To evaluate the efficacy and complication profile of excimer laser trabeculostomy (ELT), an emerging laser-based trabecular minimally invasive glaucoma surgery (MIGS), combined with cataract surgery in routine clinical practice.
Patients and methodsSingle-site, retrospective, interventional study. Preoperative and postoperative clinical data of patients with cataract and open-angle glaucoma (OAG) who underwent combined phacoemulsification and ELT were collected and analyzed at preoperative day, one week postoperatively, and after one, two, three, six, nine and 12 months. Main outcome measure was intraocular pressure (IOP). Qualified and complete success were defined as an IOP less than 21mmHg and an IOP reduction ≥20% from preoperative medicated IOP with or without adjuvant medical treatment, respectively.
ResultsThirty-four eyes of 29 patients were included; 29 eyes completed 1-year follow-up. The mean preoperative IOP under medications was 20.9±2.6mmHg (±standard deviation, SD) and decreased significantly at one year (16.3±1.9; p<0.0001). The mean number of IOP-lowering medications decreased from 1.7±0.7 to 0.3±0.8 (p<0.0001) at the 12-month follow-up. At one year, 81% of eyes were medication free. Qualified and complete success was obtained in 62% and 58% of eyes, respectively. Two eyes had postoperative hyphema, three eyes had transient IOP spikes and one patient underwent a subsequent filtering surgery at three months.
ConclusionCombining ELT with phacoemulsification in eyes with cataract and mild to moderate OAG significantly reduced IOP and medication use without meaningful complications after one-year follow-up in a real world clinical practice setting.
Evaluar la eficacia y posibles complicaciones de la trabeculostomía láser excímer (excimer laser trabeculostomy, ELT) una técnica de cirugía de glaucoma mínimamente invasiva (minimally invasive glaucoma surgery, MIGS) emergente de tipo láser, realizada en combinación con la cirugía de la catarata durante la práctica clínica habitual.
Pacientes y métodosEstudio intervencionista, retrospectivo, en un único centro. Se recopilaron los datos clínicos preoperatorios y postoperatorios de pacientes con catarata y glaucoma de ángulo abierto (GAA) que fueron sometidos a cirugía combinada de ELT y facoemulsificación, en el día preoperatorio, a una semana, y uno, dos, tres, seis, nueve y 12 meses después de la cirugía. La variable principal del estudio fue la presión intraocular (PIO). El éxito cualificado y completo se definió como una PIO menor de 21mmHg con una reducción del 20% o más de la PIO preoperatoria, con o sin tratamiento médico adyuvante, respectivamente.
ResultadosSe incluyeron 34 ojos de 29 pacientes; 29 ojos completaron el seguimiento a un año. La PIO preoperatoria media bajo medicación hipotensora fue de 20,9±2,6mmHg (± desviación estándar, DE) y disminuyó significativamente al año (16,3±1,9; p<0,0001). El número medio de medicamentos reductores de la PIO disminuyó de 1,7±0,7 a 0,3±0,8 (p<0,0001) en el seguimiento de 12 meses. Al año, el 81% de los ojos estaban libres de medicación. Se obtuvo un éxito cualificado y completo en el 62% y el 58% de los ojos, respectivamente. Dos ojos presentaron hipema postoperatorio, tres ojos sufrieron un aumento transitorio de PIO y un paciente requirió de cirugía filtrante en los tres meses sucesivos.
ConclusiónLa combinación de ELT con facoemulsificación en ojos diagnosticados de catarata y glaucoma de ángulo abierto (GAA) leve a moderado redujo significativamente la PIO y el uso de medicamentos sin complicaciones significativas después de un año de seguimiento en un entorno de práctica clínica habitual.
Glaucoma is the second leading cause of blindness worldwide, and the only treatments with demonstrated evidence of slowing its progression are based on IOP reduction. In open-angle glaucoma (OAG) the mechanism of IOP elevation has been found to be related to an increase in resistance, particularly between the trabecular meshwork (TM) and the Schlemm canal (SC) inner wall.1 Although prolonged use of IOP-lowering drugs remains first-line treatment, this approach is hampered by compliance issues, side effects including those involving the ocular surface and reduced filtering surgery success rates.2 Furthermore, glaucoma and cataract coexistence increases with age, and an estimated 20% of patients who undergo cataract surgery are receiving IOP-lowering medications, due to ocular hypertension or different levels of glaucoma.3
While cataract surgery by itself has a certain hypotensive effect, it is modest, often not allowing for topical therapy discontinuation.4 Recently, novel minimally invasive glaucoma surgeries (MIGS) have been developed. MIGS comprise a group of surgical procedures that preferably share the following properties: (1) ab interno approach; (2) minimal disruption of normal anatomo-physiology; (3) excellent safety profile; (4) moderate IOP reduction; and (5) rapid patient recovery.5 Most of these techniques are based on the introduction of small implants or goniotomies at the same time as the cataract surgery (phaco-MIGS).6 Trabecular MIGS are those that attempt to increase physiological aqueous humor flow by overcoming SC resistance as with iStent™ and iStent inject™ (Glaukos Corporation, San Clemente, California, USA) and with dilating devices such us the Hydrus microstent™ (Ivantis Inc., Irvine, California, USA). Goniotomy approaches involve cutting, tearing or attempting to remove a portion of the TM.
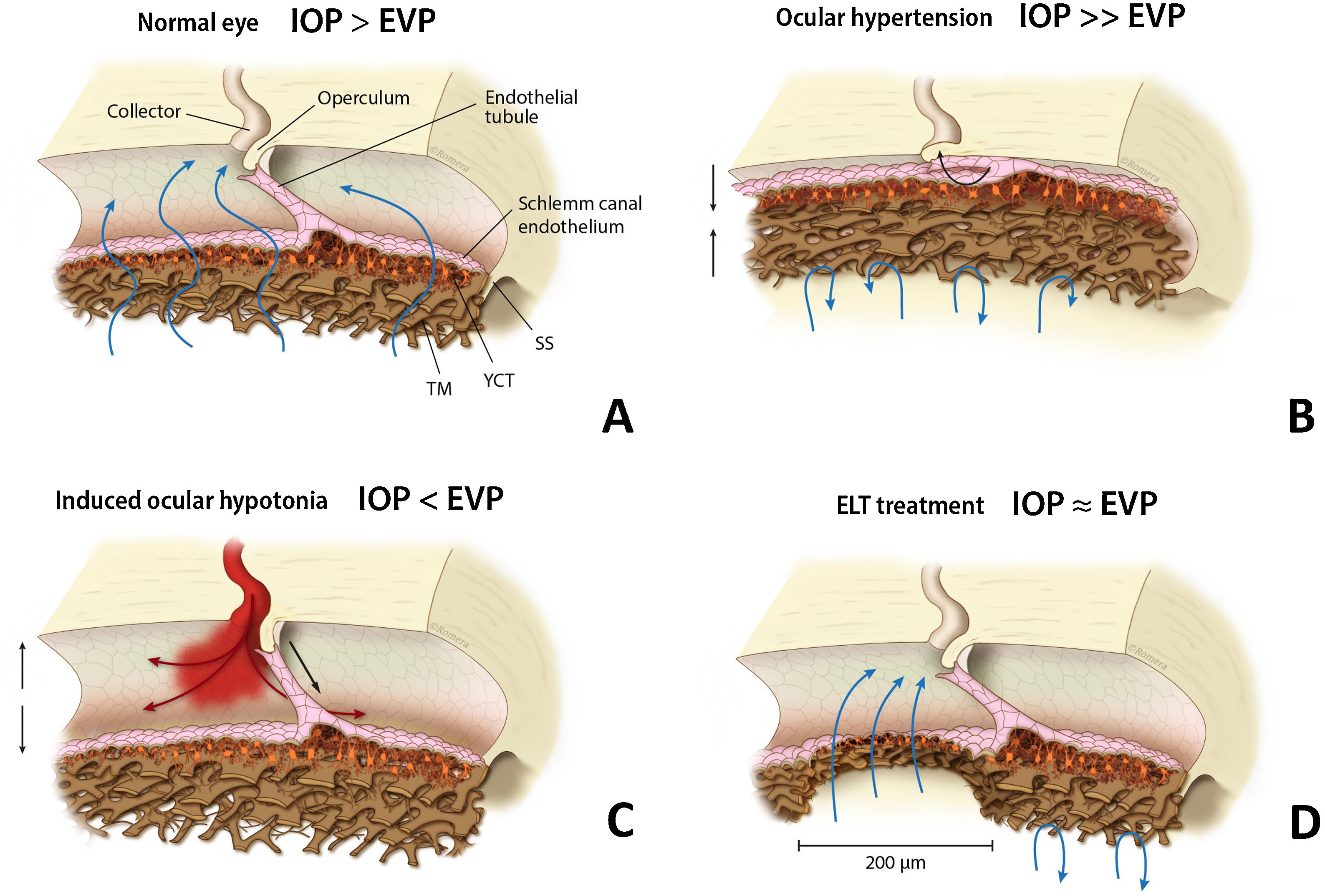
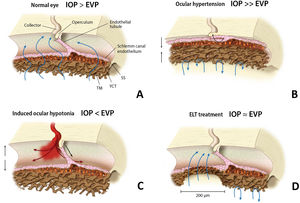
Among the trabecular MIGS, excimer laser trabeculostomy (ELT) is an implant-free microablative ab-interno procedure that uses ultraviolet cold laser energy to create permanent perforations over the iridocorneal angle, selectively ablating multifocal areas of the TM and SC inner wall,7 thus creating direct communication between the anterior chamber (AC) and collector channels (CC) (Fig. 1). Excimer ablative technology is less traumatic to tissue with less inflammation, which might result in greater effect persistence.8
EVP (episcleral venous pressure). (a) Schlemm canal anatomy in a normal eye, TM (trabecular meshwork), JCT (juxtacanalicular trabeculum), SS (scleral spur). (b) High resistance in TM and JCT with SC diameter reduction in an OAG patient. (c) Blood reflux may appear in SC during induced intraocular hypotension. (d) TM and JCT removal after ELT, a permanent and direct communication is created between anterior chamber and collector channels. IOP: intraocular pressure; EVP: episcleral venous pressure; TM: trabecular meshwork; JCT: juxtacanalicular trabeculum; SS: scleral spur; ELT: excimer laser trabeculostomy; SC: Schlemm canal.
The aim of this study is to show our experience with this evolving procedure and analyze its safety and efficacy in a real world clinical setting.
Patients and methodsStudy designThis is a retrospective interventional study that evaluated combined phacoemulsification (phaco) and ELT (ExTra Laser, MLase, AG, Germering, Germany) surgeries performed consecutively at the University Hospital of Albacete Glaucoma Department from October 28th 2017 to January 31st 2019. The device received CE marking in Europe in 2014 and at the time of this study had not been cleared for sale by the US Food and Drug Administration (FDA). Institutional ethical approval cor this study was obtained through the University Hospital of Albacete Research Committee (number 2018/12/144). All study procedures adhered to the Declaration of Helsinki for research involving humans, and written informed consent was obtained from all the study participants.
Inclusion and exclusion criteriaFirst six ELT surgeries performed were excluded as they were considered within the learning curve. After these, consecutive patients aged 18 years or older with a diagnosis of mild to moderate open-angle glaucoma (visual field [VF] mean deviation [MD] not greater than −12dB according to the Hodapp-Parrish-Anderson glaucoma severity classification system)9 and a concurrent cataract were included. Patients were enrolled in the study if they had visually significant cataract and if their IOP was above target or if there was glaucomatous progression in their VF.
Exclusion criteria were a diagnosis of ocular hypertension (elevated IOP without glaucomatous optic disc or VF changes) and those patients with prior argon trabeculoplasty or glaucoma surgery, less than 3 months of follow-up, neovascular glaucoma or narrow angles (Shaffer grade of less than 3 preoperatively).
Preoperative assessmentPreoperatively, patient demographics including age, gender, eye laterality, glaucoma severity, last recorded non-medicated IOP, preoperative IOP (with or without treatment, i.e., baseline IOP), number of glaucoma medications and biometric axial length (IOLMaster 500, Carl Zeiss Meditec AG, Jena, Germany) were recorded.
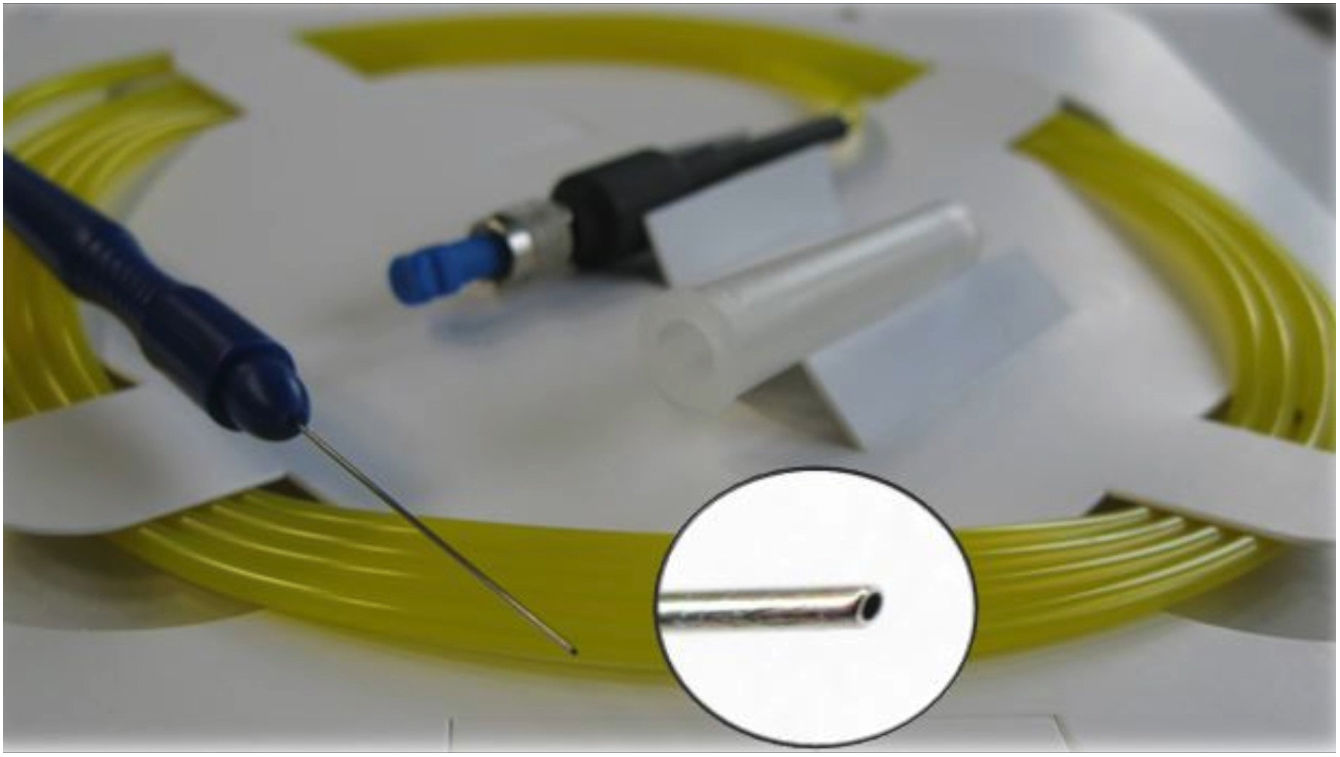
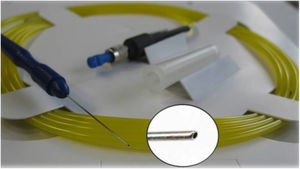
ELT ExTraLaser and single-use ELT Fido ProbeELT is performed using a short-pulsed (20Hz, 60–120ns) 308nm xenon chloride (XeCl) excimer laser, which delivers photoablative energy (1.27–1.31mJ) to precisely remove the tissue obstructing aqueous outflow with minimal thermal damage to adjacent tissue. The ELT “Fido” is a single-use fiberoptic probe with a metallic 65° angled tip of 500μm outer diameter and 210-μm fiber core diameter, designed to optimize contact with the trabecular meshwork (Fig. 2). The console program allows 10 laser discharges (shots) to be performed for each probe, after which the probe is discarded.
Surgical techniqueAll surgeries were performed in a similar fashion by 3 expert glaucoma surgeons: NPA, AMV and IIKA. A standard bimanual 2.2 or 2.75mm clear-cornea phaco (Infiniti Vision System, Alcon Inc., Fort Worth, Texas, USA) with intracapsular lens implantation (AcrySof IQ SN60WF, Alcon Inc.) was performed first. Inmediately following phaco, the operating microscope was tilted 35° away from the surgeon and the patient's head was turned in the opposite direction of the operating eye to maximize the visualization of the nasal angle. The ExTra laser device was calibrated, and a sterile Fido was coupled to the laser console. A direct gonioprism (Transcend TVG Surgical Gonio Lens, Volk Optical Inc., Mentor, Ohio, USA) was placed on the cornea with the non-dominant hand and the anatomic landmarks, including the trabecular meshwork, were brought into focus looking for blood reflux. Under sodium hyaluronidate 2% ocular viscoelastic device (OVD) (AJL VISC 2%, AJL Ophthalmic, SA, Araba, Spain) the ELT Fido was introduced via a 0.8–1mm temporal corneal clear incision (or via the same principal incision used for the phaco procedure), keeping the probe handpiece marking on top. The fiber tip was directed toward the pigmented TM and advanced until coming into contact with it. Ten trabeculostomies were performed over a span of 90° to 120° of the nasal quadrant. Blood reflux was desired to ensure that perforations had reached CC. The Fido fiberoptic was then removed from the eye and the OVD along with residual traces of blood reflux were aspirated using the irrigation/aspiration handpiece. Finally, the anterior chamber depth was restablished with BSS and the eye was pressurized above 20mmHg. Intraoperative complications were recorded at the end of the procedure.
Postoperative assesmentThe postoperative medical treatment regimen consisted of a fixed combination of tobramycin and dexamethasone sodium phosphate (1mg/ml) eye drops (q.i.d.), tapered over the course of one month. IOP-lowering medications were withheld at day one if IOP was lower than 22mm Hg and reintroduced if an IOP spike occured. Follow-up visits were scheduled at postoperative day 1, week 1 and months 1, 2, 3, 6, 9 and 12. At all visits, examinations included best corrected visual acuity (BCVA) using LogMAR charts, IOP measured by Goldmann applanation tonometry and slit-lamp examination including gonioscopy and funduscopy. IOP-lowering medications were reintroduced after steroid washout if the IOP was higher than the patient's pre-specified target over 2 consecutive visits. If IOP remained insufficiently controlled during follow-up, a secondary glaucoma surgery was performed at the discretion of the surgeon. The number of hypotensive medications, postoperative complications and subsequent procedures were assessed at each visit.
The main outcome measure was IOP. Secondary study endpoints were need for ocular hypotensive medication, BCVA, intra- and postoperative complications, and requirement for subsequent interventions. IOP spikes, defined as an IOP increase ≥10mmHg above baseline IOP, were also taken into account.
Complete success was defined as an IOP less than 21mmHg and an IOP reduction ≥20% from preoperative medicated IOP and no IOP-lowering medications needed, while qualified success was defined as IOP less than 21mmHg and an IOP reduction ≥20% with medical treatment. The proportion of eyes achieving each of these success criteria was reported at each time point.
Statistical analysisNormal distribution of the variables was assessed using the Shapiro–Wilk test. For categorically distributed variables, counts and percentages are presented and outcome variables were tested using the Fisher exact test. For continuous variables, means and standard deviations are given. Within-group differences were tested using a Student t test if normality was assumed or by the Mann–Whitney U test as a non-parametric test. The before and after paired mean changes comparison were analyzed using a Student paired t test or Wilcoxon test. Survival was analyzed using the Klaplan-Meier estimator. Differences were considered significant when the p-value was <0.05. SPSS statistical software (version 18.0, IBM Corporation, Armonk, New York, USA) was used to analyze the results.
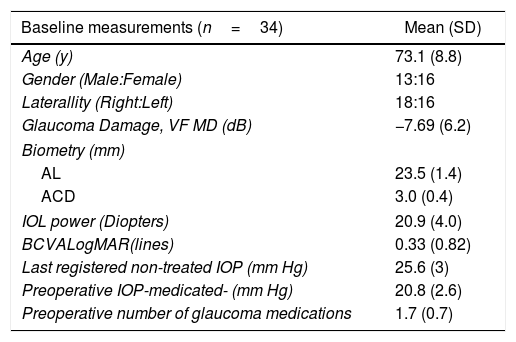
ResultsOverall, data from 34 eyes of 29 patients were included in this analysis. Mean follow up was 11.5±0.5 months (four patients lost the one-year follow-up visit due to COVID-19 pandemic onset). Patient's Demographic characteristics are shown in Table 1.
Demographics and baseline characteristics.
| Baseline measurements (n=34) | Mean (SD) |
|---|---|
| Age (y) | 73.1 (8.8) |
| Gender (Male:Female) | 13:16 |
| Laterallity (Right:Left) | 18:16 |
| Glaucoma Damage, VF MD (dB) | −7.69 (6.2) |
| Biometry (mm) | |
| AL | 23.5 (1.4) |
| ACD | 3.0 (0.4) |
| IOL power (Diopters) | 20.9 (4.0) |
| BCVALogMAR(lines) | 0.33 (0.82) |
| Last registered non-treated IOP (mm Hg) | 25.6 (3) |
| Preoperative IOP-medicated- (mm Hg) | 20.8 (2.6) |
| Preoperative number of glaucoma medications | 1.7 (0.7) |
IOP: intraocular pressure; MD: Mean Desviation; AL: Axial lenght; ACD: Anterior chamber Depth; BCVA: Best corrected visual acuity; IOL: Intraocular lens.
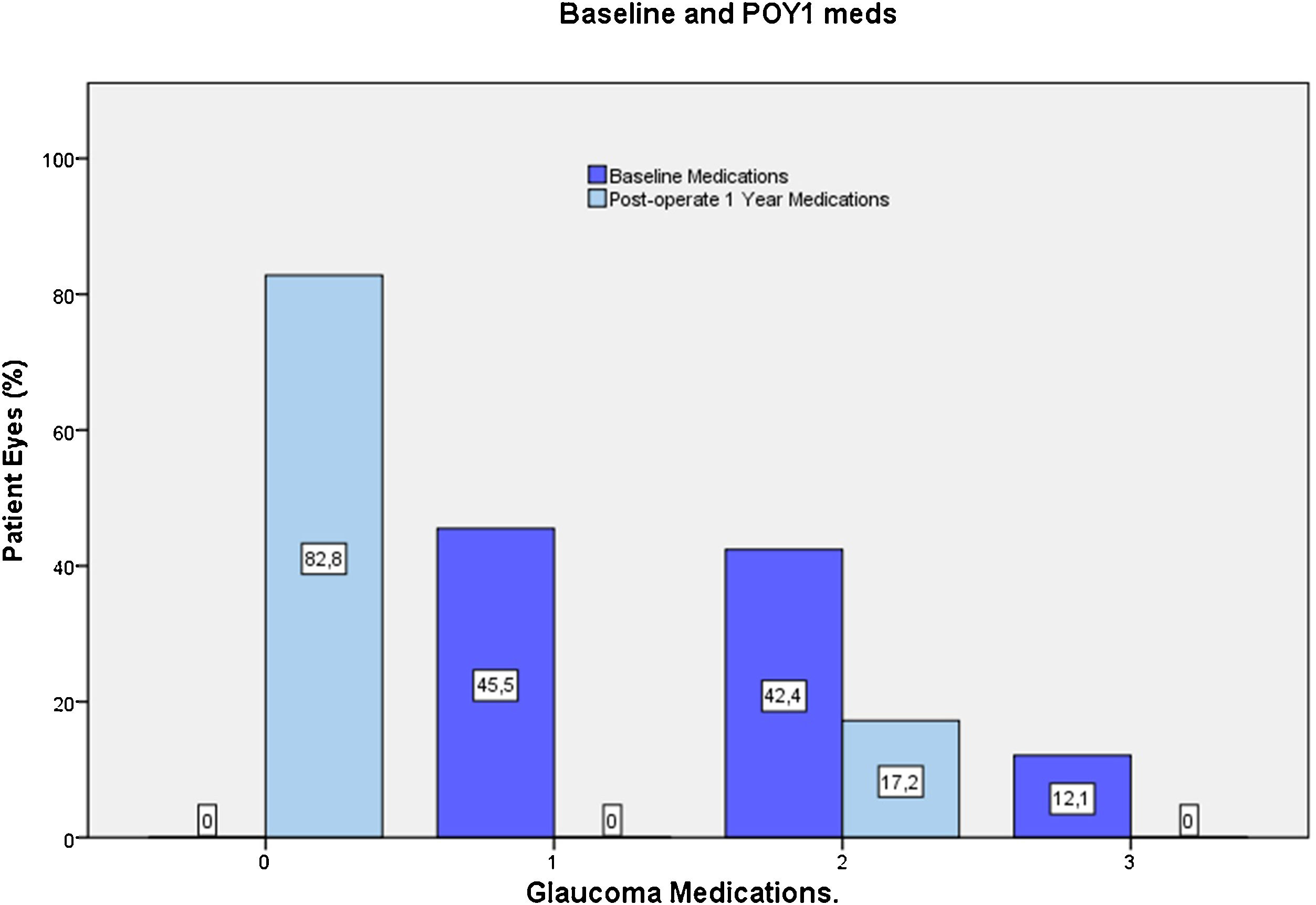
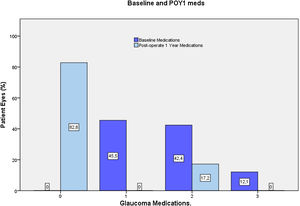
Most eyes had primary OAG (n=32), except for one case of pseudoexfoliative glaucoma and one eye with pigmentary glaucoma. Mean last recorder unmedicated IOP was 25.7±3.1mmHg (data available for 23 eyes). All the included eyes were using IOP-lowering medication at preoperative visit, most commonly a prostaglandin analog (76.5%) or a beta-blocker (36%). For 54.5% of eyes, more than one hypotensive medication were used (Fig. 5).
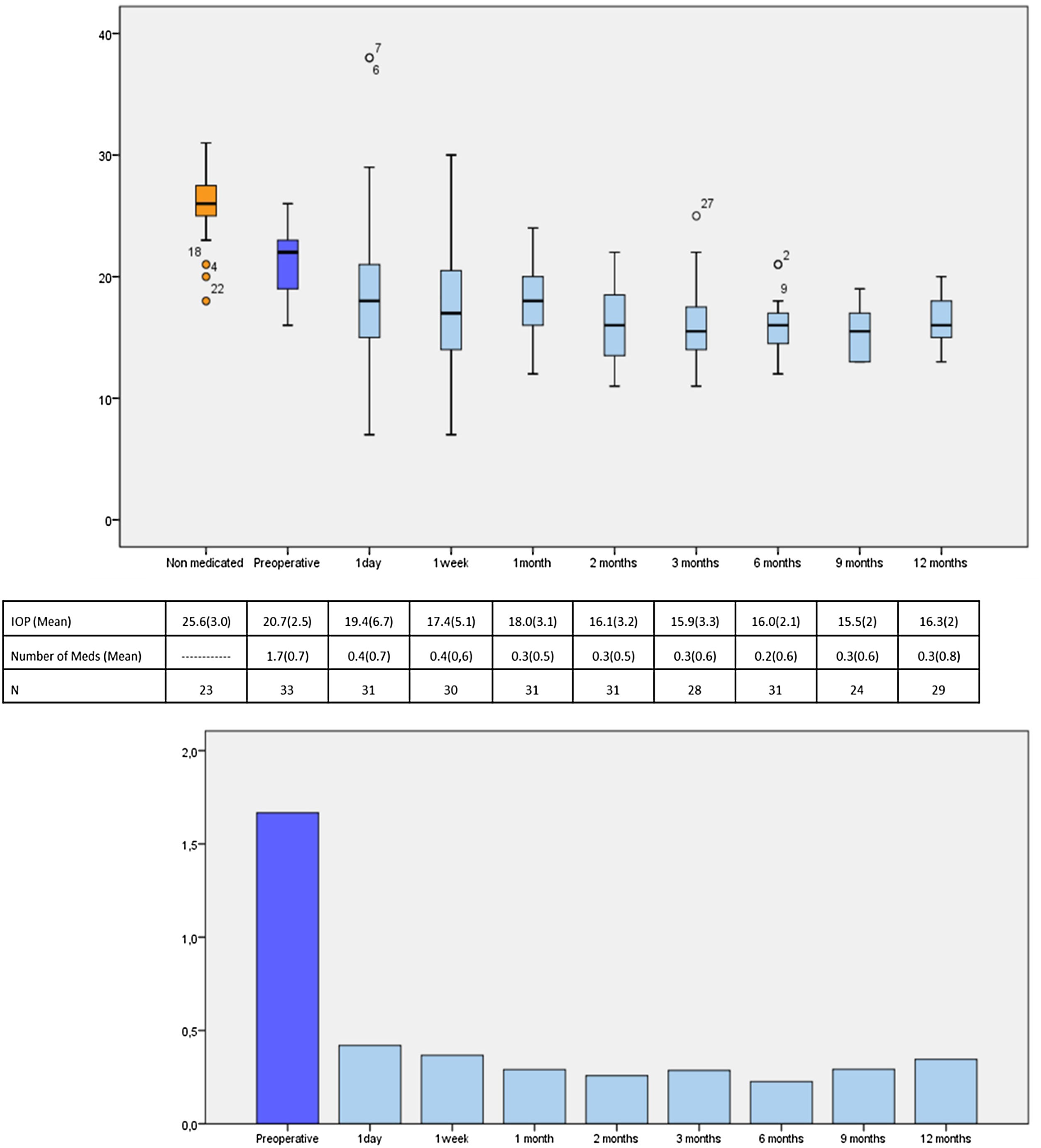
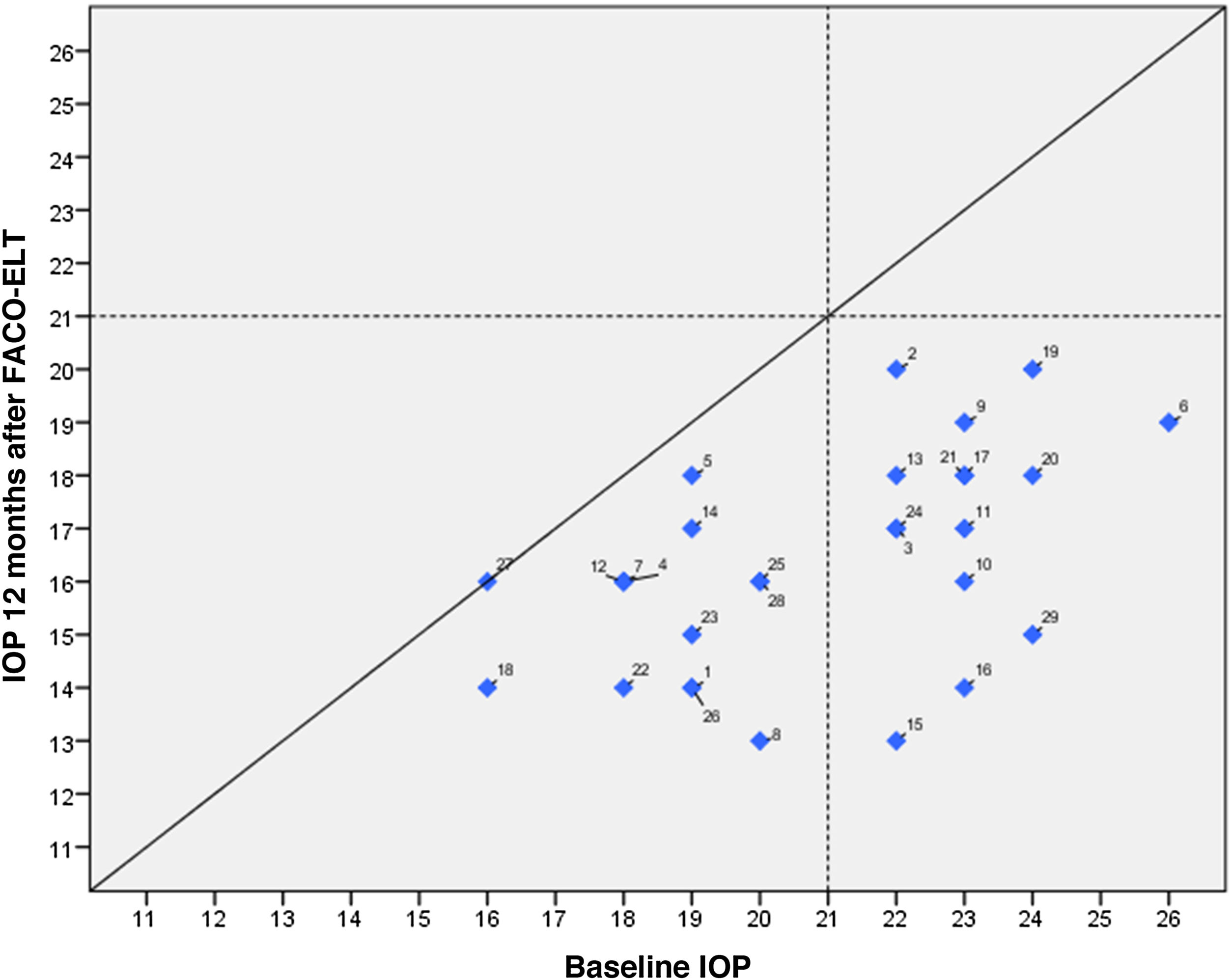
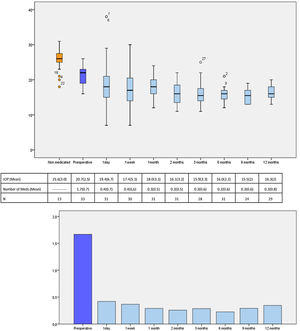
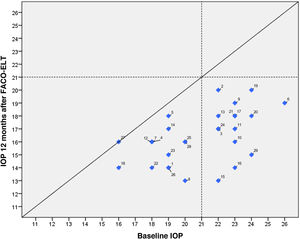
IOP and number of glaucoma medications data at preoperative and at each follow-up time point are shown in Fig. 3. IOP change in each case one year after surgical treatment is displayed in Fig. 4. From a mean preoperative IOP of 20.8±2.5mmHg while using 1.7±0.7 topical hypotensive medications, statistically significant IOP reduction was achieved in all eyes as soon as postoperative day one and remained significant at every postoperative time point through 12 months follow-up ranging from 13.3% to 26.6% (2.8 to 5.6mmHg). IOP values leveled off around 8 weeks after surgery, stabilizing at 16.1±3.2mmHg. Overall, 1 year after phaco-ELT we found a 22% IOP reduction in IOP from baseline (mean preoperative IOP 20.8±0.4mmHg; mean IOP at 12 months 16.3±2mmHg, p<0.0001). The number of eyes with IOP measurements over 21mmHg changed from 50% at baseline to 0% at 12 months. One year after surgery, 100% of eyes had IOPs under 21mmHg while 50% were under 16mmHg and 25% under 14mmHg (Fig. 4).
Number of patients, mean IOP and mean number of IOP-lowering medications at each time point. Top figure: Box-and-whisker plot of mean IOP at each time point (expressed in mmHg). Center table: Mean IOP and number of IOP-lowering drops during the study and the participants studied at each time point, expressed as mean (standard deviation). Bottom figure: Graphical representation of the mean number of medications over time. IOP: intraocular pressure; N=number of available patients.
Similarly, the mean number of hypotensive medications was significantly lower at every postoperative visit compared to baseline, ranging from 0.23 to 0.43 (p=0.000). At 12 months, eyes required 0.3±0.8 IOP-lowering medications on average (a mean reduction of 1.3 medications from baseline, p<0.001) (Fig. 3).
At the end of the follow up, 82% of eyes were medication free (only 5 eyes remained under medication) (Fig. 5). In those eyes without medication at 12 months, mean registered untreated IOP was reduced from 25.3±2.5mm Hg up to 16.2±2.3mmHg, (36% reduction, p<0.0001, n=23).
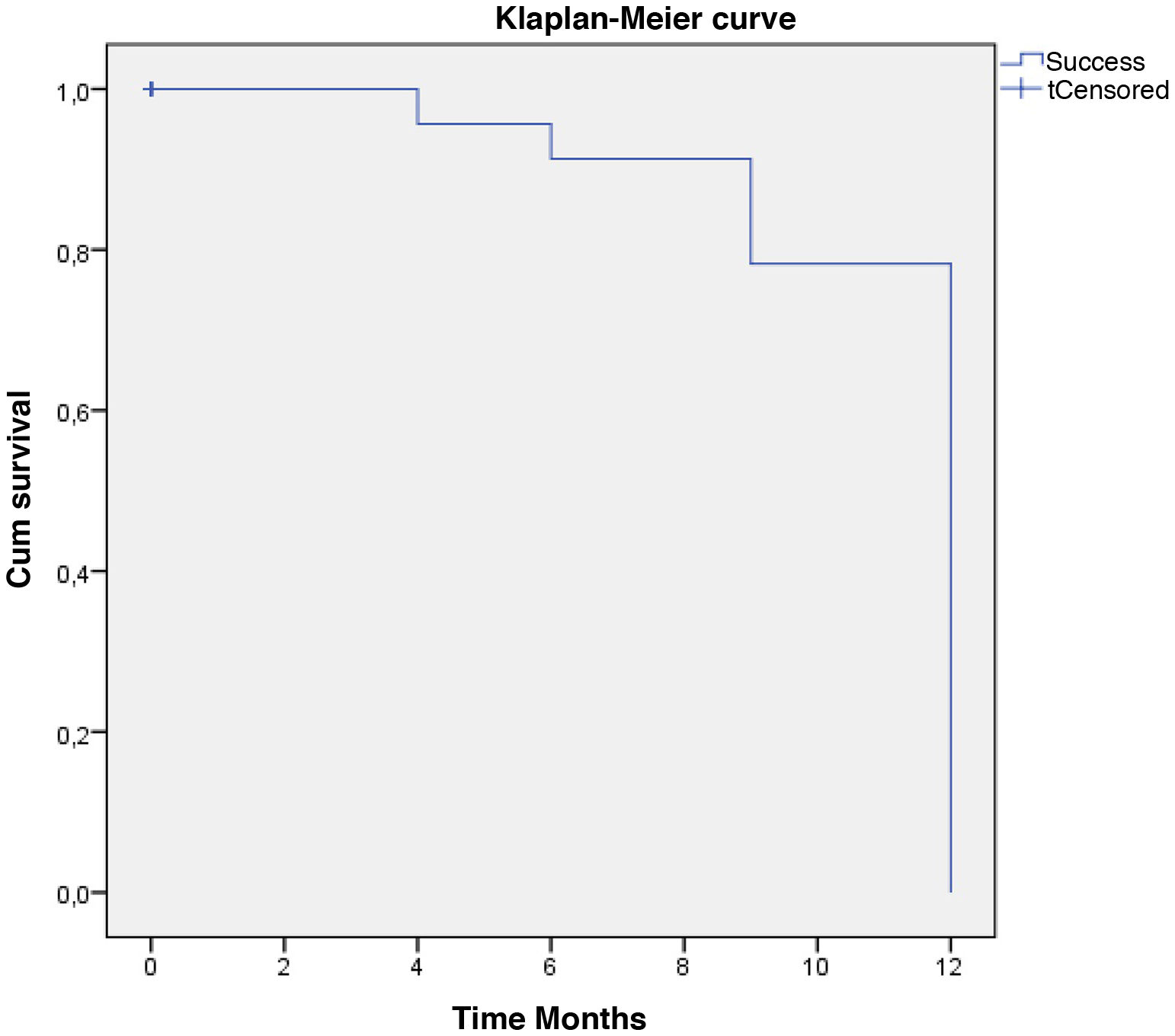
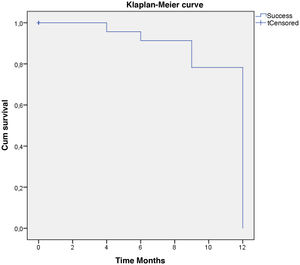
Qualified success was reached by 62% and complete success by 58% of the eyes with one year follow-up. In the Kaplan–Meier survival model, defined event as “Qualified or complete success”, the probability of survival for surgical success at twelve months was 79.3%. (Fig. 6).
Mean BCVA improved from 0.33±0.82 preoperatively to 0.19±0.70 in the first 24h, and significantly to 0.12±0.82 as soon as one week after phaco-ELT (p=0.005). All patients recovered normal activity within the first week. At one year, mean BCVA was 0.07±1.30, representing a significant improvement from preoperative visit (p<0.001).
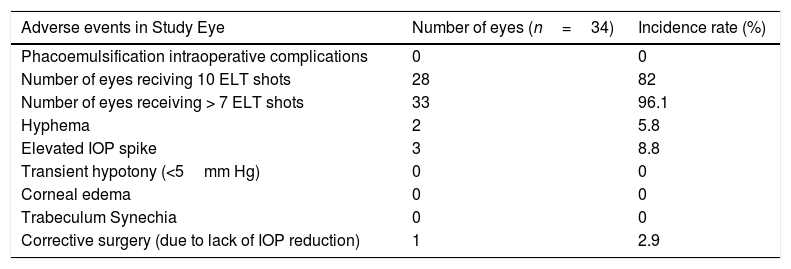
Adverse events were non-sight threatening and self-limited. Intraoperatively, the 10 available laser shots could be applied in 82% of cases; however, it was not possible to perform all of them in 5 cases due to insufficient visualization or poor positioning (see more detailed information in Table 2). Although intraoperative blood reflux was recorded in 58% of surgeries during intraoperative induced hypotony, only 2 significant hyphemas (more than 1mm) were evidenced 24h after surgery. Three IOP spikes occurred, which resolved within 48h with topical medical treatment. No complications were observed in the late postoperative period and no rescue maneuvers were necessary. No synechiae were documented on gonioscopic follow up. In some cases, blood reflux during slit lamp gonioscopy was documented at more than 6 months after surgery. Only in one case a deep sclerectomy was performed as a secondary surgical procedure after not reaching the preset target IOP (excluded from analysis).
Intraoperative and postoperative ocular adverse events.
| Adverse events in Study Eye | Number of eyes (n=34) | Incidence rate (%) |
|---|---|---|
| Phacoemulsification intraoperative complications | 0 | 0 |
| Number of eyes reciving 10 ELT shots | 28 | 82 |
| Number of eyes receiving > 7 ELT shots | 33 | 96.1 |
| Hyphema | 2 | 5.8 |
| Elevated IOP spike | 3 | 8.8 |
| Transient hypotony (<5mm Hg) | 0 | 0 |
| Corneal edema | 0 | 0 |
| Trabeculum Synechia | 0 | 0 |
| Corrective surgery (due to lack of IOP reduction) | 1 | 2.9 |
Prolonged medical treatment in glaucoma care has several disadvantages such as compliance issues, cost and tolerance-related problems. Most importantly, chronic hypotensive topical treatment has been reported to have a negative effect on the ocular surface and an association with increased subsequent conventional surgery failure rates.10 Recently, with the advent of MIGS, a more interventional approach to glaucoma management has been proposed,11 and in patients with co-existent cataract and glaucoma, the time of phacoemulsification may offer a window of opportunity to treat glaucoma in a more proactive way. The option to perform only cataract surgery in these patients overall achieves a poor IOP-lowering effect, with mild reductions of only 14% and removal of less than 0.5 medications at 1 year, as shown in a recent meta-analysis.4 At the other extreme, combined procedures with phacoemulsification and trabeculectomy may be less effective than a stand-alone glaucoma filtering surgery, possibly due to the release of inflammatory mediators during cataract surgery12,13 and are associated with a higher complication rate.14 Intermediate phaco-MIGS alternatives are now available, and although validated algorithms optimizing the times for the different techniques are not yet available, a reasonable first option for early cases could be to combine phacoemulsification with a trabecular MIGS, thus preserving conjunctiva for future filtering surgeries.5 These include stenting approaches as well as cutting approaches.
ELT is an implant-free trabecular MIGS technique that uses a selective laser treatment to create direct communication between the AC and the collector channels. In a recent systematic review evaluating 3069 studies on MIGS, only 33 were included in the meta-analysis and of those, three were ELT studies (low risk of bias and only one randomized clinical trial [RCT]), showing one of the highest scores in the efficacy analysis.15 In these and other studies, ELT has been shown to achieve an average IOP reduction of approximately 30%, which appears to increase further when combined with cataract surgery.16–22 However, like all the trabecular MIGS, this hypotensive effect is variable depending on how different the preoperative IOP is from the episcleral venous pressure (EVP), since there is a floor effect that limits hypotensive efficacy.19 For this reason, RCTs and other studies in which medication wash-out is carried out before ELT treatment may overestimate the hypotensive effect of the intervention compared to real-world studies in which medical treatment is normally not interrupted.
Consequently, the purpose of our study was to evaluate the IOP-lowering effect and the safety profile of excimer laser trabeculostomy (ELT) combined with cataract surgery in a clinical practice setting. In our study, from a mean preoperative (with medical treatment) IOP of 21mmHg, we achieved a mean IOP decrease of 4.5mmHg (22%) and a reduction of 1.3 IOP-lowering medications, with approximately 80% of eyes not requiring medical treatment. These results are superior to those reported for phaco as a solo procedure4 and quite similar to the phaco-ELT work published by Töteberg et al. in which they reported an analogous mean IOP decrease of 4mmHg with 1 drug removal.18 Previously, in a phaco-ELT case series (n=50), Wilmsmeyer et al. obtained a higher IOP reduction of 27% but without a significant change in the number of hypotensive medications.17 Unlike these two retrospective series, we specifically excluded ocular hypertension patients to know the effect of ELT in open-angle glaucoma, being the first to provide biometric data from eyes, such as axial length, anterior chamber depth and intraocular lens power, supporting that our sample excluded narrow angles that could have benefited from phacoemulsification hypotensive effect. In addition, due to the close monitoring carried out during the initial period, we found that the maximum ELT effect occured at 8 weeks following treatment, approximately one month after corticosteroid withdrawal.
Interestingly, blood reflux through the trabeculostomies has been observed during gonioscopy maneuvers more than 6 months after surgery. That finding may indicate that these laser trabeculostomies do not heal, supporting the idea that excimer laser does not enhance scarring. These findings correlate with the recent publication of a comparative study in which the non-thermal phaco-ELT procedure showed superior efficacy at 1 year over phaco-ab-interno trabeculotomy with the Trabectome device (NeoMedix, Tustin, California, USA) in the Kaplan–Meier statistics.23
Similar to those seen with other trabecular MIGS methods, the most frequent postoperative complications were hypertensive peaks and hyphema,20 which developed in 8.8% and 5.8% of eyes, respectively. Such frequencies were very similar to those reported by other investigators.15–19 Hyphema usually occurs due to blood reflux through the trabeculostomies when the pressure gradient is reversed intraoperatively, which may be minimized if rapid pressurization of the anterior chamber is applied at the end of surgery. As in most combined procedures, IOP spikes are believed related to OVD retention or inflammatory debris and in our series, they were transient and controlled with medical treatment. In contrast to other trabecular MIGS techniques,24–26 we did not observe anterior synechiae in any of our patients at any of the postoperative gonioscopic follow-ups. These safety data results are similar to those reported by Töteberg et al. in which phaco-ELT was compared to conventional phaco-trabeculectomy, and the former was found to have fewer complications without the need for associated rescue maneuvers.19
Regarding impact on patient quality of life, rapid recovery in both visual acuity and normal activity occurred in all our patients, ‘which are anticipated outcomes for all MIGS, including ELT.5 Additionally, most patients were medication free 1 year after the surgery, which would very likely lead to an improvement of their ocular surface and quality of life.
Regarding the complete and qualified success, we have used the same criteria what evaluate glaucoma surgery in clinical trials. Due to the lack of a washout period in the clinical practice and the potential decrease in IOP estimated for this technique, the results of complete success would have been better if we had excluded from our study patients receiving treatment with more than two drugs or non medicated IOP above 30mmHg. These findings will be useful to adjust surgical indications especially when the main goal is to remove the IOP lowering medication completely.
The limitations of the study include the retrospective non-controlled design, the relatively small sample and the lack of a control group. However, since our study included a very well characterized consecutive series from a routine clinical setting, our results may more closely approximate real-life conditions and may be more clinically applicable.
In conclusion, combined ELT and phacoemulsification provided a significant IOP-lowering effect and reduction of preoperative IOP-lowering medications without meaningful complications. Additional prospective randomized clinical trials are needed to corroborate these findings.
FundingThere was no source of funding.
Conflict of interestThe authors AMV and IIKA are consulting physicians for ELT Sight, Inc.
NP and AMV had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors alone are responsible for the content and writing of the paper. This research received no specific grant from any funding agency. The authors thanks to Michael Berlin, MD for introducing and training us to this surgical technique.
Please cite this article as: Moreno-Valladares A, Puerto Amorós N, Mendez Llatas M, Pazos-López M, Ahmed IIK. Cirugía combinada de trabeculostomía láser excímer y facoelmusificación: datos a un año en el mundo real de una MIGS de tipo láser. Arch Soc Esp Oftalmol. 2021;96:631–639.