Radiofrequency ablation of scar related right atrial flutter is challenging. Long procedures, prolonged fluoroscopic times and high percentages of recurrences are of concern. We present a simple and progressive approach based on a single electroanatomic map of the right atrium.
MethodsTwenty-two consecutive patients with atrial flutter and history of cardiac surgery were included. An electrophysiologic study was performed to define localization (left or right) and cavo-tricuspid isthmus participation using entrainment mapping. After a critical isthmus was localized, ablation was performed with an external irrigated tip catheter with a power limit of 30W. Potential ablation sites were confirmed by entrainment.
ResultsThe predominant cardiopathy was atrial septal defect. All arrhythmias were localized in the right atrium; mean cycle length of the clinical flutter was 274±31ms. Only 40% had cavo-tricuspid isthmus participation. None of the patients with successful ablation had recurrences after 13±9.4 months of follow-up.
ConclusionsA progressive approach with only one activation/voltage CARTO® map of the atrium and ablation of all potential circuits is a highly effective method for ablating scar related macroreentrant atrial arrhythmias.
La ablación con radiofrecuencia de flutter auricular relacionado con cicatrices posquirúrgicas es compleja. Procedimientos prolongados, con tiempos de fluoroscopia altos y una tasa de recurrencia elevada son problemas habituales. Mostramos un abordaje simple y progresivo basado en un solo mapa de cartografía electroanatómica de la aurícula derecha.
MétodosSe incluyeron 22 pacientes consecutivos con flutter auricular e historia de cirugía cardiaca. Se realizó estudio electrofisiológico para definir la localización del circuito de flutter (derecho o izquierdo) y la participación o no del istmo cavotricuspideo mediante encarrilamiento. Una vez localizado la zona de conducción lenta o critica del circuito, se realizó ablación con radiofrecuencia con catéter de irrigación externa a 30W. Posteriormente se llevó a cabo ablación de todos los circuitos potenciales.
ResultadosLa cardiopatía más dominante fue la comunicación interauricular. Todas las arritmias se localizaron en la aurícula derecha. El ciclo de flutter fue de 274±31ms. En solo 40% de los casos se demostró participación del istmo cavotricuspideo. No se observaron recurrencias de la arritmia durante un seguimiento de 13±9.4 meses.
ConclusionesEste abordaje escalonado con un solo mapa CARTO® de activación/voltaje de la aurícula y la ablación de todos los circuitos potenciales es altamente efectivo para el tratamiento de arritmias por macrorreentrada relacionadas con cicatriz posquirúrgica.
Patients who have undergone cardiac surgery, especially for correcting congenital abnormalities, could have rhythm disturbances long after the procedure, with an increase in morbidity and mortality.1–4 Electrophysiological sequelae of reparative surgery for congenital heart disease include the presence of multiple lines of conduction block, related to scars and fibrosis, which favor reentrant arrhythmias.5 Radiofrequency catheter ablation (RFCA) of these arrhythmias is challenging and associated with long procedures, prolonged fluoroscopic times and high percentages of recurrences.6 Electroanatomic mapping has been used to better define areas of conduction block that delineate possible channels of conduction that could be the target for RFCA.7 Even so, the presence of multiple potential circuits, and the induction of different arrhythmias with high recurrence rate represent a challenge.8 In this paper we present our approach for the ablation of these complex arrhythmias.
MethodsTwenty-two consecutive patients with atrial flutter and previous history of cardiac surgery were submitted to an electrophysiologic (EP) study. All patients were anticoagulated with acenocumarin to maintain an International Normalized Ratio (INR) between 2 and 3 for at least 3 weeks before the procedure and received antiarrhythmic drugs to control ventricular rate. The protocol was approved by the Ethics Committee and all patients signed an informed consent. Three femoral venous punctures were done and three 7F sheets were placed in the femoral vein. A decapolar catheter (Polaris, Boston Scientifics, USA) was placed in the coronary sinus. A duodecapolar catheter (Halo, Biosense Webster Inc., Diamond Bar, CA, USA) was positioned in the right atrium, and a Navistar irrigated-tip catheter was used for mapping and ablation. A bolus of 2500U of heparin was administered intravenously. Initial assessment of the clinical arrhythmia was made determining cycle length (CL) and activation wave front (AWF). Entrainment was performed in the cavo-tricuspid isthmus (CTI), proximal coronary sinus, low lateral and high lateral walls to establish the possible site of origin and critical isthmus. An activation/voltage map was obtained. Scar was defined as the smallest local potential that could not be distinguished from noise. The circuit of the clinical arrhythmia was defined in the CARTO® map and the critical isthmus (CTI or other) targeted for ablation. Radiofrequency energy was applied with power control limited to 30–35 watts with a Stockert 70 RF generator (Biosense Webster Inc., Diamond Bar, CA, USA) until the arrhythmia stopped or until a change in CL or AWF were observed. If a second arrhythmia appeared, either spontaneously or with atrial stimulation, the voltage map was used to define potential responsible channels, and the critical isthmus was confirmed with entrainment. Voltage map settings were fixed from 1mV or higher for normal tissue. In order to “close” the targeted channel, lines of ablation were performed between a scar and an anatomical barrier or between two scars or lines of block. Same strategy was used for any other arrhythmias induced, always with the same voltage map, until no arrhythmias could be induced with fast atrial pacing up to an A1–A1 of 200ms. Finally, if we considered that there were any other potential channels in the voltage map, a line of ablation was also performed.
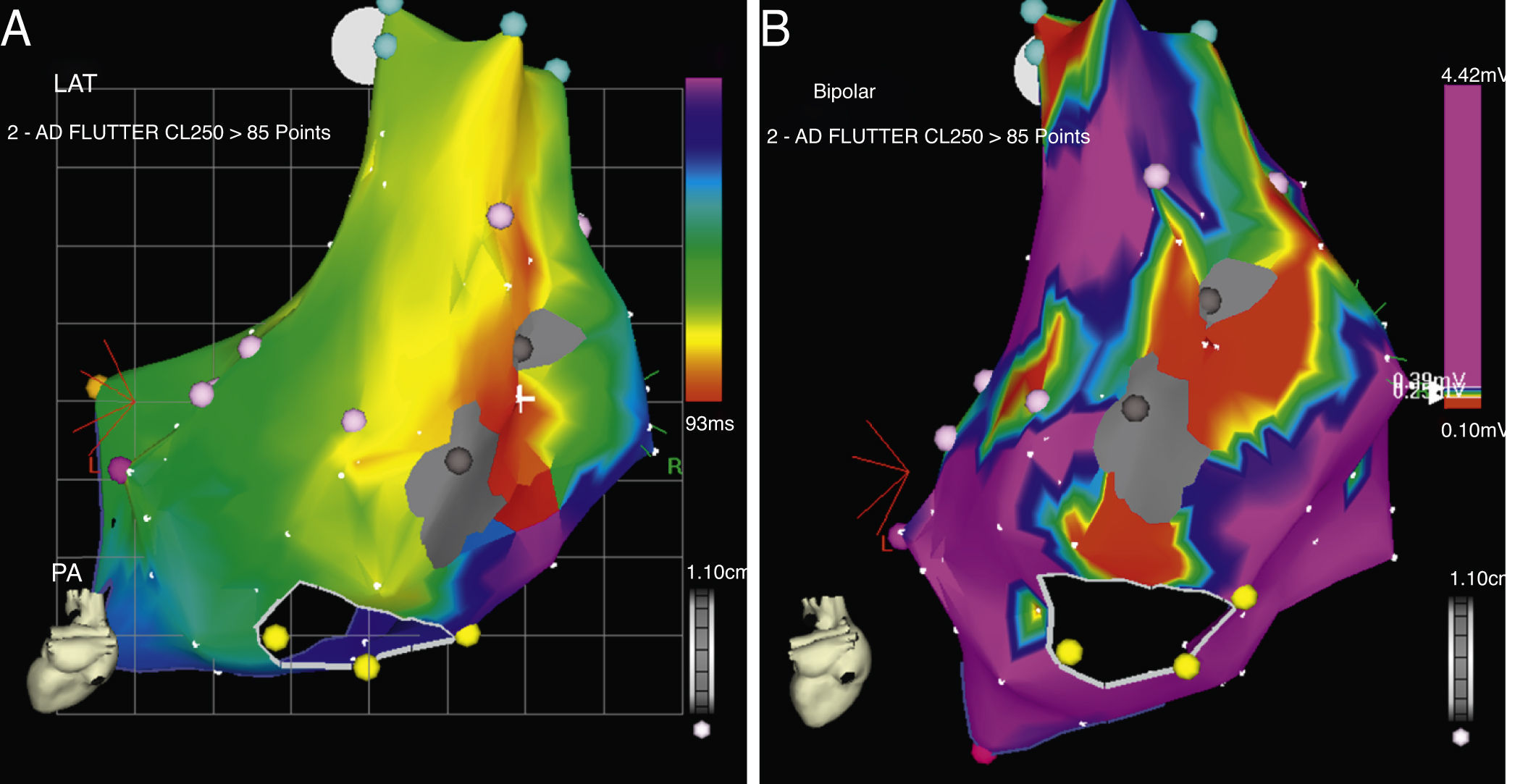
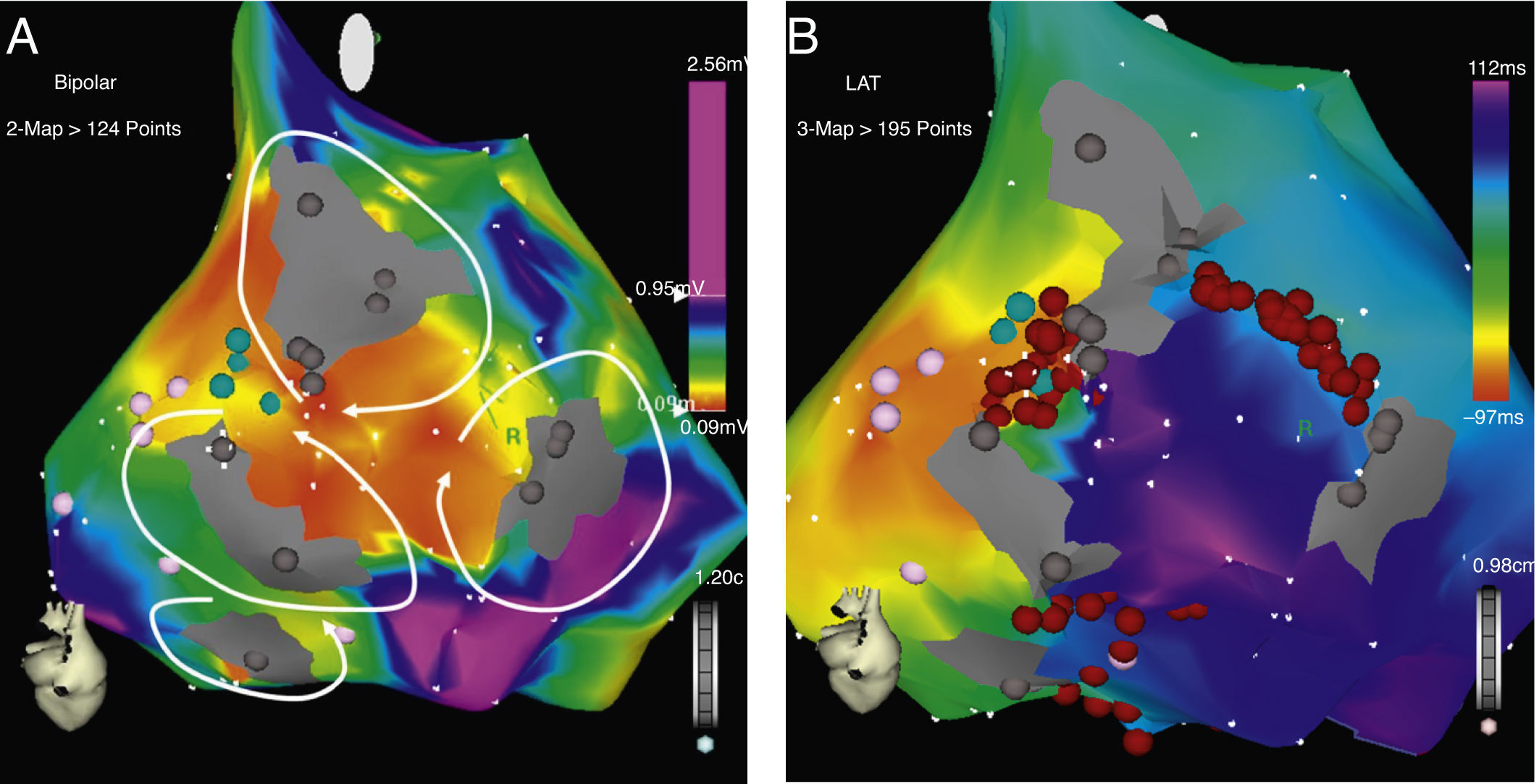
ResultsFifteen out of the 22 patients (68%) were women, mean age 43±16 years (Table 1). The predominant cardiopathy was atrial septal defect (ASD) with its different varieties. All the clinical arrhythmias were localized in the right atrium. Mean CL of the clinical flutter was 274±31ms. CTI participation was only documented in 40% of the arrhythmias. The rest were localized in the lateral or posterolateral right atrial wall, between lines of block, scars and anatomical barriers (Figs. 1 and 2). AWF during the clinical arrhythmia was counterclockwise in 23%, clockwise in 27% and other in 50% of the patients. CARTO® maps had an average of 119±32 points and 2±1.3 potential circuits different from the clinical arrhythmia were found. All potential circuits were targeted for ablation. In 82% of patients the procedure was considered successful, with no arrhythmias induced after the procedure. Mean procedure duration was 180min with a fluoroscopic time of 38±21min. None of the patients with successful ablation have had recurrences after 13±9.4 months of follow-up. Two patients (9%) required a pacemaker after the procedure because of severe and non-reversible sinus node dysfunction.
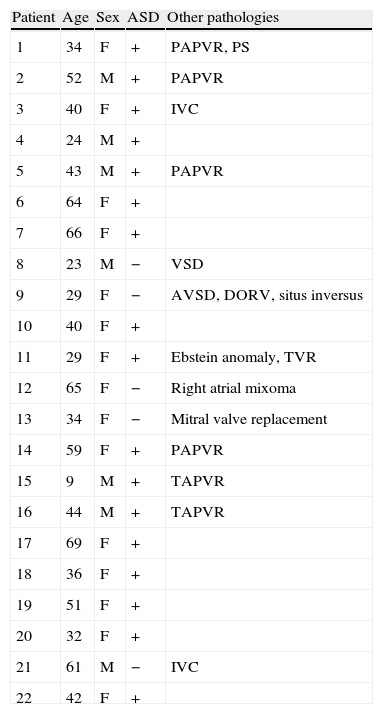
Clinical characteristics of the study group.
| Patient | Age | Sex | ASD | Other pathologies |
| 1 | 34 | F | + | PAPVR, PS |
| 2 | 52 | M | + | PAPVR |
| 3 | 40 | F | + | IVC |
| 4 | 24 | M | + | |
| 5 | 43 | M | + | PAPVR |
| 6 | 64 | F | + | |
| 7 | 66 | F | + | |
| 8 | 23 | M | − | VSD |
| 9 | 29 | F | − | AVSD, DORV, situs inversus |
| 10 | 40 | F | + | |
| 11 | 29 | F | + | Ebstein anomaly, TVR |
| 12 | 65 | F | − | Right atrial mixoma |
| 13 | 34 | F | − | Mitral valve replacement |
| 14 | 59 | F | + | PAPVR |
| 15 | 9 | M | + | TAPVR |
| 16 | 44 | M | + | TAPVR |
| 17 | 69 | F | + | |
| 18 | 36 | F | + | |
| 19 | 51 | F | + | |
| 20 | 32 | F | + | |
| 21 | 61 | M | − | IVC |
| 22 | 42 | F | + |
ASD, atrial septal defect; VSD, ventricular septal defect; PAPVR, partial anomalous pulmonary venous return; TAPVR, total anomalous pulmonary venous return; PS, pulmonary stenosis; AVSD, atrio-ventricular septal defect; TVR, tricuspid valve replacement; DORV, double outlet right ventricle.
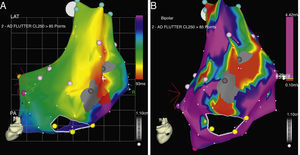
CARTO® map reconstruction of the right atrium in a patient with surgical correction of an ASD: (A) activation map showing a macro-reentrant circuit involving two dense scars in the posterolateral wall. (B) Voltage map showing multiple channels of low voltage arrowed the scars and anatomical barriers.
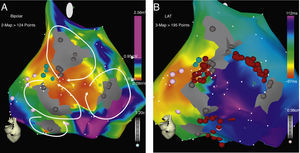
Atrial macroreentrant arrhythmias are frequently observed in patients after cardiac surgery, especially in those with congenital heart disease. Patients with surgical correction of ASD have a high incidence of these arrhythmias years after the procedure. Gatzoulis et al. reported a series of 218 adults with ASD correction with an incidence of atrial fibrillation or flutter of 19% previous to surgery, of which 60% persisted with the arrhythmia after the procedure and 2.8% developed the arrhythmia during a 4-year follow-up.9 Medeiros et al. reported an incidence of rhythm disturbances of 6.23% previous to surgery, being atrial flutter the most frequent (34.5%).1 After surgery, 14% developed atrial arrhythmias, and again atrial flutter was the most frequent (44.6%). Age, pulmonary hypertension and arrhythmias before the procedure are the variables associated with postoperative atrial flutter.10 RFCA is highly effective for curing cavo-tricuspid dependent flutter.11 In patients with surgical correction of congenital heart disease, participation of the CTI could be as high as 70%, although if ablation only targets the CTI, recurrences could be as high as 40%.12,13 This could be explained due to the presence of wide areas of scar tissue, slow conduction and block that act as true channels of preferential conduction that favor new arrhythmia circuits. In our series, the CTI participated in the circuit of the clinical arrhythmia in only 40% of patients. This could be due to a selection bias. Different investigators have proposed the ablation of all the circuits to improve the success of the ablation.8,13,14 Different techniques have been proposed for this approach; the use of electroanatomical tools for 3D reconstruction of the atria, using either multiple activation maps for each arrhythmia induced or high point density (>200 points) voltage maps to locate channels have been described with good results. These techniques, although effective, are associated with long procedural times, and are very demanding for the EP team, and not free of recurrences. Our technique is based on the initial assessment of the clinical arrhythmia with traditional entrainment techniques, and the elaboration of only one activation/voltage map of intermediate point density (<200pts). Ablation is oriented to the critical isthmus of the arrhythmia (CTI or other) in the activation map and all other arrhythmias induced are approached using the voltage map as a guide to localize the potential new circuit with its participation confirmed by entrainment. Ablation lines are made “sealing” all responsible channels until no arrhythmias could be induced. If any other potential channels are localized in the voltage map, an empirical line of RF is done to seal the channel (Figs. 1–3). With this method, we identified that in 60% of our patients the clinical arrhythmia was not localized in the CTI, and that there were at least an average of 2 potential circuits per patient that participated in other than the clinical arrhythmia. Delacretaz et al.8 reported 2.4 morphologies per patient and Verma et al.13 report that up to 27% of patients with only one demonstrable circuit at the time of the ablation, had recurrence of another type of flutter during follow-up and a high incidence of recurrence, up to 83%, in patients in which only the clinical circuit was treated, suggesting an empiric approach of ablating all potential flutter circuits, regardless of the presenting atrial flutter. Thus, a progressive ablation of all circuits guided by the voltage map is very attractive and in our series, resulted in the non-inducibility of arrhythmias in 82% of the patients with no recurrence of flutter in all these patients at almost 1.5 years of follow up and comparable to others with similar patients.8,13,14 The elaboration of only one map of intermediate density helps for guidance to the potential circuits and keeps the procedure short and with acceptable fluoroscopic times comparable to others.8
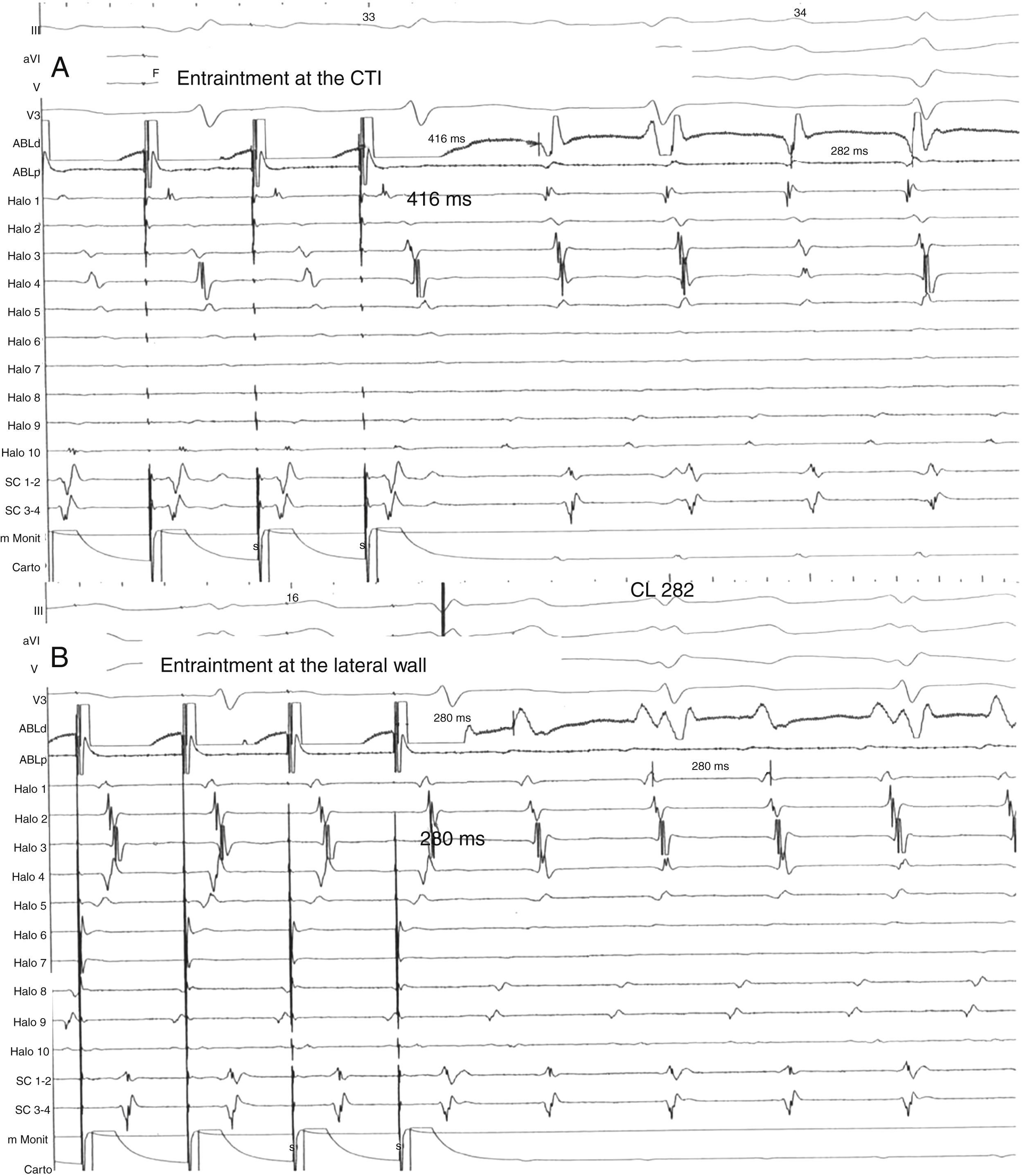
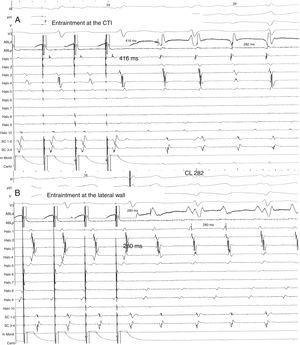
Same patient as Fig. 1. (A) Entrainment mapping is performed first at the cavo-tricuspid isthmus (CTI). Note manifest fusion and a long return cycle (416) in comparison with tachycardia cycle (282). (B) Entrainment mapping at the right lateral wall, between the scars. Observe concealed fusion and a return cycle equal to tachycardia cycle length.
Limitations: These results cannot be extrapolated to all kinds of surgical corrections like Mustard or Senning procedures, where the damage to the atria is much more extensive, because our series is practically composed of ASD patients.
ConclusionA progressive approach based on only one activation/voltage CARTO® map of the right atrium, with ablation of all potential circuits, is a highly effective method to eliminate scar-related macroreentrant atrial arrhythmias associated with congenital heart disease.
FundingNo endorsement of any kind received to conduct this study/article.
Conflict of interestThe authors declare no conflict of interest.