients with DD and 14,4 % without DD had died after LT (NS, Table 4). The causes of death did not differ between groups. 1 of the 2 transplanted patients with DD who died post-LT died because of a cardiovascular complication.
Outcome of transplanted patients with and without DD by the revised criteria of 2019.
| Revised criteria – 2019 | ||
|---|---|---|
| N = 206 | ||
| No DD (n = 194) | DD (n = 12) | |
| Outcome at data collection (n) (%) Alive Deceased | 166 (85,6)28 (14,4) | 11 (91,7)1 (8,3) |
| Cause of death (n) (%) Multiple organ failure Cardiovascular Non-cardiovascular Unknown | 9 (32,1)4 (14,3)12 (42,9)3 (10,7) | 0 (0)1 (100)0 (0)0 (0) |
| Follow-up time until data collection or death (days) | 990 (1178) | 1395 (1791) |
| MACE (n) (%) Yes No | 27 (13,9)167 (86,1) | 2 (16,7)10 (83,3) |
Median (IQR).
Of the 206 patients who underwent transplantation, 177 (85,9 %) had survived at data collection. Median follow-up post-LT was 1012 days. In the group of patients who died post-LT, 1 patient (3,4 %) had DD pre-LT according to the revised 2019 criteria. The presence of DD pre-LT was not associated with death post-LT. Patients who died had significantly lower e’ lateral (mean 9,849 vs 11,796 cm/s, P = 0,050).
Major adverse cardiovascular events (MACE, including ischemic heart disease, heart failure, cardiac death) occurred in 2/12 (16,7 %) patients with pre-LT DD in the post-transplantation phase, and in 27/194 (13,9 %) patients without pre-LT DD (NS). No association could be found between the presence or absence of DD and the occurrence of MACE by regression analysis.
3.4Evolution of DD post-transplant12 patients with DD according to the revised definition underwent LT. In 9/12 transplanted patients with DD pre-LT, we had a follow-up with sufficient data to re-evaluate the presence of DD (at median follow-up of 1395 days post-transplant). Only 1 patient of them fulfilled the criteria for DD post-LT.
4DiscussionUsing the revised criteria, the prevalence of DD in this study was 6,4 %, indicating a significant shift in the reported prevalence of DD compared to earlier studies using the original criteria (up to 65 %) [1,4].
Conflicting data exist regarding the clinical impact of DD in LT candidates. Mittal et al. reported an association between DD pre-LT and mortality post-LT [5]. However, other studies could not establish a clear association between the presence of DD and mortality in cirrhotic patients, regardless of using the original or revised criteria [6–11]. Our study also could not demonstrate an association between pre-transplant DD and overall post-transplant mortality. Recent reports have suggested that DD-CCM, defined by the new criteria, increases the risk of MACE including cardiovascular death after LT [6,9]. This could not be confirmed in our cohort.
Age-related changes in cardiac structure and function contribute to DD development. Our study revealed that patients with DD were older. As the age limit for LT extends, we believe that particularly the older population should receive special attention in future studies analyzing the impact of DD.
We observed that patients who had died after LT had significantly lower e' lateral. We found a notable association between lower e' lateral before LT and post-LT mortality. These findings are consistent with those of Spann et al., who demonstrated associations between septal e', cardiovascular complications and all-cause mortality [6]. These associations further support the importance of proper echocardiographic assessment in cirrhotic patients undergoing LT.
The current understanding regarding whether cardiac function improves after LT in patients with DD remains unclear. Some studies have reported on the potential for improvement in DD post-LT. Izzy et al. found that DD normalized in 6/22 affected patients at 1 year post-LT [9]. Ali et al. reported resolution of CCM in 13/23 patients post-LT [12]. We retrieved a follow-up echocardiogram after LT in 9/12 patients with DD pre-LT. Only 1 patient fulfilled the criteria post-LT. Although this reports on a very small number of patients, it suggests that DD could potentially improve post-LT. However, due to the limited number of patients in our study, further research with a larger sample size is necessary to investigate this hypothesis more thoroughly.
Our study adds to the discussion on the clinical impact of DD in LT. While we found no direct link between pre-LT DD and post-LT mortality, an association was observed between lower e’ lateral values before LT and increased post-LT mortality. This observation might suggest the need for closer cardiac evaluation during the pre-transplant phase for these patients, potentially informing follow-up care and medical management.
We recognize that a single-center, retrospective design is a limitation. Indeed, to date, investigations assessing the impact of DD on LT outcomes have been limited to single-center studies. However, the field of DD remains understudied, and any additional data further improve our understanding of the clinical impact of these liver-induced cardiac alterations. Strengths of this study include the long follow-up of a large cohort of well-characterized cirrhotic patients. Future multicenter initiatives are essential to generating more robust data, enhancing our understanding of the disease, and elucidating its implications on LT outcomes.
5ConclusionsIn conclusion, our study revealed that 6,4 % of LT candidates were diagnosed with DD based on the revised criteria. We found that the presence of DD did not significantly impact overall post-LT survival. However, we observed a potential association between lower e' lateral values before LT and increased post-LT mortality. Additional prospective studies will help explore whether DD has varying effects on outcomes after LT in specific subsets of patients.
FundingXV is funded by a translational clinical mandate of the Foundation Against Cancer Belgium. SL is supported by a grant from the Research Foundation – Flanders (FWO) (1227824 N). AG, HVV and SR are senior clinical investigators of the FWO (1805718 N, 1801721 N and 1802624 N).
Author contributionsFV, MK, MDP, XV, HD, SL, AG, HVV, SR: study concept and design
FV, MK, SR: acquisition of data; analysis and interpretation of data; statistical analysis
FV, MK, SR: drafting the manuscript
MDP, XV, AG, HVV: administrative, technical and material support
MDP, XV, HD, SL, AG, HVV: critical revision of the manuscript for important intellectual content
FV, MK, MDP, XV, HD, SL, AG, HVV, SR: final approval of the version to be published
The authors thank Evelyn Vander Straeten for her help in creating the database.