The influenza virus has accompanied humans since time immemorial, in the form of annual epidemics and occasional pandemics. It is a respiratory infection with multiple repercussions on people's lives at an individual and social level, as well as representing a significant burden on the health system. This Consensus Document arises from the collaboration of various Spanish scientific societies involved in influenza virus infection. The conclusions drawn are based on the highest quality evidence available in the scientific literature and, failing that, on the opinion of the experts convened. The Consensus Document addresses the clinical, microbiological, therapeutic, and preventive aspects (with respect to the prevention of transmission and in relation to vaccination) of influenza, for both adult and pediatric populations. This Consensus Document aims to help facilitate the clinical, microbiological, and preventive approach to influenza virus infection and, consequently, to reduce its important consequences on the morbidity and mortality of the population.
El virus de la gripe ha acompañado al ser humano desde tiempo inmemorial, en forma de epidemias anuales y pandemias ocasionales. Se trata de una infección respiratoria con múltiples repercusiones sobre la vida de las personas a nivel individual y social, así como una importante sobrecarga para el sistema sanitario. El presente documento de consenso surge de la colaboración de diversas sociedades científicas españolas implicadas en la atención de la infección por virus de la gripe. Las conclusiones extraídas se han fundamentado en las evidencias de mayor calidad disponibles en la literatura científica y, en su defecto, en la opinión de los expertos convocados. En el documento de consenso se abordan los aspectos clínicos, microbiológicos, terapéuticos y preventivos (respecto de la prevención de la transmisión y con relación a la vacunación) de la gripe, tanto para población pediátrica como para adultos. Este documento de consenso pretende ayudar a facilitar el abordaje clínico, microbiológico y preventivo de la infección por virus de la gripe y, consecuentemente, a disminuir sus importantes consecuencias sobre la morbimortalidad de la población.
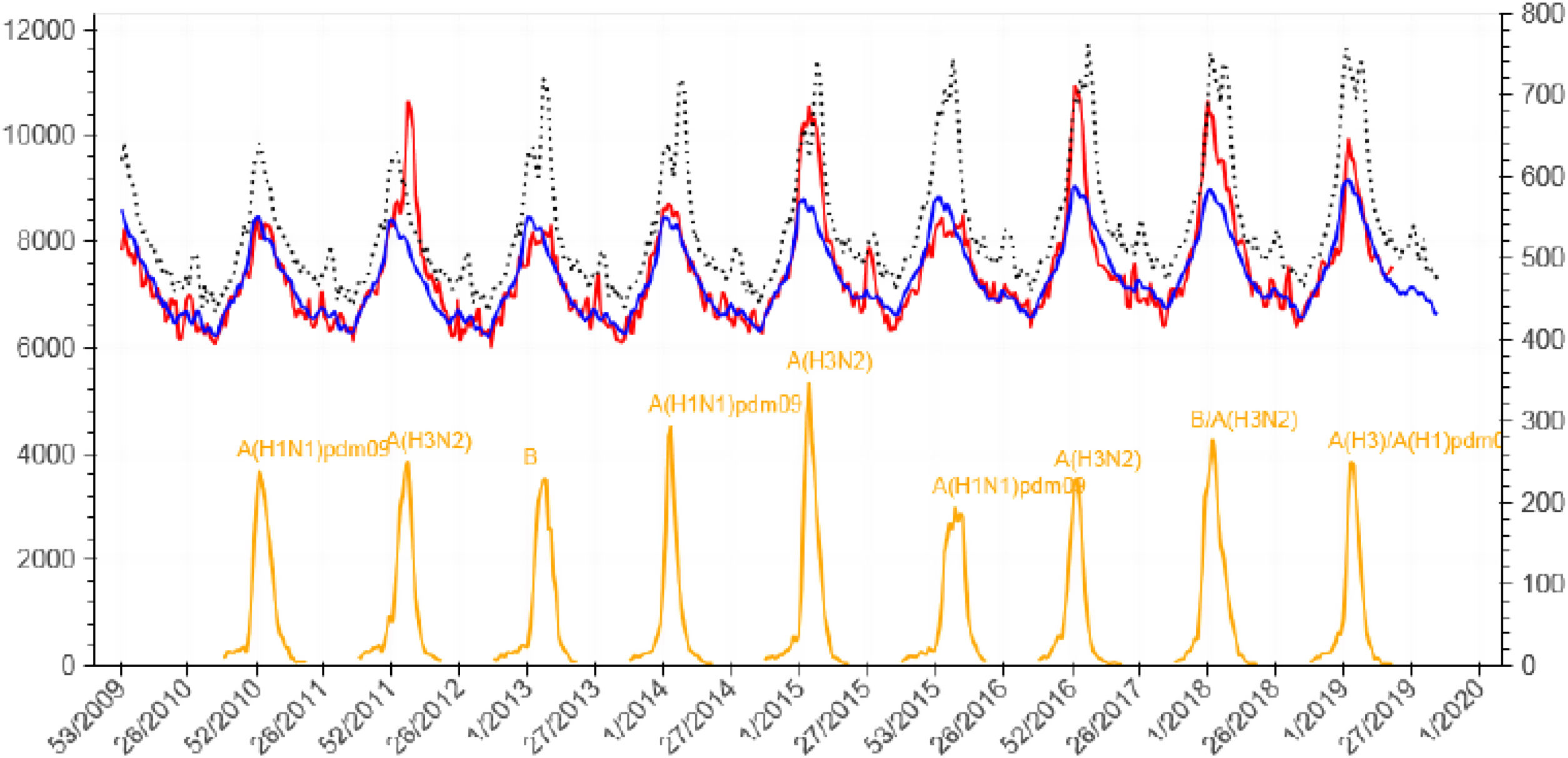
Infection by influenza virus has accompanied humanity from time immemorial, producing annual epidemics that can cause severe infection, mainly in the elderly, pregnant women, or in those with previous comorbidities. Moreover, from time to time, it produces periodic pandemics related to genomic mutations that can give rise to a devastating disease, mostly in young people without previous exposure to that type of virus. There is probably no other infectious disease that better correlates with population mortality as influenza virus infection does. As shown in Fig. 1, there is a tiny correlation between the daily oscillation of mortality for the general population and the weekly rate of influenza virus infection.1 Only the recent pandemic of COVID-19 by coronavirus SARS-Cov-2 has presented a comparable effect on the mortality of the general population in modern times.
Daily global mortality by any cause in Spain (2010–2019) and weekly incidence of influenza virus infection. Source: National Center of Epidemiology, Health Institute Carlos III, Ministry of Science, Spain.1 Footnote: red line: detected mortality; blue line: expected mortality; yellow line: incidence of influenza; x-axis: week/year; left y-axis: absolute number of deaths; right y-axis: number of cases of influenza infection per 100,000 inhabitants.
Despite these facts, influenza virus infection is still considered a benign unimportant infection by a large proportion of citizens and, even more worrisome, by physicians.
In the last few decades, we have witnessed a huge development in the diagnostic, preventive, and therapeutic tools for influenza virus infection that have demonstrated their usefulness in reducing the incidence, morbidity, and mortality of this infection. Meanwhile, a powerful media movement has made a big fuss based on non-scientific statements, provoking mass rejection to the application of these tools that could benefit public health. A recent study estimated that seasonal influenza produces between 300,000 and 600,000 deaths annually worldwide.2
This Consensus Statement arose as an initiative of the Spanish Society of Medical Microbiology and Infectious Diseases (SEIMC) and was enthusiastically taken on by the following scientific societies: the Spanish Society of Pediatric Infectious Diseases (SEIP), the Spanish Association of Vaccinology (AEV), the Spanish Society of Family and Community Medicine (SEMFYC), and the Spanish Society of Preventive Medicine, Public Health and Health Management (SEMPSPGS). The result is this Consensus Document that jointly approaches influenza virus infection from different complementary perspectives.
In the opinion of the authors of this Consensus Statement and their supporting Scientific Societies, this document represents a great opportunity for the diffusion of systematized scientific knowledge to the medical community, in order to improve the approach toward influenza virus infection in the twenty-first century.
We invite the readers to consult the whole text of this Consensus Document that includes the rationale for all the recommendations. The whole text is available at Appendix A.
MethodsThe development of this Consensus Statement was an initiative of the Executive Committee of SEIMC. They appointed an Infectious Diseases expert (FLM) and a Microbiology specialist (TP) as coordinators of the working group for the drafting of the manuscript in April 2018. Moreover, the Executive Committee of SEIMC contacted other Scientific Societies in order to develop a unified document approaching influenza virus infection from a holistic point of view. The following Scientific Societies were contacted: the Spanish Society of Pediatric Infectious Diseases (SEIP), the Spanish Association of Vaccinology (AEV), the Spanish Society of Family and Community Medicine (SEMFYC) and the Spanish Society of Preventive Medicine, Public Health and Health Management (SEMPSPGS). The Executive Committee of each of these societies appointed experts who were contacted and agreed to join the working group. The coordinators appointed by SEIMC prepared the index of the Consensus Statement and wrote out the queries to be answered by the panel of experts. Each group of experts worked in their field of expertise and a unified draft was constructed. The multidisciplinary panel of experts held a teleconference (May 2019) and a face-to-face meeting (June 2019) to discuss the aspects of the document in which consensus had not been achieved. Apart from the literature evidence (up to June 2022), the clinical experience and personal expertise of the members of the panel were taken into consideration when high quality evidence could not be found in the literature. In case of discrepancy, the criteria of the coordinators were applied.
The panel experts were asked to perform a systematic review of the scientific literature, with no time limit, in order to answer the assigned queries according to the best evidence available. PubMed, Embase, and the Cochrane Database for Systematic Reviews were consulted. The literature search was updated up to June 2022. The strength of the recommendations and the quality of evidence were graded based on the US Public Health Service Grading System (Table 1). Apart from the method for grading the recommendations, the document was written following the Appraisal of Guidelines Research and Evaluation (AGREE II) tutorial.
Strength of the recommendations and quality of the evidence.
| Category/grade | Definition |
|---|---|
| Strength of recommendations | |
| A | Good evidence to support a recommendation for or against use |
| B | Moderate evidence to support a recommendation for or against use |
| C | Poor evidence to support a recommendation |
| Quality of evidence | |
| I | Evidence from one or more properly randomized controlled trial |
| II | Evidence from one or more well-designed clinical trial without randomization; from cohort or case-controlled analytical studies (preferably from more than one center); from multiple time-series; or from dramatic results from uncontrolled experiments |
| III | Evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or reports from expert committees |
The target of the Consensus Statement is the diagnosis, treatment, and prevention of seasonal influenza virus infection. It was not designed to address the management of pandemic outbreaks by non-previously circulating influenza virus or the management of exceptional infections by strains of influenza virus of animal origin (“avian flu”).
All the members of the panel participated in the building of the Consensus Statement and approved the final version. The document was sent for audit by external peer reviewers. All the members of the Scientific Societies involved in the preparation of the manuscript had the opportunity to review the draft and make comments before publication. The final version was revised and approved by the Executive Committee of SEIMC and the other societies involved in the consensus (SEIP, AEV, SEMFYC and SEMPSPGS) prior to publication and adoption as an official document by the respective societies.
Clinical diagnosis and management of influenza virus infection in adultsWhen should influenza virus infection be suspected in an adult?Recommendations
- 1.
Influenza infection does not have specific clinical symptoms and its clinical picture might be undistinguishable from that produced by another respiratory virus. From an epidemiological point of view, the World Health Organization (WHO) case definition of influenza-like illness (ILI) for influenza sentinel surveillance refers to an acute respiratory infection with a temperature greater than or equal to 38°C and cough, with sudden onset within the previous 10 days (see Table 2 at full text in Appendix A) (A-II).
- 2.
The symptoms that most accurately predict an influenza infection are cough and a temperature greater than or equal to 39°C. Nevertheless, a lower temperature or even the absence of fever does not exclude the possibility of influenza virus infection (A-II).
- 3.
During influenza season, influenza infection can be considered in people with fever and acute exacerbation of underlying chronic lung disease, in elderly people with new or worsening respiratory symptoms (including exacerbation of congestive heart failure or altered mental status, with or without fever), in severely ill people with fever or hypothermia, and hospitalized adults who develop febrile respiratory illness after hospital admission (A-II).
- 4.
At any time of the year, in people with acute febrile respiratory symptoms who are epidemiologically linked to an influenza outbreak (healthcare workers, household and close contacts of people with suspected influenza, travelers returning from countries where influenza viruses may be circulating, participants in international mass gatherings, and cruise ship passengers) (A-II).
Recommendations
- 1.
Among adult patients with influenza-like illness, clinical findings are not particularly useful to differentiate influenza virus infection from another respiratory virus infection (B-II).
Recommendations
- 1.
An adult patient should be sent to the Emergency Department of a hospital if the patient might benefit from hospital admission due to the development of pneumonia as a complication of influenza virus infection (A-II).
- 2.
From a clinical point of view, this possibility should be suspected in the presence of shortness of breath, pain or pressure in the chest, sudden dizziness, confusion, and/or severe or persistent vomiting. It should also be considered in case of influenza virus infection symptoms that improve but then relapse in the form of fever and/or worsening lower respiratory tract symptoms (A-II).
- 3.
A patient with a suspected or diagnosed influenza virus infection with a chest X-ray performed outside the hospital environment showing pneumonia should be sent to the Emergency Room of a hospital to consider the need for hospital admission (A-III).
- 4.
An adult patient with influenza virus infection should be sent to the Emergency Department of a hospital if he/she presents exacerbation of underlying chronic diseases that might require hospital admission (A-II).
Recommendations
- 1.
Pneumonia should be considered in every patient with suspected influenza virus presenting with clinical features suggestive of lower respiratory tract infection in the context of the annual epidemic period of influenza (A-II).
- 2.
Pneumonia should be considered in every patient with confirmed influenza virus infection presenting with clinical features suggestive of lower respiratory tract infection (A-II).
- 3.
The possibility of influenza virus infection should be considered in everyone with a diagnosis of pneumonia in the context of the annual epidemic period of influenza (A-II).
Recommendations
- 1.
Although certain presenting clinical features may enable recognition of influenza pneumonia, no single symptom or scoring system is sufficient to differentiate between influenza and bacterial pneumonia (B-II).
Recommendations
- 1.
Influenza should be suspected in any child that presents acute fever with or without respiratory symptoms during the annual epidemic influenza period (A-II).
- 2.
The definition of influenza-like illness (ILI) has a very low diagnostic yield in children, especially in those younger than 5 years (A-II).
- 3.
In infants younger than 6 months, influenza may present as a sepsis-like syndrome (A-II).
Recommendations
- 1.
Many of the respiratory viral illnesses in children share similar signs and symptoms and although there are clinical differences that are specific to some viruses, physicians cannot usually confirm or rule out a particular viral infection on clinical grounds alone (A-I).
- 2.
It is essential to be able to obtain a microbiological diagnosis in patients where a specific diagnosis may modify patient management (specifically, the possibility to initiate antiviral influenza treatment) (A-I).
Recommendations
- 1.
Infants, children, or adolescent patients should be sent to the Emergency Department of a hospital if they could benefit from inpatient treatment due to the development of pneumonia or any other complication of influenza virus infection (A-II).
- 2.
Infants, children, and adolescent patients with risk factors (immunosuppressed patients, chronic lung disease, hemodynamically significant heart disease, severe neurological pathology, nephropathies and chronic liver diseases) should be microbiologically tested in the Primary Care environment or sent to the Emergency Department for a microbiological confirmation of influenza virus infection if this might modify the management of these patients (admission to hospital, initiation of antiviral treatment, performance of chest X-ray, etc.) (B-II).
- 3.
From a clinical point of view, this possibility should be suspected in the presence of poor general condition, signs of sepsis, altered level of consciousness or seizures, dehydration, shock, respiratory distress (tachypnea, chest retractions, hypoxemia, and episodes of apnea), or any alarming sign in clinical evolution according to medical criteria (see Table 3 at full text in Appendix A) It should also be considered in case of influenza virus infection symptoms that improve but then relapse in the form of fever and/or worsening lower respiratory tract symptoms (A-II).
- 4.
A pediatric patient with suspected or X-ray confirmed pneumonia should be sent to the Emergency Room of a hospital to consider the need for hospital admission if he or she is in poor clinical condition (A-II).
- 5.
Infants younger than 3 months of age with fever of unknown origin should be sent to the Emergency Department as, based on clinical grounds, influenza virus infection might be indistinguishable from other potentially life-threatening conditions (A-II).
Recommendations
- 1.
Pneumonia should be considered as a possibility in every pediatric patient with suspected influenza virus presenting with clinical features suggestive of lower respiratory tract infection in the context of the annual epidemic period of influenza (A-II).
- 2.
Pneumonia should be considered as a possibility in every patient with confirmed influenza virus infection presenting with clinical features suggestive of lower respiratory tract infection (A-II).
- 3.
The possibility of influenza virus infection should be considered in every child with the diagnosis of pneumonia in the context of the annual epidemic period of influenza (A-II).
- 4.
Influenza pneumonia and bacterial pneumonia may present overlapping clinical symptoms. Differential diagnosis may require a chest X-ray, and laboratory and microbiological tests, and cannot be defined only on a clinical basis (B-II).
Recommendations
- 1.
Microbiological diagnosis is indicated when the result of the test might change the clinical care of the patient or influence the clinical approach to other subjects exposed to the patient tested (see Table 4 at full text in Appendix A) (A-II).
- 2.
Microbiological diagnosis is indicated in cases of severe clinical course and for people at high risk of developing influenza-related complications (for instance, those with underlying cardiopulmonary diseases or immunocompromised subjects) (see Table 4 at full text in Appendix A) (A-II).
- 3.
Microbiological diagnosis should be attempted in every case with clinical suspicion of influenza virus infection in subjects admitted to hospital (A-II).
- 4.
Microbiological diagnosis should be attempted in healthcare workers (HCWs) with a clinical suspicion of influenza virus infection when they are taking care of patients with risk factors for developing severe forms of influenza, and when taking care of patients admitted to hospital or to long-term care facilities (B-III).
- 5.
Microbiological diagnosis is not indicated for non-immunocompromised subjects and subjects not presenting risk factors for the development of severe forms of influenza virus infection when they are not going to be admitted to hospital and/or they do not present a severe clinical condition (A-II).
- 6.
An accurate microbiological diagnosis of influenza virus infection and other respiratory viruses might help to avoid unnecessary antibiotic treatment and might help to accurately prescribe specific antiviral influenza treatment when indicated (A-III).
- 7.
For epidemiological purposes, cases of influenza virus infection should be microbiologically diagnosed, starting at week 40 and ending on week 20 of the following year (for the Northern hemisphere) and by designated reference laboratories, in order to establish the type of virus strain circulating and the moment of initiation of the epidemic period (A-II).
Recommendations
- 1.
Nasopharyngeal (NPS) or oropharyngeal (OPS) specimens collected by using sterile polyester swabs with plastic or aluminum shafts (not wooden shafts) are the preferred samples for non-invasive microbiological diagnosis of influenza virus infection in adults (A-I).
- 2.
NPS aspirate or washing is an alternative specimen that can be used for diagnosis. Collection of this specimen is especially well tolerated by children (A-II).
- 3.
A correct technique for NPS sampling must be highlighted as a factor that directly correlates with the yield of the microbiological diagnosis (A-III) – see Fig. 2 at full text in Appendix A.
- 4.
Alternatively, saliva specimens may be used but they are associated with a lower yield for microbiological diagnosis (A-II).
- 5.
Swabs must be transported to the Microbiology laboratory in sterile transport tubes with virus transport medium. Dry tubes for the transport of samples for bacterial diagnosis are not adequate (A-II).
- 6.
Lower respiratory tract specimens (bronchoalveolar lavage or tracheobronchial aspirate, depending on clinical status of patient) should be collected for viral microbiological diagnosis from hospitalized patients with respiratory failure receiving mechanical ventilation, including subjects presenting a sever clinical condition with a previous negative virus detection in an upper respiratory tract specimen sampled during the ongoing infectious episode (A-II).
- 7.
The yield of the microbiological diagnosis is inversely related to the time elapsed since the beginning of the symptoms. The earlier the sampling, the higher the yield of the microbiological diagnosis (A-II).
- 8.
Blood, plasma, serum, urine, stool, and cerebrospinal fluid are not suitable specimens for routine influenza virus infection diagnosis (A-III).
- 9.
Single or paired serum samples for serological diagnosis are only indicated for epidemiological purposes (A-III).
Recommendations
- 1.
Nucleic acid amplification test (NAAT) is the method of choice for the microbiological diagnosis of influenza virus infection. It should be able to identify type A and type B influenza virus. It is advisable to use a test that is able to identify type A influenza virus and distinguish subtypes H1 and H3 (A-II).
- 2.
Rapid molecular assays detect influenza virus infection with high sensitivity and specificity. These tests are recommended to be used in hospitalized patients with suspected influenza virus infection and may be a better alternative to the other rapid influenza diagnostic tests in outpatient settings (A-II).
- 3.
Antigen detection tests should be restricted to pediatric patients with samples collected within 24–48h following the onset of symptoms, when NAAT is not available (A-III).
- 4.
Viral culture should not be used for primary diagnosis in the clinical setting. It should be reserved for cases in which further antigenic or genetic characterization is needed (A-III).
- 5.
Serological testing for influenza is not generally recommended except for research purposes and for Public Health surveillance (A-II).
Recommendations
- 1.
Resistance to neuraminidase inhibitors should be considered when a microbiological diagnostic test continues to be positive more than 8–10 days after initiation of treatment with this type of antivirals (particularly when the antiviral dose is suboptimal) (B-III).
- 2.
Resistance to neuraminidase inhibitors should also be considered when a microbiological diagnostic test is positive while on or immediately after prophylaxis with this type of antivirals (C-III).
- 3.
Resistance to antivirals should be especially considered in the immunocompromised population with evidence of persistent viral replication (e.g., 7–10 days after initiation of treatment) (B-III).
- 4.
Periodic tests to detect resistance in influenza virus from random samples from community circulating virus should be performed. This surveillance should be limited to the reference laboratories designated by regional or national government authorities or by international Public Health organizations (C-III).
- 5.
Antiviral resistance testing can be performed by specific gene sequencing, real-time single-nucleotide polymorphism (SNP) detection, polymerase chain reaction, or by genome-wide genotyping (C-III).
Recommendations
- 1.
Genomic assays are preferred over antigen detection assays as rapid diagnostic tests when used for microbiological diagnosis of influenza virus infection at point-of-care (A-III).
- 2.
Rapid diagnostic tests performed by clinicians at point-of-care must be implemented and used under the quality control of a reference laboratory of virology, in both the primary care setting and emergency facilities (B-III).
Recommendations
- 1.
Detection of influenza virus by genomic tests (at the type and subtype level) for seasonal strains should be available for laboratories performing microbiological diagnosis (A-II).
- 2.
SNP assays for well-established single mutations associated with viral resistance should be implemented in large regional hospitals.
- 3.
Deep genetic and antigenic characterization (clades and subclades or minor antigenic variants) as well as specific serological assays should be limited to the reference laboratories designated by regional or national government authorities or by international Public Health organizations (A-II).
Recommendations
- 1.
Active viral surveillance of influenza virus is the cornerstone for detecting emerging influenza virus strains with pandemic potential (A-I).
- 2.
Viral surveillance is the backbone for the selection of candidate viruses for the next-season vaccine (A-III), and also provides relevant and crucial information for interpreting vaccine effectiveness.
- 3.
Seasonal influenza virus surveillance is necessary in order to establish when the epidemic annual period starts. It can also determine the proportions of type, subtype, and lineage of circulating viruses and assess antigen or genetic mismatch of circulating viruses with those included in the seasonal vaccine (A-I).
- 4.
Virologic surveillance should be limited to the reference laboratories designated by regional or national government authorities or by international Public Health organizations (A-II).
Recommendations
- 1.
Adults diagnosed with non-complicated influenza virus infection within the community should start specific antiviral treatment as outpatients if they present risk factors for the development of a complicated infection (see Table 5 at full text in Appendix A) (A-II).
- 2.
Neuraminidase inhibitors are the first line drugs to be prescribed for those in whom treatment is indicated as outpatients (A-I).
- 3.
Oral oseltamivir (see recommended doses in Table 6 at full text in Appendix A) is preferred over inhaled zanamivir for adults who can take oral drugs (A-III).
- 4.
The earlier the initiation of treatment with neuraminidase inhibitors, the greater the beneficial effect (A-II).
- 5.
Treatment with neuraminidase inhibitors should ideally be started within the first 48h after the onset of symptoms but a clinical benefit might be obtained even if started later than 48h after the onset of symptoms (A-II).
- 6.
Competent health authorities should adopt the measures to ensure access to these drugs for those in whom treatment is indicated, in the context of the National Health System (A-III).
Recommendation
- 1.
Adults fulfilling the criteria for outpatient treatment of the influenza virus infection (see 6.1) should start antiviral treatment as soon as possible when they are evaluated throughout the period of annual influenza epidemic, providing a microbiological diagnosis to confirm or exclude the infection is not available in less than 6h (A-III).
Recommendation
- 1.
Symptomatic treatment is recommended to alleviate the symptoms of influenza (C-II).
- 2.
Symptomatic treatment of influenza for fever, headache, and myalgia is appropriate with paracetamol, ibuprofen, or dipyrone (B-II).
- 3.
Cough can be relieved with honey and dextromethorphan, but the use of over-the-counter medications should be carefully weighed against the risk of adverse effects (B-II).
- 4.
Treatment with antibiotics is not indicated unless bacterial superinfection is suspected (A-III).
Recommendations
- 1.
Selected previously healthy patients with a confirmed early diagnosis of seasonal influenza during the epidemic period may start specific antiviral treatment as outpatients in the first 24h after the start of the clinical picture. It must be considered that expected benefit is limited to the reduction of time of illness or the development of acute otitis media and not to a reduced rate of hospitalization or other complications. Parents must be informed of the benefit-risk balance obtained with the treatment. The Panel of this Consensus Statement considers this benefit does not justify the recommendation for the indiscriminate use of antiviral treatment in the general pediatric population (A-II).
- 2.
Selected children diagnosed with non-complicated influenza virus infection within the community may start specific antiviral treatment as outpatients if they present significant risk factors for the development of a complicated infection (immunosuppressed patients, chronic lung disease, hemodynamically significant heart disease, severe neurological pathology, nephropathies, and chronic liver diseases) (A-II).
- 3.
Neuraminidase inhibitors are the first line drugs to be prescribed for those in whom treatment is indicated as outpatients (A-I).
- 4.
Oral oseltamivir (capsules or oral suspension – see posology in Table 7 at full text in Appendix A) is preferred over inhaled zanamivir (not indicated in any case for those under 5 years of age) for children who can take oral drugs (A-III).
- 5.
The earlier the initiation of treatment with neuraminidase inhibitors, the greater the beneficial effect (A-II).
- 6.
Treatment with neuraminidase inhibitors should ideally be started in the first 48h after the onset of symptoms but a clinical benefit might be obtained even if started later than 48h after symptom onset (A-II).
- 7.
Competent health authorities should adopt the measures to ensure access to these drugs for children in whom treatment is indicated, in the context of the National Health System (C-III).
Recommendation
- 1.
It is not indicated for the general pediatric population (C-III).
- 2.
It is indicated in exceptional cases where pediatric patients present risk factors for an adverse outcome in the context of a strong clinical suspicion of influenza virus infection while simultaneously presenting an impossibility of performing diagnostic tests (C-III).
Recommendations
- 1.
Symptomatic treatment of influenza for fever, headache, and myalgia is appropriate with paracetamol, ibuprofen, or dipyrone (B-II).
- 2.
Cough can be relieved with honey and dextromethorphan, but the use of over-the-counter medications should be carefully weighed against the risk of overdose (B-III).
- 3.
The use of salicylates and codeine should be avoided in patients younger than 18 years of age because of risk of fatal outcomes (C-III).
- 4.
Treatment with antibiotics is not indicated unless bacterial superinfection is suspected (A-III).
Recommendations
- 1.
Prompt use of antivirals is recommended for adult patients admitted to hospital with suspected or confirmed influenza virus infection (A-II).
- 2.
Neuraminidase inhibitors are the first-line drugs to be prescribed for those in whom treatment is indicated when admitted to hospital (A-I).
- 3.
Oral oseltamivir is preferred over inhaled zanamivir for adults who can take oral drugs (A-III).
- 4.
Oseltamivir can be administered as an oral solution through a nasogastric tube for those unable to swallow the capsules or to inhale zanamivir (A-II).
- 5.
The earlier the initiation of treatment with neuraminidase inhibitors, the greater the beneficial effect. Neuraminidase inhibitors should be started as soon as possible, preferably within the first 6h after arrival at the Emergency Room (A-II).
- 6.
Treatment with neuraminidase inhibitors should ideally be started in the first 48h after the onset of symptoms but, for those admitted to hospital, treatment must be started regardless of duration of symptoms (A-II).
- 7.
Adults fulfilling the criteria for treatment of influenza virus infection when admitted to hospital should start antiviral treatment as soon as possible when they are evaluated during the period of annual influenza epidemic (A-III).
- 8.
Competent health authorities should adopt the measures to ensure access to these drugs for those in whom treatment is indicated, in the context of the National Health System (A-III).
Recommendations
- 1.
Corticosteroids should not be added to influenza treatment in hospitalized patients, unless indicated for other reasons (A-III).
- 2.
Adding macrolides and naproxen to oseltamivir might be of benefit in patients with simultaneous pneumonia and influenza virus infection (C-I).
- 3.
Passive immunotherapy and sirolimus need further studies to be recommended in cases of severe influenza virus infection (B.II).
- 4.
Other therapeutic measures studied in humans, such as statins, nitazoxanide and herbal medicines, have not been consistently proven to improve prognosis in hospitalized adults with influenza infection, and therefore are not routinely recommended (C-III).
- 5.
Cough can be relieved with dextromethorphan, but the use of over-the-counter medications should be carefully weighed against the risk of adverse effects (B-II).
Recommendations
- 1.
Adults presenting a clinical picture of a severe respiratory infection (extensive pneumonia, respiratory failure, hypotension) while infected by influenza virus should receive early antibiotic treatment in addition to antiviral therapy.
- 2.
In adults with influenza virus infection whose respiratory symptoms deteriorate after an initial improvement, antibiotic therapy should be considered (A-III).
- 3.
Microbiological diagnostic tests to confirm bacterial coinfection or superinfection must be performed in these situations in patients admitted to hospital (A-III).
- 4.
If started when indicated, antibiotic treatment of adults with influenza virus infection should be active against commonly influenza-associated bacteria, such as Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, and Haemophilus influenzae (A-II).
- 5.
In case of nosocomial superinfection, the possibility of methicillin-resistant Staphylococcus aureus should be considered (A-II).
- 6.
Aspergillus spp. coinfection should also be considered, especially in immunosuppressed patients and those admitted to an intensive care unit (A-II).
Recommendations
- 1.
Antiviral treatment is recommended for children presenting risk factors for a complicated course (immunosuppressed, chronic lung disease other than asthma, hemodynamically significant heart disease, severe neurological pathology, nephropathies, and chronic liver diseases) when admitted to hospital due to influenza virus infection (B-III).
- 2.
Antiviral treatment may also be considered for children admitted to hospital due to influenza virus infection but not fulfilling the risk factors for a complicated course when presenting pneumonia or respiratory failure or at the time of admission into the critical care unit (B-III).
- 3.
Neuraminidase inhibitors are the first line drugs to be prescribed for those in whom treatment is indicated when admitted to hospital (A-I).
- 4.
Oral oseltamivir is preferred over inhaled zanamivir for children who can take oral drugs (C-III).
- 5.
Oseltamivir as an oral solution might be a better option than capsules for the pediatric population (C-III).
- 6.
Zanamivir is not indicated, under any circumstances, for children younger than five years of age (A-III).
- 7.
Oseltamivir can be administered as an oral solution through a nasogastric tube for those unable to swallow the capsules or to inhale zanamivir (A-II).
- 8.
The earlier the initiation of treatment with neuraminidase inhibitors, the greater the beneficial effect. When indicated, neuraminidase inhibitors should be started as soon as possible, preferably within the first six hours after arrival at the Emergency Room (A-II).
- 9.
When indicated, treatment with neuraminidase inhibitors should ideally be started within the first 48h after the onset of symptoms but, for severely ill children, treatment might be started regardless of duration of symptoms (A-II).
- 10.
Microbiologically confirmed influenza diagnoses should ideally be made before antiviral indication, due to the lack of specificity of symptoms. Etiological diagnosis also enables patient isolation in seasonal influenza period, which overlaps with other viruses, such as Respiratory Syncytial Virus (A-I).
- 11.
Exceptionally in patients who are critically ill and/or have risk factors, a strong clinical suspicion of influenza, and impossibility of performing a diagnostic test, antivirals could be prescribed without microbiological confirmation (C-III).
- 12.
Competent health authorities should adopt the measures to ensure access to these drugs for those in whom treatment is indicated, in the context of the National Health System (C-III).
Recommendations
- 1.
Symptomatic treatment of influenza for fever, headache, and myalgia is appropriate with paracetamol, ibuprofen or dipyrone (B-II).
- 2.
The use of salicylates should be avoided in children younger than 18 years of age because of the risk of developing Reye's syndrome (C-III).
- 3.
Supported sitting position and gentle suction of the nares when secretions block them can be useful (B-II).
- 4.
Intravenous fluid therapy is indicated if adequate oral intake is not possible, and oxygen therapy or mechanical ventilation as indicated (B-II).
- 5.
Other drugs such as antihistamines, nasal decongestants, antitussives, expectorants, or mucolytics are not generally recommended (B-II).
- 6.
Corticosteroids should not be added to influenza treatment in hospitalized patients, unless indicated for other reasons (A-III).
Recommendations
- 1.
Antibiotic treatment is indicated in proven or strongly suspected secondary bacterial infections cases (including bacterial otitis media, sinusitis, and pneumonia). Empiric antibiotics should generally be directed at the most common bacterial pathogens following influenza: Streptococcus pneumoniae, Staphylococcus aureus, and Streptococcus pyogenes (A-I).
- 2.
There is no indication for prescribing antibiotics in order to prevent secondary bacterial complication (A-I).
- 3.
In hospitalized children with influenza infection when bacterial pneumonia is suspected, complementary tests are recommended, as symptoms and signs of virus and bacteria often overlap. No complementary test on its own is enough to define bacterial coinfection (B-II).
- 4.
The best performing clinical decision rule for the diagnosis of bacterial coinfection or superinfection combines C-reactive protein (CRP) higher than 13mg/dl, procalcitonin higher than 0.52ng/ml, and/or alveolar consolidation in chest X-ray (B-II).
- 5.
In children with influenza virus infection whose respiratory symptoms deteriorate after an initial improvement, antibiotic therapy should be considered (A-III).
- 6.
Microbiological diagnostic tests to confirm bacterial coinfection or superinfection must be performed in these situations, in patients admitted to hospital (A-III).
Recommendations
- 1.
Annual influenza vaccination of people in high-risk groups is recommended (A-I) – see Section 10.
- 2.
It is recommended to perform hand hygiene after contact with respiratory secretions by means of hand washing with soap and water (or alcohol-based hand sanitizers containing at least 60% ethanol or isopropanol when soap and water are not available) (A-II).
- 3.
People should cover their nose and mouth when coughing or sneezing using tissues or flexed elbow (if a tissue is not available) in order to contain respiratory secretions, followed by hand hygiene. Touching eyes, nose, or mouth should be avoided where possible (B-II).
- 4.
Routine cleaning of frequently touched surfaces and objects that might be contaminated with respiratory secretions (at home, schools, childcare facilities, and workplaces) is recommended (B-II).
- 5.
Post-exposure chemoprophylaxis could be considered in asymptomatic people at high risk of developing complications from influenza and for those in whom influenza vaccination is contraindicated, unavailable, or expected to have low effectiveness (e.g., people who are significantly immunocompromised) (C-II).
- 6.
Clinicians can also consider post-exposure chemoprophylaxis for people who are unvaccinated and are household contacts of a patient at very high risk of complications from influenza (e.g., severely immunocompromised patients) (C-II).
- 7.
A 10-day regimen with a neuraminidase inhibitor is recommended as post-exposure chemoprophylaxis. It should be initiated as soon as possible (within 48h of exposure for oral oseltamivir [see recommended dosage for adults at Table 8 and for children at Table 9, both in full text in Appendix A] or within 36h for inhaled zanamivir) (A-III).
Recommendations
Vaccination
- 1.
Annual influenza vaccination of healthcare workers and people in high-risk groups is recommended (A-I) – see Section 10.
- 2.
Annual influenza vaccination and pneumococcal vaccine of residents in long term care facilities is recommended (A-II) – see Section 10.
Chemoprophylaxis
- 1.
Post-exposure antiviral chemoprophylaxis should not be used routinely (B-III). Antiviral prophylaxis can be considered after exposure (see criteria in Table 10 at full text in Appendix A) to a person with influenza in some circumstances, such as asymptomatic patients, healthcare workers at high risk of developing complications from influenza, or for those in whom influenza vaccination is contraindicated, unavailable, or expected to have low effectiveness (e.g., people who are significantly immunocompromised) (A-II).
- 2.
A 10-day regimen with a neuraminidase inhibitor is recommended as post-exposure chemoprophylaxis. It should be initiated as soon as possible (within 48h of exposure for oral oseltamivir or within 36h for inhaled zanamivir) (A-I) – see recommended dosage for adults at Table 8 and for children at Table 9, both in full text in Appendix A).
Standard precautions, hand hygiene, and respiratory hygiene/cough etiquette
- 1.
Reinforce effective hand hygiene and cough etiquette when in contact with patients, visitors, and staff (Catch it, Bin it, Kill it) (B-II).
- 2.
Provide disposable tissues, no-touch receptacles for disposal of tissues, and alcohol-based hand rubs (B-II).
- 3.
Provide instructions to cover mouths/noses when coughing or sneezing, use disposable tissues, and perform hand hygiene (i.e., by posting signs at entrances and in strategic places) (B-II).
- 4.
Standard cleaning and disinfection procedures as well as food handling, laundry, and waste management are adequate when attending patients with suspected or confirmed influenza (B-II).
Triage for rapid identification of patients with influenza-like illness (ILI)
- 1.
Instruct people to inform healthcare professionals upon arrival if they present symptoms of respiratory infection so that preventive actions can be taken (B-III).
- 2.
Offer masks to coughing persons upon entry to hospital (B-II).
- 3.
Enable differentiated spaces in waiting rooms for patients with symptoms of respiratory infection (B-III).
- 4.
It is recommended that patients be separated one or more meters from each other and by physical barriers (B-III).
Infection prevention and control precautions when caring for patients with ILI or confirmed influenza infection
- 1.
Droplet precautions are required for all cases of ILI that are known or suspected to be influenza virus infection until influenza has been excluded or the patient is no longer deemed contagious (A-II).
- 2.
Place patients with suspected or confirmed influenza in individual rooms or specific areas. If an individual room is not available, consult the Infection Prevention and Control Team for assessing isolation by cohort (B-III). In long-term care and other residential settings, make decisions regarding patient placement on a case-by-case basis after considering infection risks of other patients in the room and available alternatives (C-III).
- 3.
Patients with suspected or proven influenza who require non-invasive ventilation should have priority for negative-pressure rooms (if available) and/or rooms with 100% exhaust capability (B-II).
- 4.
For aerosol generating procedures, use of FFP2 face mask or a respirator, fluid repellent gown, disposable gloves, and eye protection (B-III).
- 5.
Closed-ventilation suction circuits should be used where available, with bacterial and viral filters placed over the expiratory port (B-III).
Peri- and postpartum care
- 1.
A pregnant woman with suspected or confirmed influenza virus infection admitted to hospital should be attended according to the recommendations for the general population before, during, and after delivery. These measures include standard and droplet precautions (B-II).
- 2.
After delivery, due to the risk of serious complications were the newborn to become infected by influenza, temporary separation from the baby should be considered, in accordance with the mother's wishes. The baby should be cared for by a healthy caregiver whenever possible (B-III).
- 3.
Mothers with the intention to breastfeed should express their milk in order to establish and maintain the milk supply. This breastmilk can be fed to the newborn by the healthy caregiver (B-III).
- 4.
In case the baby remains in the same room (due to the mother's wishes or for logistic reasons), standard and droplet precautions should be established in order to minimize transmission (B-III). The hospital must implement measures to reduce viral exposure of the newborn including physical barriers (i.e., a curtain between the mother and the newborn), maintaining at least 2 meters between the mother and the newborn, and ensuring another adult is present to care for the newborn.
- 5.
If breastfeeding is maintained while the mother presents influenza virus infection, she should wear a surgical face mask and practice hand hygiene before each feeding or contact with her newborn (B-III).
Containment measures
- 1.
During periods of increased influenza activity, minimize visits by patients seeking care for mild influenza-like illness who are not at increased risk of complications (B-III).
- 2.
Limit visitors with acute respiratory symptoms and/or with high risk of influenza complications (B-III).
- 3.
Healthcare workers presenting symptoms that suggest influenza virus infection should stop patient care activities, don a facemask, and immediately notify their supervisor (and infection control personnel) to determine appropriateness of contact with patients, temporary reassignment, or exclusion from work until criteria for a non-infectious status are met (B-III).
Training and education of healthcare workers (HCWs)
- 1.
Educate healthcare workers on the importance of source control measures to contain respiratory secretions so as to prevent droplet and fomite transmission of respiratory pathogens (B-II).
- 2.
Staff education and training on infection control methods, policies, and procedures should be delivered to all staff members (B-II).
- 3.
Healthcare settings must establish mechanisms to find out about influenza virus activity in the community as well as for the prompt detection of outbreaks in healthcare settings (B-III).
A nosocomial outbreak is defined by the diagnosis of healthcare-associated influenza infection (at least one of the cases with microbiological confirmation) in two or more patients admitted to the same ward in a period of less than 48h (A-II).
What measures should be adopted to control an influenza outbreak?A bundle of measures, rather than one measure alone, must be implemented when a nosocomial influenza outbreak is detected in an institution (A-II). This includes administrative, pharmacological, and non-pharmacological measures (see Table 11 at full text in Appendix A) (A-II).
Acute care hospitalsRecommendations
- 1.
Non-pharmacological measures must be used to prevent virus dissemination (B-II).
- 2.
Administer post-exposure prophylaxis as soon as possible to patients in close contact with a confirmed or suspected case of influenza and risk factors for developing serious complications in case of infection (see Table 11 at full text in Appendix A) (A-II).
- 3.
Post-exposure prophylaxis should be used in healthcare workers with comorbidities who are prone to complications in case of influenza infection (see Table 11 at full text in Appendix A) (A-II).
- 4.
Routine pre-exposure prophylaxis for all patients or staff is not recommended, not even in an outbreak situation, but could be considered in wards admitting immunocompromised patients or when staff members are suspected of being involved in maintaining an outbreak (B-II).
Recommendations
- 1.
Patients admitted to neonatal or pediatric intensive care units should be placed in individual rooms whenever they develop influenza virus infection (B-II).
- 2.
Mask, gown, and gloves should be worn when taking care of patients with influenza virus infection admitted to neonatal or pediatric intensive care units (B-II).
- 3.
Post-exposure prophylaxis should be administered as soon as possible to unvaccinated exposed neonates or infants admitted to pediatric intensive care units (A-III).
- 4.
Post-exposure prophylaxis should be used in healthcare workers whose comorbidities for high-risk influenza complications are present in themselves or in their household members (A-III).
- 5.
Administer antiviral prophylaxis to unvaccinated healthcare workers and family members including those vaccinated in the previous two weeks or if vaccine failure is suspected (A-III).
- 6.
Massive prophylaxis for all neonates or infants admitted to pediatric intensive care units and their staff should be considered in case of a persistent outbreak despite other more restrictive measures or in case the staff are suspected to be involved in maintaining the outbreak (C-III).
- 7.
Entry to the ward must be restricted to people presenting respiratory symptoms (A-III).
Recommendations
- 1.
Whenever a case of influenza virus infection is detected in a resident of a long-term care facility or nursing home, the rest of the residents should receive antiviral prophylaxis, regardless of their vaccination status (A-I).
- 2.
Post-exposure prophylaxis should be administered to healthcare workers with comorbidities who are prone to complications in case of influenza infection (A-II).
- 3.
Routine pre-exposure prophylaxis for all staff is not recommended, not even in an outbreak situation, but could be considered when staff members are suspected to be involved in maintaining an outbreak (B-II).
- 4.
Reinforce hand hygiene and the use of face masks among staff (B-II).
- 5.
Vaccination of staff and residents when the first cases of influenza virus infection are detected should not be considered an adequate control measure (A-I).
- 6.
Implementation of other non-pharmacological measures such as social distancing and cohorting could be considered (B-III).
Recommendations
- 1.
Vaccination is recommended for children between 6 months and 18 years of age in certain circumstances (see criteria in Table 12 at full text in Appendix A) (A-III).
- 2.
Vaccination of healthy children between six months and five years of age is universally recommended (AIII).
- 3.
Both political authorities and healthcare workers should redouble their efforts in order to boost vaccination against influenza virus among children belonging to target groups (A-III).
Recommendations
- 1.
Vaccination is recommended for all adults aged 65 years old or older (A-I).
- 2.
Vaccination is recommended for adults between 19 and 64 years of age in certain circumstances (see criteria in Table 13 at full text in Appendix A) (A-II).
- 3.
Both political authorities and healthcare workers should redouble their efforts in order to boost vaccination against influenza virus among adults belonging to target groups (A-III).
Recommendation
- 1.
Vaccination of children and adolescents with quadrivalent vaccine (against influenza virus A H3N2, influenza A H1N1pdm09, influenza B/Victoria lineage, and influenza B/Yamagata lineage) is recommended (B-III).
Recommendations
- 1.
For those older than or equal to 19 years of age in whom vaccination is indicated, a quadrivalent vaccine (against influenza A H3N2, influenza A H1N1pdm09, influenza B/Victoria lineage, and influenza B/Yamagata lineage) is recommended (B-III).
- 2.
For adults for whose age group the vaccine is licensed, a quadrivalent (against influenza A H3N2, type A H1N1pdm09, influenza B/Victoria lineage, and influenza B/Yamagata lineage) enhanced seasonal influenza vaccine is recommend (either adjuvant (B-III), high-dose (B-II), or recombinant (B-II)).
Recommendations
- 1.
One dose of the vaccine and another dose separated from the first one by an interval of four weeks is recommended for children between six months and eight years of age, if they have never before received a dose of influenza vaccine (A-I).
- 2.
A single annual dose is recommended for younger than nine-year-olds who have been vaccinated in previous influenza seasons (A-I).
- 3.
For everyone older than nine years of age, a single annual dose of the influenza vaccine is recommended regardless of vaccination in previous seasons (A-I).
- 4.
A full dose of 0.5ml of the influenza vaccine is recommended for everyone, independently of their age (A.I).
- 5.
The vaccine should be administered in October-November for those living in the Northern Hemisphere (A-III).
- 6.
Vaccination is indicated until the end of the annual influenza season for those who did not receive the vaccine in October-November (A-III).
Recommendations
- 1.
Influenza virus vaccination should be avoided in those who previously developed a severe allergic reaction (e.g., anaphylaxis) to a previous influenza vaccine or any of its components (A-III).
- 2.
Currently, egg allergy is not considered a contraindication for the administration of egg-cultured influenza vaccine (A-III).
- 3.
Any acute disease of moderate or severe intensity (e.g., asthmatic crisis, decompensated heart failure, acute diarrhea), with or without fever, constitutes a temporary contraindication for the administration of the vaccine. In these circumstances, vaccination should be postponed until the acute illness is resolved (A-III).
Future studies should address several points concerning influenza infection.3 From an epidemiological point of view, it will be necessary to develop tools for a better prediction of epidemics, pandemics, and interactions with other respiratory viruses. We also need the development of new tools, for example machine learning, in order to diagnose influenza virus infection more accurately in the clinical context. In order to improve the diagnosis of infection, the development of easy-to-use “point-of-care” techniques that can give reliable information to the clinician to adopt immediate therapeutic decisions are necessary. The therapeutic armamentarium against influenza virus needs to be expanded with new oral antivirals to be administered in the early phases of the infection. New evidence is needed regarding the transmission of the virus (via droplets or aerosols) in order to set more accurate recommendations for isolation and personal protective equipment. Finally, vaccines that produce an enhanced immunological response are required, along with universal vaccines presenting activity against different types of influenza virus in order to avoid annual re-vaccination. Some lessons learned from the SARS-Cov-2 pandemic should be applied to dealing with the influenza virus in the future.
FundingThere is not funding source.
Conflict of interestsFrancisco López-Medrano, Diego van Esso-Arbolave, Santiago Alfayate, Manuel García Cenoz, Santiago Melón, Judith Chamorro-Camazón, Alfredo Tagarro, Marta Cruz, Diego Viasus, Jordi Carratalà, Elisa Cordero, Germán Schwarz-Chávarri, M Ángeles Marcos and Nemesio Moreno-Millán declare no conflict of interests. María Fernández-Prada has been a member of advisory boards and has given talks for continuing medical education for Pfizer, GSK, Seqirus, MSD, Sanofi, and Sanofi-Genzyme. Jaime Pérez-Martín has given talks for continuing medical education for Seqirus and Sanofi. Tomàs Pumarola has been a member of advisory boards for Seqirus and Sanofi. Juan Rodríguez García has been a member of advisory boards or has given talks for continuing medical education for Pfizer, GSK and Sanofi.
We would like to thank the Executive Committee of the Spanish Society for Clinical Microbiology and Infectious Diseases (SEIMC) for trusting us to develop this Consensus Statement (especially José Ramón Blanco and Antonio Aguilera). We are particularly grateful to Carlota Gudiol, Ma Nieves Larrosa, Manuel Crespo, and Juan Carlos Rodríguez Díaz (former members of the Executive Committee of SEIMC). We also acknowledge the enthusiastic welcome to SEIMC's proposal of the other Scientific Societies involved in drawing up the document. Many thanks to Nuria Jiménez, secretary of SEIMC, for her assistance with logistic affairs. Moreover, we acknowledge José María Martínez-Ávila for his original illustrations specifically designed for this Consensus statement and Meryl Jones for the review of the English version of the manuscript.
Article previously published in Enfermedades Infecciosas y Microbiología Clínica, Anales de Pediatría and Revista de Medicina Preventiva y Salud Pública, with the consent of the authors and publishers.