To validate a translated and culturally adapted version of the Morisky Medication Adherence Scale for use in Spanish population, and to examine the psychometric properties of this scale in patients with type 2 diabetes mellitus in Spain.
DesignThis cross-sectional study was conducted in a single university hospital in Spain. Patients diagnosed with type 2 diabetes mellitus at least 1 year before inclusion, being treated with anti-diabetic medication were included.
InterventionWe used the Spanish version of the scale to measure treatment adherence.
Principal measurementsthree level categorical scale is broken down into low adherence (score of <6), medium adherence (score of 6 to <8) and high adherence (score of 8). To validate the questionnaire, we measured internal consistency through Cronbach's α, confirmed construct validity through an exploratory principal component analysis and assessed test–retest reliability.
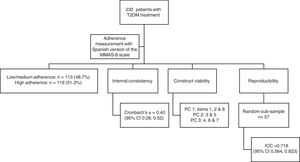
Results232 patients met the inclusion criteria. The Cronbach's α coefficient was 0.40 (95% CI 0.28–0.52). The exploratory principal component analysis showed three components. The intraclass correlation coefficient was 0.718 (95% CI 0.564–0.823).
Conclusionsthe Spanish version of the Morisky Medication Adherence scale showed low internal consistency, the exploratory factor analysis identified three dimensions, and the test–retest reliability was acceptable, therefore, psychometric properties of MMAS-8 are not suitable for measuring medication adherence in type 2 diabetes mellitus patients from Spain.
Validar la herramienta de adherencia terapéutica de Morisky tras una adaptación cultural y lingüística para su uso en la población española, y examinar sus propiedades psicométricas en pacientes con diabetes mellitus tipo 2 en España.
DiseñoSe realizó un estudio transversal en un hospital universitario en España.
ParticipantesSe incluyeron pacientes diagnosticados de diabetes mellitus tipo 2 al menos un año antes del estudio y tratados con antidiabéticos orales.
IntervenciónSe utilizó la versión traducida de la escala para medir la adherencia al tratamiento.
Mediciones principalesNivel de adherencia categorizado a través de 3 escalas, baja (<6 puntos), media (6 a <8 puntos) o alta (8 puntos). La consistencia interna del cuestionario se evaluó a través del coeficiente α de Cronbach, y se realizó un análisis exploratorio de los componentes principales para confirmar la validez del constructo, y se evaluó la fiabilidad a través del test-retest.
ResultadosDoscientos treinta y dos pacientes cumplieron con los criterios de inclusión. El coeficiente α de Cronbach era 0,40 (IC 95%: 0,28-0,52). El análisis exploratorio mostró 3 componentes principales. El coeficiente de correlación intraclase era de 0,718 (IC 95%: 0,564-0,823).
ConclusionesLa versión adaptada de la escala de adherencia terapéutica de Morisky mostró una consistencia interna baja, el análisis factorial exploratorio identificó 3 dimensiones y la fiabilidad test-retest fue aceptable, por lo tanto, las propiedades psicométricas de MMAS-8 no son adecuadas para medir la adherencia a la medicación en pacientes con diabetes mellitus tipo 2 en España.
The World Health Organization defines diabetes mellitus as “a chronic disease that occurs either when the pancreas does not produce enough insulin, or when the body cannot effectively use the insulin it produces”.1 An estimated 422 million adults were living with diabetes worldwide in 2014, compared with 108 million in 1980. The age-standardised global prevalence of diabetes practically doubled during this period, increasing from 4.7% to 8.5% in the adult population.1 In 2012, Soriguer et al.2 reported that the overall prevalence of diabetes was 13.8% (adjusted for age and sex) in Spain.
For patients with diabetes to fully benefit from their drug treatment, they must follow the prescription provided by their health care provider; the extent to which they do so is known as medication adherence.3 Unfortunately, poor therapeutic adherence is common among people suffering from chronic diseases, particularly type 2 diabetes patients.4–6 This has both health-related consequences – failure of treatment, rehospitalisation or death – and financial consequences, as it can result in expensive emergency medical interventions.7,8 Previous reviews and meta-analyses have examined interventions to promote adherence and improve glycaemic control, self-care behaviours and other key outcomes in diabetes mellitus patients.9,10
Several approaches can be adopted to assess adherence to medication regimens. Direct measures include assaying body fluids to detect the presence of the drug and observing how patients use their medication. Indirect measures include patient self-reports (e.g. with validated scales), pill counts (manual or with electronic devices such as the Medication Event Monitoring System MEMS), and reviews of dispensing or prescribing records or claims data.3,11,12
Standardised questionnaires are a common choice because they are cheap and fast. An updated version of the Morisky-Green-Levine13 questionnaire, the eight-item Morisky Medication Adherence Scale (MMAS-8),14 has been used in several studies to assess adherence to medication in chronic diseases. The MMAS-814,15 has been translated and adapted for use in diabetes patients in many countries16–19 but has not yet been validated for Spanish-speaking populations, which account for around 560 million people worldwide (at least 8.5% of whom have diabetes). In this study, we aimed to validate a translated and culturally adapted version of the MMAS-8 for use in Spanish population, and to examine the psychometric properties of this scale in patients with type 2 diabetes mellitus in Spain.
Materials and methodsEthicsThis study was approved by the Human Research Ethics Committee of the university hospital and was performed in accordance with the Helsinki Declaration of 1975. Potential participants were given an information sheet, and those who provided written informed consent were included in the study.
Study designThis cross-sectional study was conducted in a single university hospital in Spain between September 2016 and May 2017. The hospital provides primary and secondary care for patients with diabetes mellitus. We identified eligible patients from the electronic health records, and invited them to participate at their routine diabetes check-up.
The inclusion criteria were: patients diagnosed with type 2 diabetes mellitus at least 1 year before inclusion, being treated with anti-diabetic medication (at least one drug), aged 18 years or older, who gave informed consent, and whose clinical data was included in the electronic medical records. The Spanish national healthcare system covered the cost of all drug prescriptions. The exclusion criteria were: patients with neurological or psychological impairments and/or cognitive defects that could prevent them from completing the questionnaire; patients with a life expectancy of less than one year; patients whose pathological situation could interfere with their participation in the study (physical, social or psychological problems that could prevent them from adhering to treatment); patients who did not understand the questionnaire due to language or literacy; institutionalised geriatric patients; patients who did not want to respond to the survey or who did not complete the questionnaire; patients with in-hospital prescriptions, a private healthcare centre or other insurer, or taking non-prescription drugs; and patients not covered by the Spanish national healthcare system.
Study variablesAt the inclusion visit, after the routine check-up, a study investigator asked participants to fill out a questionnaire including the following variables: age (years), sex (man/woman), level of education (none/primary/secondary/university), employment status (working/retired/unemployed), smoking status (smoker/non-smoker/former smoker) and exercise (high/moderate/low). We collected the following variables from the check-up report (from the same day): body mass index (BMI, in kg/m2) and HbA1c levels (<7%/≥7%).
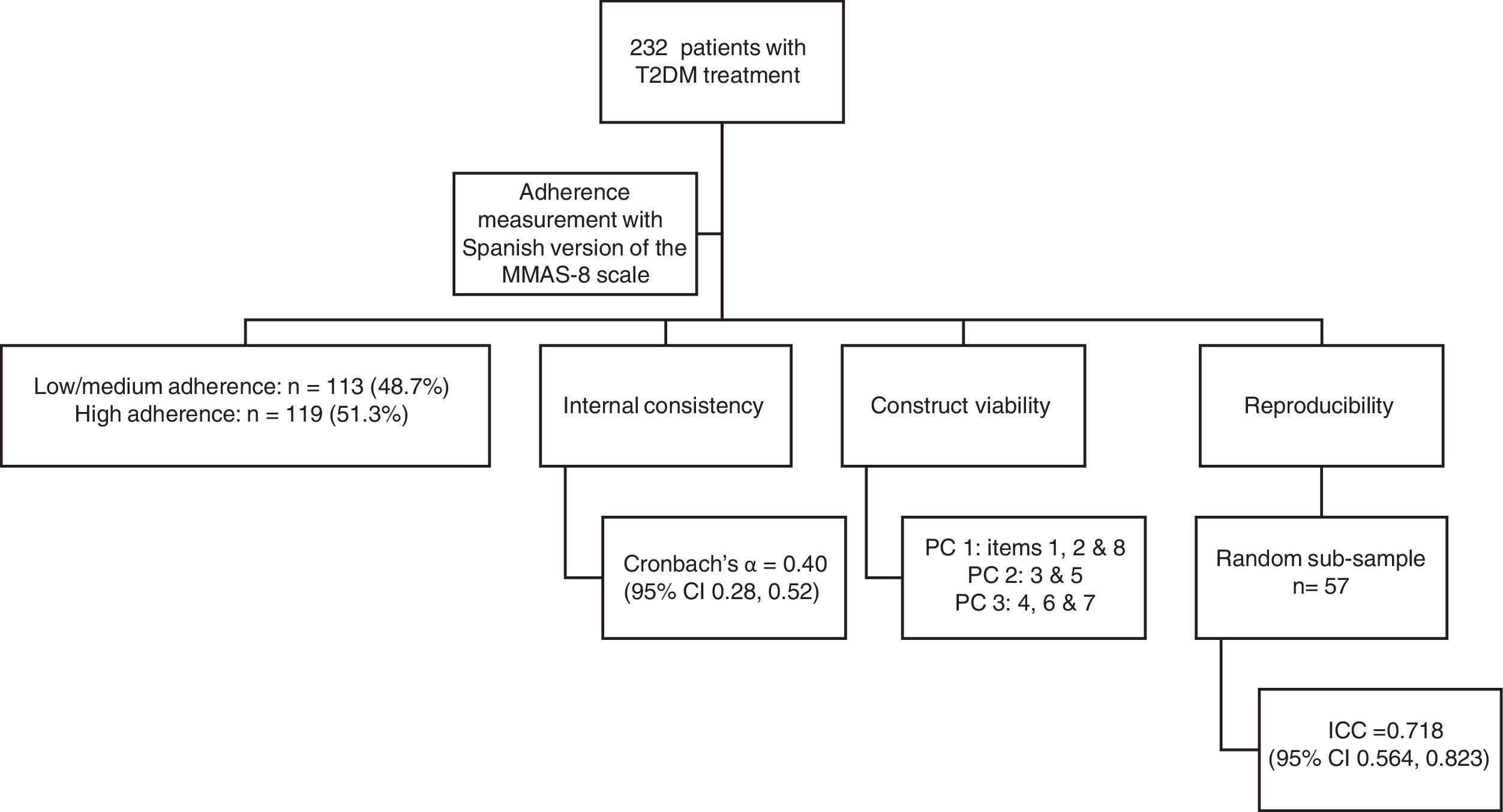
Adherence measurement instrumentWe used the Spanish version of the MMAS-8 scale to measure treatment adherence. Response categories are yes/no for each item with a dichotomous response and a 5-point Likert response for the last item. The three level categorical scale is broken down into low adherence (score of <6), medium adherence (score of 6 to <8) and high adherence (score of 8). Use of the ©MMAS is protected by United States copyright laws. Professor Donald E. Morisky, who developed the MMAS-8, provided us with the Spanish translation and gave us permission to use it in this study (Supplemental Data 1). Before beginning the main study, we conducted a pilot study in 25 type 2 diabetes patients aged over 18 years (Fig. 1).
Statistical analysisWe included 232 patients in our study which represents a ratio (number of patients: number of items) of 29:1- or 29 patients per item – to assess the validity of the questionnaire. This ratio is higher than the recommended ratio by the guideline of tool validation and adaptation20 due to the ease of recruitment of patients. Thus, this sample size ensured a proportion of correct factor structures of 70%, as recommended by Costello et al.,21 and an accuracy of ±0.038 was achieved for a 95% confidence interval of a Cronbach's α coefficient of 0.8.
To validate the questionnaire, we first measured internal consistency through Cronbach's α and its 95% CI for the questionnaire as a whole and deleting each item individually. This coefficient indicates whether each item is appropriate for determining the underlying concept of the scale.22 Generally, values greater than or equal to 0.5 indicate satisfactory internal consistency.23 Secondly, we confirmed construct validity through an exploratory principal component (PC) analysis and by applying the Kaiser criterion, extracting components with eigenvalues greater than 1; by applying varimax rotation we were able to better interpret the components. We calculated the scores for each item in each component and the percentage of item variance explained by the components. Thirdly, we assessed test–retest reliability by administering a second MMAS-8 to a random sample of participants who had been contacted and scheduled for a second visit at four weeks after the initial visit. The same investigator carried out the test and retest interviews. We calculated the intraclass correlation coefficient (ICC) for the before/after total score, and the Kappa coefficient and 95% CI for each item. All statistical analyses were carried out with SPSS v.18 R v. 3.2.5.
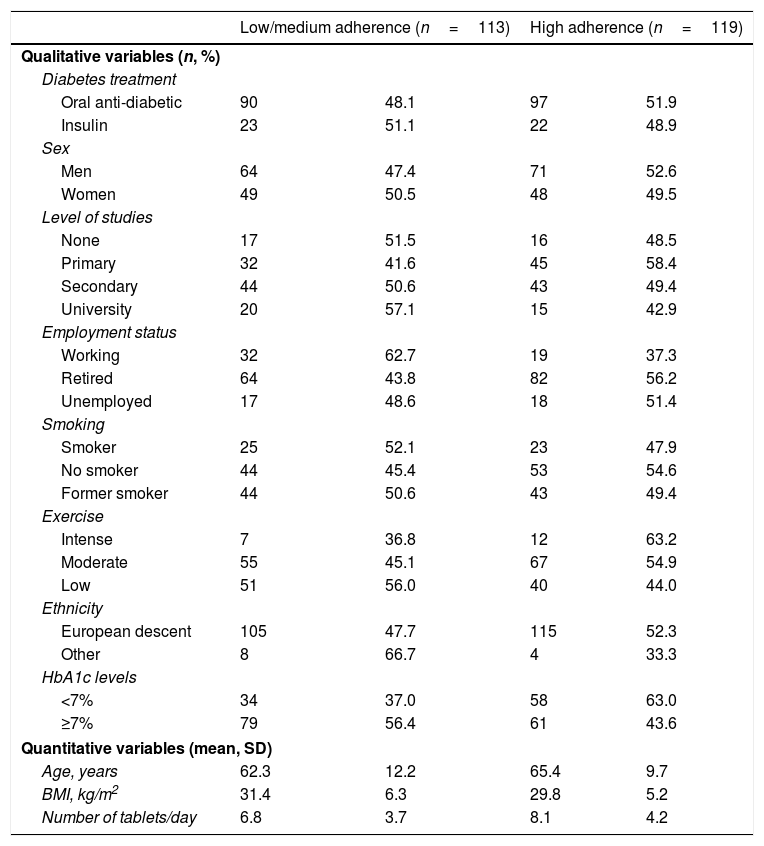
ResultsTherapeutic adherence assessmentA total of 232 patients with type 2 diabetes mellitus met the inclusion criteria during the recruitment period. Mean age of participants was 65.4 years (range 23–85 years); 58.2% (n=135) were men; mean BMI was 30.6kg/m2; 20.7% (n=48) were smokers; and 52.6% had secondary or university education. Of the 232 participants, 113 (48.7%) and 119 (51.3%) were in the low/medium and high adherence categories, respectively. Table 1 shows the characteristics of the participants according to therapeutic adherence category.
Characteristics of participants according to therapeutic adherence category (N=232).
| Low/medium adherence (n=113) | High adherence (n=119) | |||
|---|---|---|---|---|
| Qualitative variables (n, %) | ||||
| Diabetes treatment | ||||
| Oral anti-diabetic | 90 | 48.1 | 97 | 51.9 |
| Insulin | 23 | 51.1 | 22 | 48.9 |
| Sex | ||||
| Men | 64 | 47.4 | 71 | 52.6 |
| Women | 49 | 50.5 | 48 | 49.5 |
| Level of studies | ||||
| None | 17 | 51.5 | 16 | 48.5 |
| Primary | 32 | 41.6 | 45 | 58.4 |
| Secondary | 44 | 50.6 | 43 | 49.4 |
| University | 20 | 57.1 | 15 | 42.9 |
| Employment status | ||||
| Working | 32 | 62.7 | 19 | 37.3 |
| Retired | 64 | 43.8 | 82 | 56.2 |
| Unemployed | 17 | 48.6 | 18 | 51.4 |
| Smoking | ||||
| Smoker | 25 | 52.1 | 23 | 47.9 |
| No smoker | 44 | 45.4 | 53 | 54.6 |
| Former smoker | 44 | 50.6 | 43 | 49.4 |
| Exercise | ||||
| Intense | 7 | 36.8 | 12 | 63.2 |
| Moderate | 55 | 45.1 | 67 | 54.9 |
| Low | 51 | 56.0 | 40 | 44.0 |
| Ethnicity | ||||
| European descent | 105 | 47.7 | 115 | 52.3 |
| Other | 8 | 66.7 | 4 | 33.3 |
| HbA1c levels | ||||
| <7% | 34 | 37.0 | 58 | 63.0 |
| ≥7% | 79 | 56.4 | 61 | 43.6 |
| Quantitative variables (mean, SD) | ||||
| Age, years | 62.3 | 12.2 | 65.4 | 9.7 |
| BMI, kg/m2 | 31.4 | 6.3 | 29.8 | 5.2 |
| Number of tablets/day | 6.8 | 3.7 | 8.1 | 4.2 |
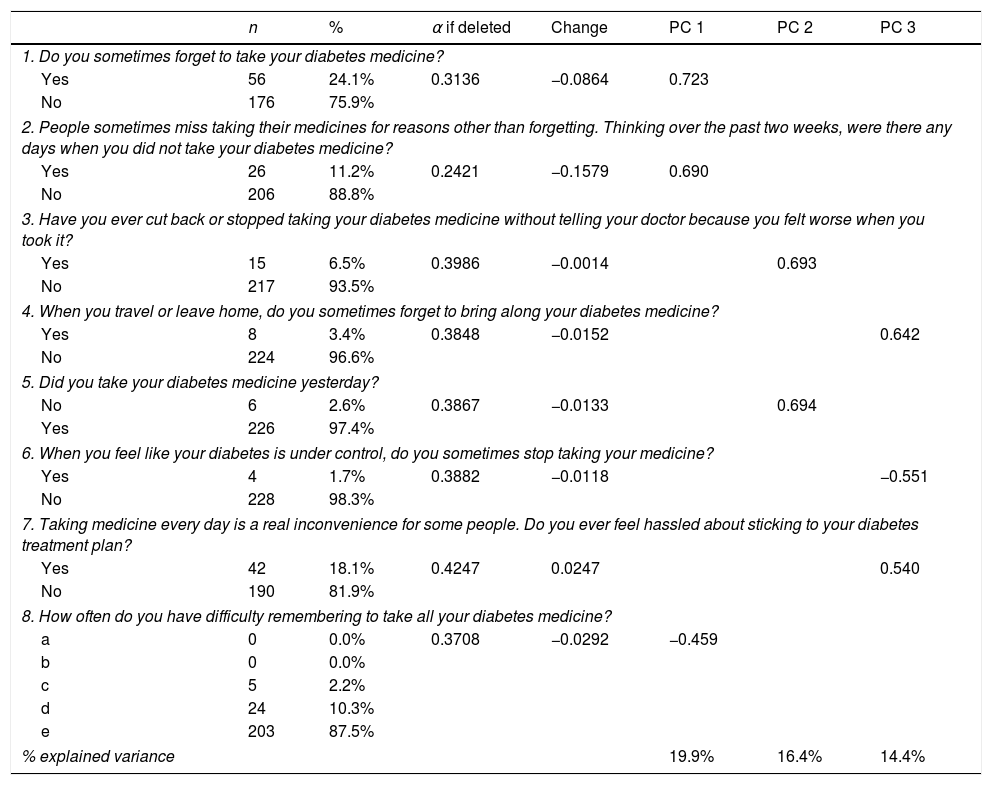
We calculated a Cronbach's α coefficient of 0.40 for the questionnaire (95% CI 0.28, 0.52), which reflects low internal consistency. Table 2 shows the number and percentage of responses in each item, and Cronbach's α if the corresponding item is deleted. Only item 7 negatively had a small effect on reliability: if it were deleted, the α coefficient would increase to 0.42.
Number and frequencies of responses; internal consistency and exploratory factor analysis.
| n | % | α if deleted | Change | PC 1 | PC 2 | PC 3 | |
|---|---|---|---|---|---|---|---|
| 1. Do you sometimes forget to take your diabetes medicine? | |||||||
| Yes | 56 | 24.1% | 0.3136 | −0.0864 | 0.723 | ||
| No | 176 | 75.9% | |||||
| 2. People sometimes miss taking their medicines for reasons other than forgetting. Thinking over the past two weeks, were there any days when you did not take your diabetes medicine? | |||||||
| Yes | 26 | 11.2% | 0.2421 | −0.1579 | 0.690 | ||
| No | 206 | 88.8% | |||||
| 3. Have you ever cut back or stopped taking your diabetes medicine without telling your doctor because you felt worse when you took it? | |||||||
| Yes | 15 | 6.5% | 0.3986 | −0.0014 | 0.693 | ||
| No | 217 | 93.5% | |||||
| 4. When you travel or leave home, do you sometimes forget to bring along your diabetes medicine? | |||||||
| Yes | 8 | 3.4% | 0.3848 | −0.0152 | 0.642 | ||
| No | 224 | 96.6% | |||||
| 5. Did you take your diabetes medicine yesterday? | |||||||
| No | 6 | 2.6% | 0.3867 | −0.0133 | 0.694 | ||
| Yes | 226 | 97.4% | |||||
| 6. When you feel like your diabetes is under control, do you sometimes stop taking your medicine? | |||||||
| Yes | 4 | 1.7% | 0.3882 | −0.0118 | −0.551 | ||
| No | 228 | 98.3% | |||||
| 7. Taking medicine every day is a real inconvenience for some people. Do you ever feel hassled about sticking to your diabetes treatment plan? | |||||||
| Yes | 42 | 18.1% | 0.4247 | 0.0247 | 0.540 | ||
| No | 190 | 81.9% | |||||
| 8. How often do you have difficulty remembering to take all your diabetes medicine? | |||||||
| a | 0 | 0.0% | 0.3708 | −0.0292 | −0.459 | ||
| b | 0 | 0.0% | |||||
| c | 5 | 2.2% | |||||
| d | 24 | 10.3% | |||||
| e | 203 | 87.5% | |||||
| % explained variance | 19.9% | 16.4% | 14.4% | ||||
% total explained variance: 50.7%.
PC, principal component.
The exploratory PC analysis (Table 2), conducted to examine construct viability, showed three PCs with eigenvalues greater than 1, explaining 50.7% of the total variance. PC 1 included items 1, 2, and 8, which are related to forgetting – and having difficulty remembering – to take medicine. PC 2 comprised items 3 and 5, which ask patients if they stop their medicine when they feel worse, and whether they took their medicine the day before. PC 3 included items 4, 6 and 7, related to patients forgetting their medicine sometimes or stopping treatment when they feel better, and the convenience of treatment (Table 2).
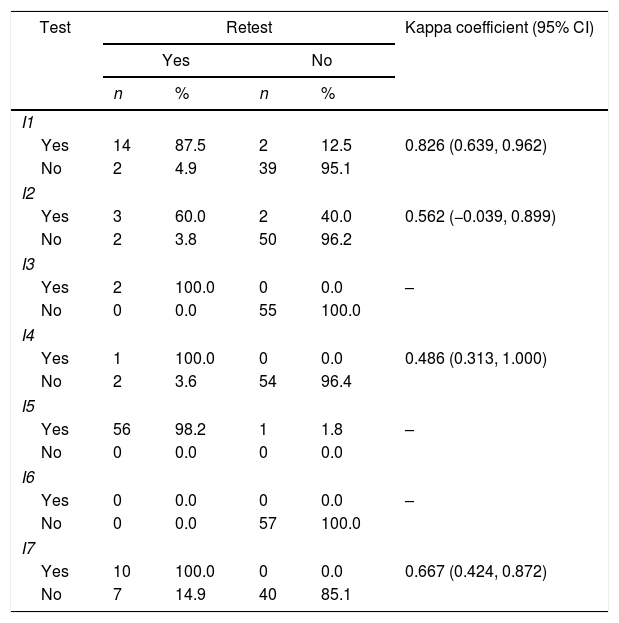
To test reproducibility, we asked 57 patients to complete the MMAS-8 in a face-to-face interview four weeks after the initial visit. This produced an intraclass correlation coefficient of 0.718 (95% CI 0.564, 0.823). Items 1, 4, 7 and 8 had a significant Kappa concordance coefficient, while items 3, 5 and 6 showed perfect concordance; only item 2 did not show a significant concordance coefficient. We also found a significant Kappa coefficient for adherence (Table 3).
Test–retest results.
| Test | Retest | Kappa coefficient (95% CI) | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| n | % | n | % | ||
| I1 | |||||
| Yes | 14 | 87.5 | 2 | 12.5 | 0.826 (0.639, 0.962) |
| No | 2 | 4.9 | 39 | 95.1 | |
| I2 | |||||
| Yes | 3 | 60.0 | 2 | 40.0 | 0.562 (−0.039, 0.899) |
| No | 2 | 3.8 | 50 | 96.2 | |
| I3 | |||||
| Yes | 2 | 100.0 | 0 | 0.0 | – |
| No | 0 | 0.0 | 55 | 100.0 | |
| I4 | |||||
| Yes | 1 | 100.0 | 0 | 0.0 | 0.486 (0.313, 1.000) |
| No | 2 | 3.6 | 54 | 96.4 | |
| I5 | |||||
| Yes | 56 | 98.2 | 1 | 1.8 | – |
| No | 0 | 0.0 | 0 | 0.0 | |
| I6 | |||||
| Yes | 0 | 0.0 | 0 | 0.0 | – |
| No | 0 | 0.0 | 57 | 100.0 | |
| I7 | |||||
| Yes | 10 | 100.0 | 0 | 0.0 | 0.667 (0.424, 0.872) |
| No | 7 | 14.9 | 40 | 85.1 | |
| a | b | c | d | e | ||
|---|---|---|---|---|---|---|
| I8 | ||||||
| a | 0 | 0 | 0 | 0 | 0 | 0.513 (0.240, 0.811) |
| b | 0 | 0 | 0 | 0 | 0 | |
| c | 0 | 0 | 0 | 1 | 0 | |
| d | 0 | 0 | 0 | 4 | 0 | |
| e | 0 | 0 | 2 | 4 | 46 | |
| Low | Medium | High | ||
|---|---|---|---|---|
| Adherence n (%) | ||||
| Low | 2 (66.7) | 1 (33.3) | 0 (0.0) | 0.633 (0.455, 0.798) |
| Medium | 3 (12.5) | 20 (83.3) | 1 (4.2) | |
| High | 1 (3.3) | 6 (20.0) | 23 (76.7) | |
I: item; 95%CI: 95% confidence interval.
The Spanish version of the MMAS-8 scale showed low internal consistency in patients from one Spanish hospital who had been diagnosed with type 2 diabetes mellitus at least one year before inclusion and were being treated with anti-diabetic medication. The exploratory factor analysis identified three dimensions, and the test–retest reliability was acceptable. The scale classified 51.3% of participants as highly adherent.
The psychometric properties of the Spanish version of the MMAS-8 differed slightly from those of other versions, especially in terms of internal consistency. The Cronbach's α was below the acceptable level of 0.7 in our study, whereas Morisky et al.14 found a Cronbach's α of 0.83 in patients diagnosed with hypertension using the original MMAS-8 (n=1367). Other authors have used different non-English language versions of the MMAS-8 in people with diabetes and reported different α values. The Malaysian (α=0.66),16 Korean (α=0.66)18 and Arabic (α=0.70)19 versions produced higher values.
Cronbach's α may underestimate the reliability of the questionnaire depending on its length and dimensionality.24 Our findings show that the Spanish version of the MMAS-8 had three dimensions with eigenvalues greater than 1, explaining 50.7% of the total variance (PC 1: items 1, 2 and 8; PC 2: 3 and 5; PC 3: 4, 6 and 7). The Malaysian16 and Korean18 scales also had three dimensions. In contrast, the original MMAS-814 was unidimensional. A multidimensional test could explain why α was lower than the acceptable level of 0.7 in our study. Although none of the items in the MMAS-8 are directly related to methods for remembering to take medicine, and this might be an important aspect of therapy adherence, the item 4 of the questionnaire addresses planning ahead and anticipation of situations patient need to be more proactive which is the most important issue.
We found that the Spanish version of the MMAS-8 had acceptable test–retest reliability (ICC=0.72), and therefore good temporal stability. The Malaysian16 and Korean18 versions obtained ICCs of 0.82 and 0.79, respectively. The Malaysian16 investigators, like us, retested after one month, while other authors22,25 who obtained higher test–retest reliability values waited only two weeks. This suggests that adherence to pharmacological recommendations is less likely to change in such a short period of time. The observed differences between results may be explained by the fact that “reliability of scales like the MMAS-8 medication adherence scale depend on health care practices, culture and education level of participants”.26
One limitation of our study was that patients were recruited in a single hospital. Although this hospital offers both primary and secondary care, further research is necessary to corroborate results obtained in different populations in different clinical settings. The strengths of our study included its prospective nature and the consecutive inclusion of patients. In addition, the Spanish national healthcare system is public and covers 99% of the population, and the whole population has access to medicines because they are partially financed by the national health system.
In conclusion, our study found that psychometric properties of MMAS-8 are not suitable for measuring medication adherence in type 2 diabetes mellitus patients from Spain.
- •
Poor therapeutic adherence is common among people with type 2 diabetes patients.
- •
To assess adherence to medication regimens is important to improve glycaemic control.
- •
The MMAS-8 has not been validated for Spanish-speaking diabetes patients.
- •
The Spanish version of the MMAS-8 scale showed low internal consistency in patients with type 2 diabetes.
- •
The Spanish version of the MMAS-8 had good temporal stability in patients with type 2 diabetes.
The present investigation has not received specific aid from agencies from the public sector, commercial sector or non-profit entities.
Conflict of interestsThe authors declare that they have no conflict of interest.
The MMAS (8-item) content, name, and trademarks are protected by US copyright and trademark laws. Permission for use of the scale and its coding is required. A license agreement is available from Donald E. Morisky, ScD, ScM, MSPH, 14725 NE 20th St Bellevue, WA 98007, USA; dmorisky@gmail.com.