Diabetes mellitus is an independent risk factor for cardiovascular disease.
ObjectiveTo compare the efficacy of devices for continuous glucose monitoring and capillary glucose monitoring in hospitalized patients with acute coronary syndrome using the following parameters: time to achieve normoglycemia, period of time in normoglycemia, and episodes of hypoglycemia.
MethodsWe performed a pilot, non-randomized, unblinded clinical trial that included 16 patients with acute coronary artery syndrome, a capillary or venous blood glucose ≥140mg/dl, and treatment with a continuous infusion of fast acting human insulin. These patients were randomized into 2 groups: a conventional group, in which capillary measurement and recording as well as insulin adjustment were made every 4h, and an intervention group, in which measurement and recording as well as insulin adjustment were made every hour with a subcutaneous continuous monitoring system. Student's t-test was applied for mean differences and the X2 test for qualitative variables.
ResultsWe observed a statistically significant difference in the mean time for achieving normoglycemia, favoring the conventional group with a P=0.02.
ConclusionContinuous monitoring systems are as useful as capillary monitoring for achieving normoglycemia.
La diabetes mellitus es un factor de riesgo independiente de enfermedad cardiovascular.
ObjetivoComparar la eficacia de los dispositivos de monitorización continua de glucosa y monitorización de glucosa capilar en pacientes hospitalizados con síndrome coronario agudo, mediante los siguientes parámetros: tiempo en lograr normoglucemia, periodo en normoglucemia y número de hipoglucemias.
MétodosEnsayo clínico no aleatorizado, no ciego, que incluyó a 16 pacientes con síndrome coronario agudo, glucosa capilar o venosa ≥140mg/dl, en tratamiento con infusión de insulina humana de acción rápida durante 48h. Se distribuyeron en 2 grupos: convencional, con medición y registro de glucosa capilar, y ajuste de insulina cada 4h, y de intervención, con medición y registro de glucosa intersticial y ajuste de insulina cada hora a través de un dispositivo de monitorización continua colocado vía subcutánea. Se aplicaron pruebas t para diferencia de medias y prueba de X2 para las variables cualitativas.
ResultadosSe observó diferencia significativa en la media del tiempo para lograr normoglucemia a favor del grupo convencional, con un valor de p=0.02.
ConclusiónLos dispositivos de monitorización continua de glucosa son tan útiles como la monitorización de glucosa capilar para lograr normoglucemia.
The worldwide prevalence of type 2 diabetes mellitus is 6% (246 million individuals).1 According to the National Health Survey (ENSANUT 2006), the prevalence in Mexico is 14.4%, with this disease being the leading cause of death (11.2%).2 Diabetes mellitus (DM) is an independent risk factor for cardiovascular disease, due to the chronic inflammatory state induced by high glucose levels and their variability. The presence of DM or hyperglycemia in patients with ischemic heart disease increases mortality by 50% per year, and causes a higher rate of re-infarction and poor response to therapeutic measures.3 More than 50% of patients with their first acute coronary syndrome have glucose intolerance or undiagnosed DM, which are factors of poor outcome in the short and long term.4
The standardized methods of glucose control that are used are capillary glucose monitoring and venous glucose monitoring5; which provide, respectively, an accurate information to be obtained directly from the sample capillary and venous blood, also, frequent self-monitoring blood glucose (SMBG) has been correlated with an improvement of metabolic control, but the optimal number of measurements of SMBG is not defined6; by the above, a real-time method that permits assessment of glucose variability in the acute coronary setting is needed to achieve proper glucose control.
Continuous glucose monitoring provides information about direction, duration, frequency and causes of variability glucose changes; in contrast to blood glucose self-monitoring that corresponds to three measurements of capillary glucose each day, continuous glucose monitoring gives greater knowledge about “all day” glucose levels. Continuous readings provide information about glucose tendency and could help to identify and prevent hypoglycemic events.7,8 One of the advantages of continuous glucose monitoring is its ability to predict future glucose levels, this cannot be done with blood glucose self-monitoring9; however, there are several difficulties with continuous glucose monitoring, as an increase in the number of insulin adjustments per day, requirement of a trained operator and calibration every 12h, which could make it less practical for in-hospital use. Several continuous glucose monitoring devices have been approved by FDA (Food and Drug Administration) for use in the United States and Europe, minimally invasive through continuous interstitial measurement involving cutaneous barrier and without puncturing a blood vessel, or with a non-invasive method through electromagnetic radiation from the skin to blood vessels. After 2h of time synchronization and specific calibration process, each sensor can provide a glucose reading every 1–10min for a 72h period. Some models have an alarm that activates if glucose levels get out of normal levels.10–13 Considering the existence of different methods of glucose monitoring and the lack of consensus about their usefulness in glycemic control of inpatients, our goal is to determine if continuous glucose monitoring devices are more effective than capillary glucose monitoring for achieving normoglycemia in patients with acute coronary syndrome in addition to preventing episodes of hypoglycemia due to the default alarms of hyper and hypoglycemia.
MethodsThis is a pilot, non-randomized, unblinded clinical trial evaluating if continuous glucose monitoring devices are more effective than capillary glucose monitoring for achieving normoglycemia in patients with acute coronary syndrome. Patients’ 20–70 years of age were enrolled if they were admitted to the emergency department of the Dr. José Eleuterio González University Hospital from January 1, 2010 to July 31, 2011 with a diagnosis of acute coronary syndrome with or without ST elevation, a blood or capillary glucose level greater than 140mg/dl, and with or without a prior history of acute coronary syndrome. Exclusion criteria included patients in cardiogenic shock with or without the use of vasopressors, acute renal failure and/or a calculated creatinine clearance less than 60ml/min according to the Crockoft–Gault formula,14 an admission diagnosis of diabetic ketoacidosis or a hyperglycemic hyperosmolar state, and patients with alcohol withdrawal syndrome, chronic liver disease, cellulitis and/or herpes at the device site placement or those unable to provide informed consent. Acute coronary syndrome,15 hypoglycemia, and diabetes mellitus were defined according to current clinical guidelines.16
The study complies with the Declaration of Helsinki and was approved by the Ethics Committee of the Universidad Autónoma de Nuevo León Medical School and Dr. José Eleuterio González University Hospital with registration number 09-025. All patients provided written informed consent.
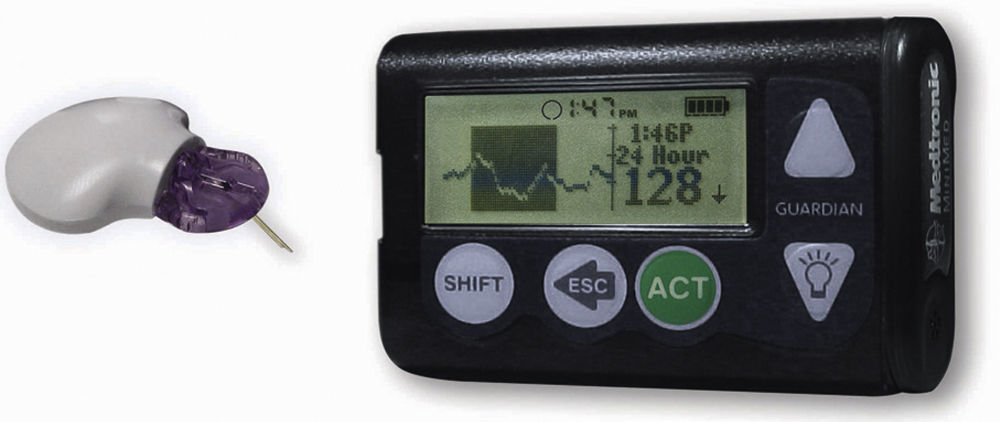
Patients were started on a continuous intravenous infusion of fast acting insulin titrated according to the Yale protocol.17 On admission patients were randomized into two groups, the conventional group, in whom measurement and recording of capillary glucose was performed every 4h, and the intervention group, in whom a Medtronic Guardian® RT device was inserted subcutaneously in the lower abdomen 2 inches from the umbilicus.18 Five minutes after electrode (sensor) insertion, the MiniLink REAL-time transmitter was connected to the sensor, allowing the electrode to have contact with interstitial fluid. The monitor was placed at the bedside of the patient and data were received every 5min through the transmitter. Two hours after the initialization time, a nurse was instructed to perform capillary glucose and insert the value into the continuous monitoring Medtronic Guardian® RT (Fig. 1) for the first calibration.19
All patients were monitored for 48h. Decisions on dose adjustment of the insulin infusion were made based on the Yale protocol; every 4h for the conventional group, and every hour for the intervention group with a glucose goal equal or less than 140mg/dl. If a hypoglycemia or hyperglycemia alarm occurred in the intervention group, the glucose value was confirmed with a capillary glucose reading and action was taken as suggested by the Yale insulin infusion protocol. Each glucose value and insulin dose was recorded on control sheets. Capillary glucose measurement was made with an Accu-Check Inform® Roche glucometer. Venous glucose readings and measurements were made every 8h for both groups and capillary glucose measurement every 12h in the intervention group for Guardian RealTime® device calibration. During monitoring, we conducted a lifestyle and prior history survey and all patients received a 25kcal/kg/day diet. A lipid profile, glycosylated hemoglobin, anthropometry, blood pressure measurements, creatinine level, and glomerular filtration rate to estimate kidney function, were performed. After discharge, each patient was followed by telephone after 30 days to assess the occurrence of complications, re-admission for acute coronary syndrome, cerebral vascular disease, arrhythmias and/or death.
The primary endpoints of the study were the time (hours) for achieving normoglycemia by group, the period of time that they remained in normoglycemia, and the presence of episodes of hypoglycemia. For study purposes normoglycemia was defined as a value less than 140mg/dl; hypoglycemia was defined by the presence of symptoms associated with hipoglycemia and capillary glucose value equal to or less than 70mg/dl according to current clinical guidelines.16
Secondary endpoints included were the day 1 to day 2 glucose difference, and the number of insulin dose adjustments per day between both groups and their difference.
Statistical analysis was performed using SPSS version 18.0. We compared the value distribution in both groups using the Kolmogorov test. Mean differences were determined in both groups with Student's t-test for independent samples; for qualitative variables, contingency tables were applied to X2 test. For analysis of variables within the same group an analysis of variance was used. All probability values reported are two-sided, and a value of P<0.05 was considered significant.
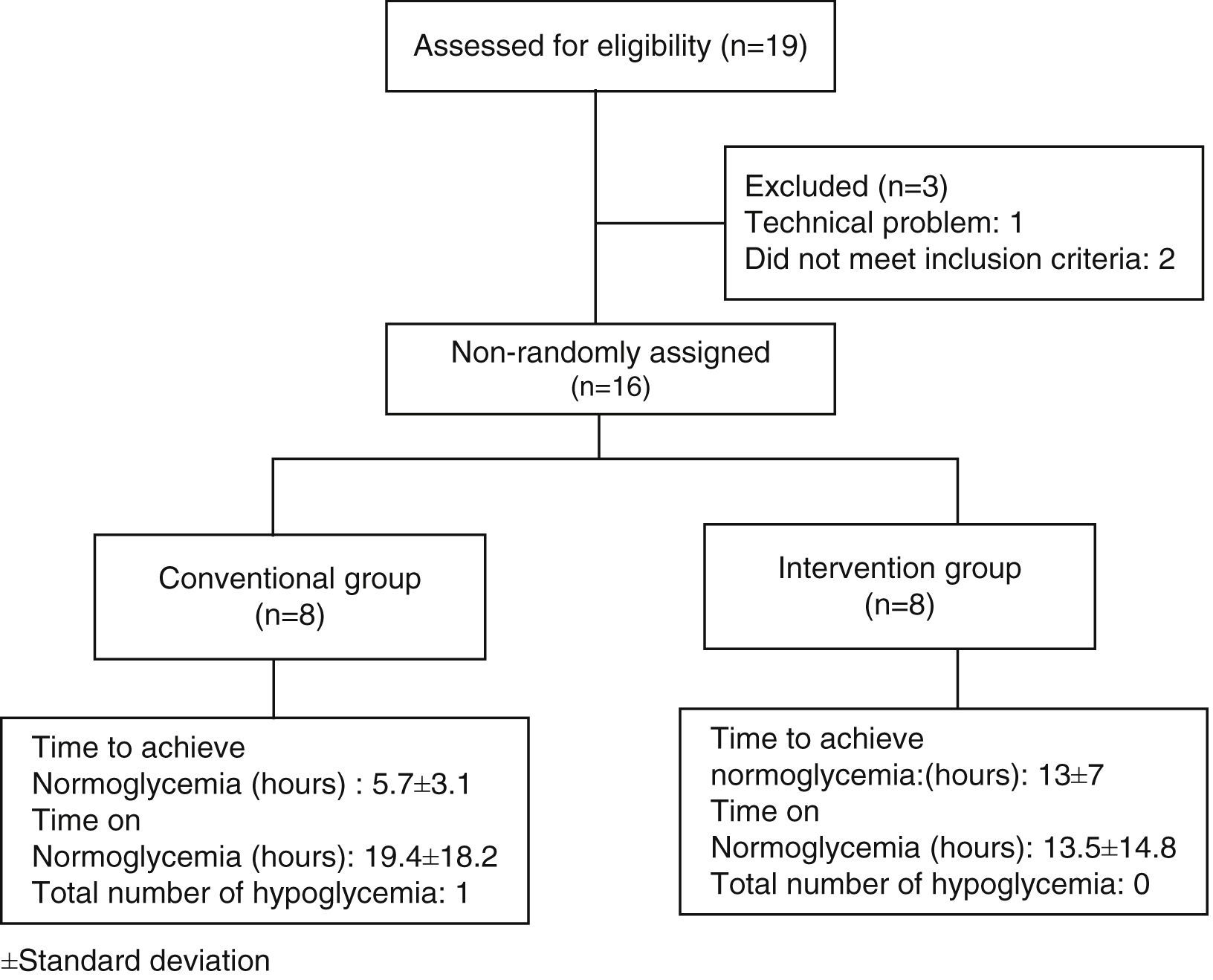
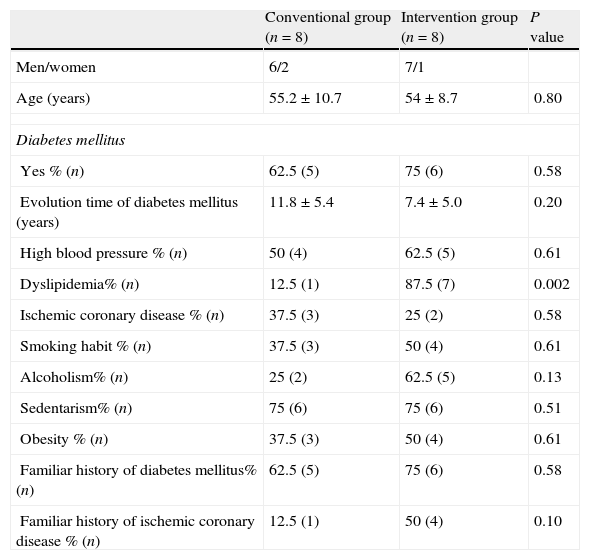
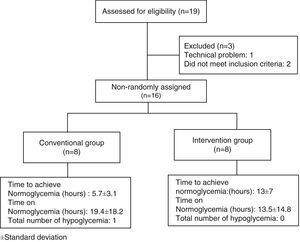
ResultsNineteen patients participated in the study, three were eliminated, one due to electrode failure, and two more for not completing the requirements. The study algorithm is shown in Fig. 2. A population of 16 patients was randomized into two groups of eight patients each. The data distribution was normal according with Kolmogorov test. The demographic characteristics of the population, with means and standard deviations are shown in Table 1. In the control group, there were six men (75%) and two women (25%), whereas in the intervention group there were seven men (87.5%) and one woman (22.5%). Mean age was 55.2 years for the conventional group, and 54 years for the intervention group. We observed a statistically significant difference for dyslipidemia in both groups, with a P value of 0.002
Demographic characteristics of the population.
| Conventional group (n=8) | Intervention group (n=8) | P value | |
| Men/women | 6/2 | 7/1 | |
| Age (years) | 55.2±10.7 | 54±8.7 | 0.80 |
| Diabetes mellitus | |||
| Yes % (n) | 62.5 (5) | 75 (6) | 0.58 |
| Evolution time of diabetes mellitus (years) | 11.8±5.4 | 7.4±5.0 | 0.20 |
| High blood pressure % (n) | 50 (4) | 62.5 (5) | 0.61 |
| Dyslipidemia% (n) | 12.5 (1) | 87.5 (7) | 0.002 |
| Ischemic coronary disease % (n) | 37.5 (3) | 25 (2) | 0.58 |
| Smoking habit % (n) | 37.5 (3) | 50 (4) | 0.61 |
| Alcoholism% (n) | 25 (2) | 62.5 (5) | 0.13 |
| Sedentarism% (n) | 75 (6) | 75 (6) | 0.51 |
| Obesity % (n) | 37.5 (3) | 50 (4) | 0.61 |
| Familiar history of diabetes mellitus% (n) | 62.5 (5) | 75 (6) | 0.58 |
| Familiar history of ischemic coronary disease % (n) | 12.5 (1) | 50 (4) | 0.10 |
±Standard deviation.
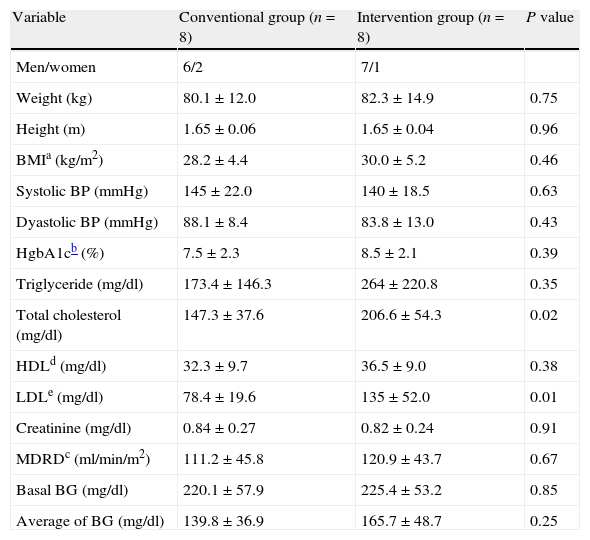
The anthropometric and clinical variables are shown in Table 2. We observed a statistically significant difference between groups in total cholesterol values, with a P value of 0.02, with a similar behavior for low-density cholesterol values, with a P=0.01.
Anthropometric and clinical characteristics of population.
| Variable | Conventional group (n=8) | Intervention group (n=8) | P value |
| Men/women | 6/2 | 7/1 | |
| Weight (kg) | 80.1±12.0 | 82.3±14.9 | 0.75 |
| Height (m) | 1.65±0.06 | 1.65±0.04 | 0.96 |
| BMIa (kg/m2) | 28.2±4.4 | 30.0±5.2 | 0.46 |
| Systolic BP (mmHg) | 145±22.0 | 140±18.5 | 0.63 |
| Dyastolic BP (mmHg) | 88.1±8.4 | 83.8±13.0 | 0.43 |
| HgbA1cb (%) | 7.5±2.3 | 8.5±2.1 | 0.39 |
| Triglyceride (mg/dl) | 173.4±146.3 | 264±220.8 | 0.35 |
| Total cholesterol (mg/dl) | 147.3±37.6 | 206.6±54.3 | 0.02 |
| HDLd (mg/dl) | 32.3±9.7 | 36.5±9.0 | 0.38 |
| LDLe (mg/dl) | 78.4±19.6 | 135±52.0 | 0.01 |
| Creatinine (mg/dl) | 0.84±0.27 | 0.82±0.24 | 0.91 |
| MDRDc (ml/min/m2) | 111.2±45.8 | 120.9±43.7 | 0.67 |
| Basal BG (mg/dl) | 220.1±57.9 | 225.4±53.2 | 0.85 |
| Average of BG (mg/dl) | 139.8±36.9 | 165.7±48.7 | 0.25 |
BG, blood glucose; BMI, body mass index; BP, blood pressure; HDL, high density cholesterol; HgbA1c, glycosylated hemoglobin; LDL, low density cholesterol; MDRD, modification of diet on renal disease.
±Standard deviation.
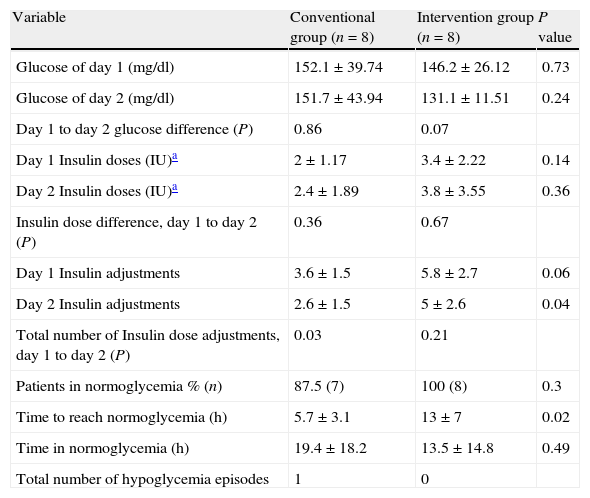
The values of glucose monitoring after performing data analysis are shown in Table 3, results were statistically significant in the mean time for achieving normoglycemia between groups, with a P value of 0.02. For variables that were analyzed in the same group (day 1 to day 2 glucose difference, insulin dose difference from day 1 to day 2, and total number of insulin doses adjustments from day 1 to day 2), we obtained significant differences in relation to day 1 to day 2 glucose in the intervention group with a P value of 0.07. With regard to the total number of insulin dose adjustments from day 1 to day 2, we observed a significant difference in the conventional group with a P value of 0.03. Only one event of hypoglycemia occurred in the conventional group.
Results of self-blood glucose monitoring vs. continuous glucose monitoring.
| Variable | Conventional group (n=8) | Intervention group (n=8) | P value |
| Glucose of day 1 (mg/dl) | 152.1±39.74 | 146.2±26.12 | 0.73 |
| Glucose of day 2 (mg/dl) | 151.7±43.94 | 131.1±11.51 | 0.24 |
| Day 1 to day 2 glucose difference (P) | 0.86 | 0.07 | |
| Day 1 Insulin doses (IU)a | 2±1.17 | 3.4±2.22 | 0.14 |
| Day 2 Insulin doses (IU)a | 2.4±1.89 | 3.8±3.55 | 0.36 |
| Insulin dose difference, day 1 to day 2 (P) | 0.36 | 0.67 | |
| Day 1 Insulin adjustments | 3.6±1.5 | 5.8±2.7 | 0.06 |
| Day 2 Insulin adjustments | 2.6±1.5 | 5±2.6 | 0.04 |
| Total number of Insulin dose adjustments, day 1 to day 2 (P) | 0.03 | 0.21 | |
| Patients in normoglycemia % (n) | 87.5 (7) | 100 (8) | 0.3 |
| Time to reach normoglycemia (h) | 5.7±3.1 | 13±7 | 0.02 |
| Time in normoglycemia (h) | 19.4±18.2 | 13.5±14.8 | 0.49 |
| Total number of hypoglycemia episodes | 1 | 0 |
±Standard deviation.
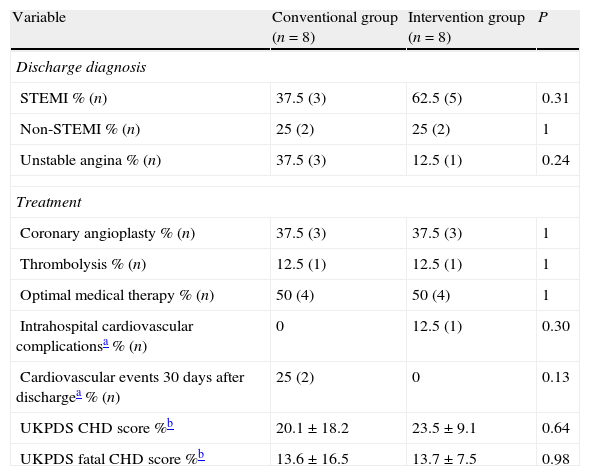
Discharge diagnoses, treatment employed, risk stratification obtained according to the UKPDS risk Engine calculator v2.020 as well as complications and mortality showed no statistically significant differences between groups, and are shown in Table 4.
Diagnosis, treatment and complications.
| Variable | Conventional group (n=8) | Intervention group (n=8) | P |
| Discharge diagnosis | |||
| STEMI % (n) | 37.5 (3) | 62.5 (5) | 0.31 |
| Non-STEMI % (n) | 25 (2) | 25 (2) | 1 |
| Unstable angina % (n) | 37.5 (3) | 12.5 (1) | 0.24 |
| Treatment | |||
| Coronary angioplasty % (n) | 37.5 (3) | 37.5 (3) | 1 |
| Thrombolysis % (n) | 12.5 (1) | 12.5 (1) | 1 |
| Optimal medical therapy % (n) | 50 (4) | 50 (4) | 1 |
| Intrahospital cardiovascular complicationsa % (n) | 0 | 12.5 (1) | 0.30 |
| Cardiovascular events 30 days after dischargea % (n) | 25 (2) | 0 | 0.13 |
| UKPDS CHD score %b | 20.1±18.2 | 23.5±9.1 | 0.64 |
| UKPDS fatal CHD score %b | 13.6±16.5 | 13.7±7.5 | 0.98 |
Our study shows that continuous glucose monitoring devices were not superior to capillary glucose monitoring for improving the time to achieve normoglycemia and the time that the patient remains there. Despite this, patients with continuous glucose monitoring devices were more stable, as demonstrated by the mean glucose levels on day 1 and day 2. In addition, all patients in the interventional group reached levels of normoglycemia in comparison with the conventional group indicating that continuous glucose monitoring allows identification of more regular glucose values with minimum variability. Continuous glucose monitoring in hospitalized patients could be a tool to prevent hypoglycemic episodes, a fact that was demonstrated in this study, as there were none in the intervention group.
All studies that compare tight glucose control have been directed at evaluating capillary glucose monitoring in the intensive care unit at different time intervals with different target glucose levels and different effects on mortality.21 The NICE-SUGAR study demonstrated a higher mortality in critically ill patients with intensive glucose control, mainly attributed to a higher number of severe hypoglycemic episodes22; however, a post hoc analysis of the DIGAMI-2 trial did not show an association between hypoglycemic episodes and adverse outcomes.23
These findings make it necessary to expand efforts toward improved glycemic control in patients with acute coronary syndrome, since continuous glucose monitoring provides a real time information about glucose variability in comparison with capillary or venous glucose.
The main strength of our study is that it is a comparative study of continuous glucose monitoring devices and conventional capillary glucose monitoring in patients with acute coronary syndrome. Existing studies have been conducted to study the accuracy of continuous monitoring devices in comparison with capillary measurements.24 This study also considered the in-hospital use of this device and the technical difficulties that may arise with it. This study shows that there is a better glycemic control with continuous glucose monitoring, suggesting less glucose variability, gaining importance for clinical evolution and prognosis; however, more studies are needed with more patients to evaluate the effects of control on glucose variability and on prognosis and outcome of patients with acute coronary syndrome.
On the other hand, our study has several limitations. It is a study with few patients, also it is an open and non-randomized study making it difficult to control all variables, obtaining wide difference in terms of some variables assessed, such as the time evolution of the DM, the presence of dyslipidemia or family history of acute coronary syndrome, glycated hemoglobin values, where there were differences that indicate a heterogeneity between groups, which is a common problem in non-randomized studies; the technical difficulties associated with using this technology should also be taken into account, which have been described in some studies; Jacobs and colleagues performed a study on the tolerability and precision of a device for continuous glucose monitoring in a rural intensive care unit, finding that despite being well tolerated by the patient, it was not very accurate for glycemic control in the context of diabetic ketoacidosis due to technical difficulties associated with calibration of the device and electrode performance.10
Randomized clinical trials that include a greater number of patient are needed, aimed at comparing continuous glucose monitoring with conventional methods (capillary and venous glucose monitoring) and their effect on glycemic control, glucose variability, evolution and prognosis of critically ill patients, including those with acute coronary syndrome, to establish the best conditions for its in-hospital use.
ConclusionFurther studies are necessary to determine the effectiveness of continuous glucose monitoring devices in hospitalized patients and to establish the most useful clinical scenarios for their use, since it is an effective way to obtain more realistic glucose control and prevent hypoglycemic episodes.
FundingDid not receive any sponsorship to conduct this study.
Conflict of interestThe author states have no conflicts of interest.
The authors are grateful to Dr. Sergio Lozano-Rodriguez for his critical review of the manuscript.