Scimitar syndrome consists in a rare malformation characterized by a partial abnormal connection in one or both right pulmonary veins to the inferior vena cava, right lung hypoplasia, and systemic circulation from the descending aorta. Scimitar syndrome is occasionally associated with other congenital malformations, such as patent ductus arteriosus (PDA).
Case reportWe report a 4-year-old patient with an “adult” variety of scimitar syndrome associated with symptomatic PDA, which was successfully occluded using a retrograde guidewire-established femoral arteriovenous loop with an Amplatzer® PDA occluder, without complications.
ConclusionsScimitar syndrome is complex and requires a complete hemodynamic study for the determination of the appropriate treatment. Pulmonary arterial hypertension is a factor associated with poor prognosis.
El síndrome de la cimitarra consiste en una rara malformación, caracterizada por una conexión anómala parcial de una o ambas venas pulmonares derechas a la vena cava inferior, hipoplasia de pulmón derecho y circulación sistémica desde la aorta descendente. El síndrome de la cimitarra en ocasiones se asocia con otras malformaciones congénitas, entre las que se incluye la persistencia del conducto arterioso (PCA).
Caso clínicoPaciente de sexo femenino de cuatro años de edad con síndrome de la cimitarra, variedad “adulto”, asociado con PCA sintomático. Se realizó exitosamente oclusión del conducto mediante un asa arteriovenosa femoral con un dispositivo Amplatzer® PDA, sin complicaciones.
ConclusionesEl manejo del síndrome de la cimitarra es complejo y amerita de un estudio hemodinámico completo para determinar el tratamiento adecuado. La hipertensión arterial pulmonar es un factor de mal pronóstico.
Patent ductus arteriosus (PDA) is considered among the most frequent cardiovascular congenital malformations, with an incidence of 5.3 to 11% of all congenital cardiac defects.1 At the Hospital Infantil de Mexico Federico Gomez (HIMFG), PDA is the second most frequent congenital cardiac defect according to the biostatistics file, only topped by interventricular communication.
Furthermore, the scimitar syndrome (SS) consists in a rare malformation characterized by the connection of the right pulmonary veins to the inferior vena cava (which appears as a Muslim curved saber on a radiography), right lung hypoplasia with dextrocardia and anomalous collateral systemic circulation from the descending aorta to the right lung base. The reported incidence is of 1–3 patients born alive and 3 to 5% of all partial anomalous connections of the pulmonary veins.2 Despite its rarity, the coexistence of SS and Fallot's tetralogy has been reported with malformations, such as aortic coarctation, left ventricular hypoplasia and right ventricle double outlet.3 Other malformations, such as interatrial communication or PDA, are considered common associations to the SS and sometimes are necessary to maintain hemodynamic stability in the patient.
As well as a surgical plan for the SS are very complex and must be designed according to clinical symptoms and associated anomalies. In general terms, two clinical types of SS have been described. The pediatric form in which pulmonary arterial hypertension (PAH) is predominant, which in turn increases considerably the mortality risk and the adult form, which has a good prognosis with less or no symptoms at all, with the absence of PAH and in which surgical treatment is irrelevant.
We describe the case of a 4-year-old patient with moderate PDA, who underwent the placement of an Amplatzer® device using an arteriovenous loop since the exaggerated dextrocardia impeded the usual anterograde placement of the guide and sheath.
2Clinical caseThe case of a 48-month-old female toddler, product of the fourth pregnancy without anomalies, whose birth weight was 2900 g and height 48 cm is described. The mother referred that the patient suffered from bronchopneumonia when she was one year old without complications. The patient had no cardiovascular symptoms and was referred to the HIMFG when she was 18 months old, when a cardiac murmur was detected. The initial studies revealed dextrocardia on the chest X-ray situs solitus and augmented pulmonary flow; the electrocardiogram showed right ventricular hypertrophy and the Doppler echocardiogram confirmed SS associated with a moderate persistent ductus arteriosus, a 5 mm interatrial communication, and the connection between the right pulmonary veins with the inferior vena cava. Based on this data, it was decided to perform the closure of the persistent ductus arteriosus.
At the time of the arrival for the catheterism, the patient had a normal appearance, weighed 15.7 kg and a height of 108 cm. She had an I Ross functional class without cyanosis or acropachy, wide pulses on the four extremities, precordial right parasternal hyperactivity with precordial deformation because of left hemithorax augmentation and suprasternal fremitus. No visceromegaly was detected; at chest auscultation, a continuous IV/VI heart murmur was revealed at the second right intercostal space. The left lung was normally ventilated, and at auscultation, the murmur and cardiac sounds were detected at the right hemithorax.
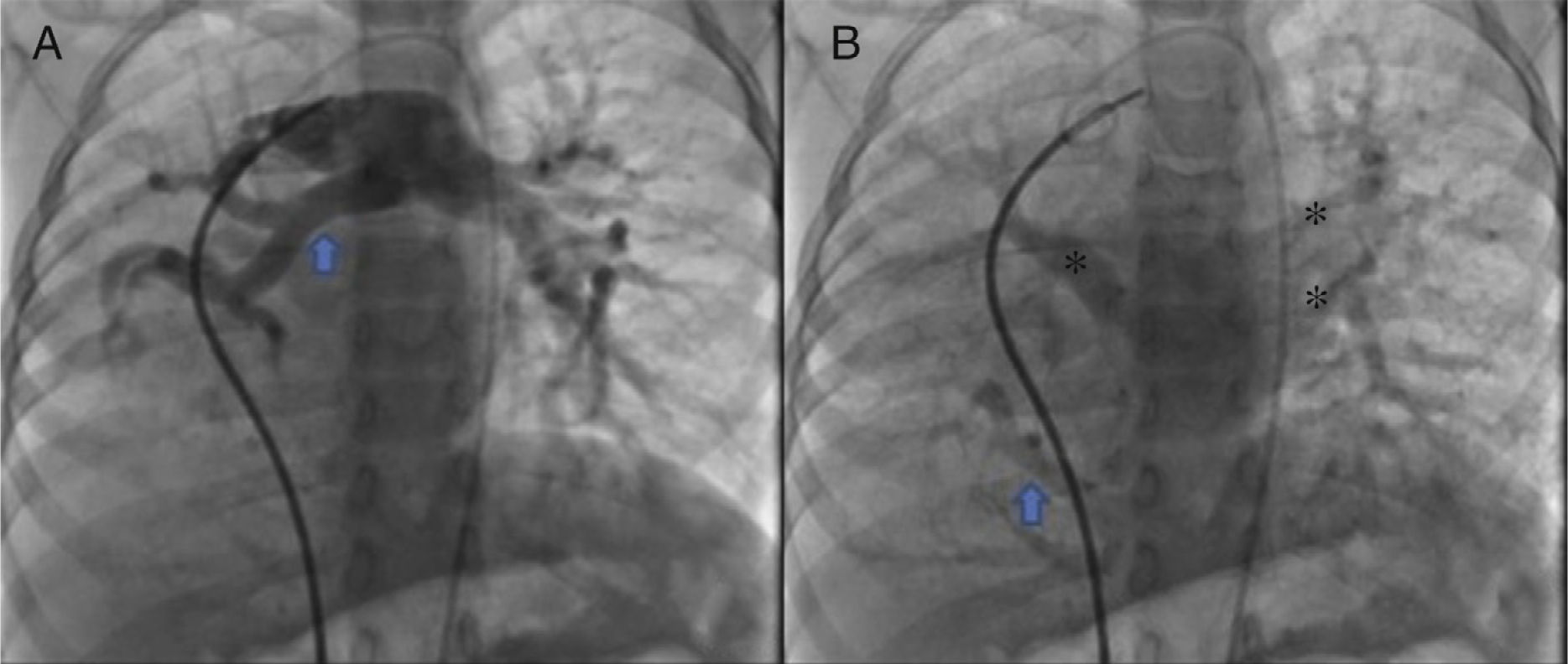
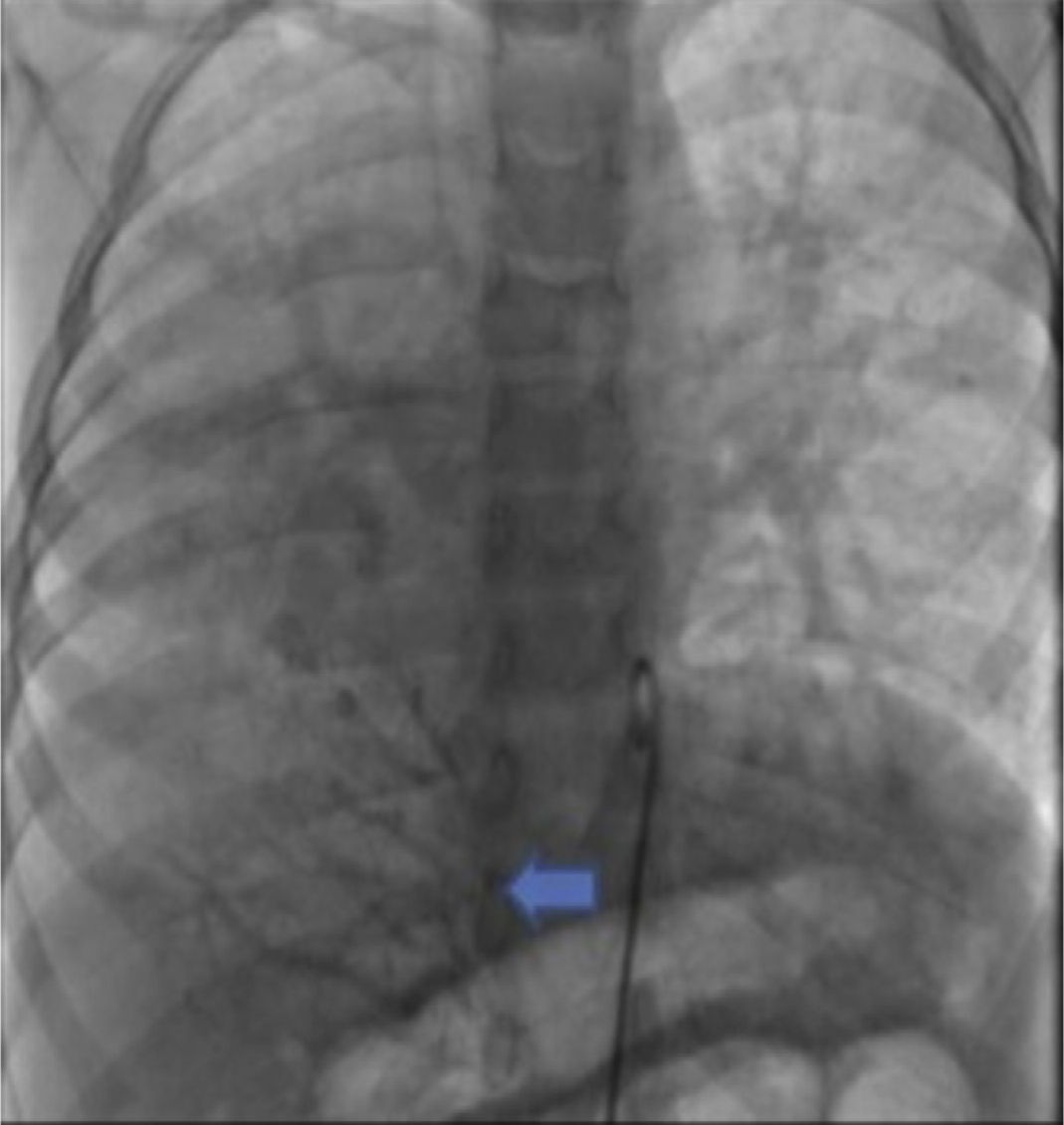
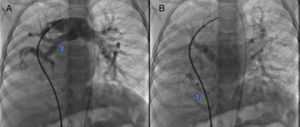
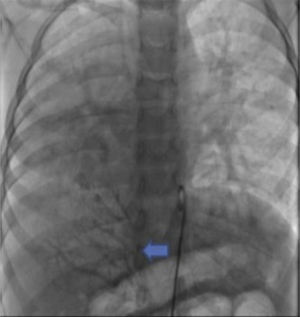
The parents signed the informed consent, and cardiac catheterism was performed according to an established protocol from the hemodynamics lab at the HIMFG, which consists in the administration of general balanced anesthesia and orotracheal intubation. We took samples for oximetry and probed cavity pressures obtaining these results: atrial pulmonary pressure of 38/14 with mean of 27 mmHg against the aortic of 70/25/45; oxygen saturation of 74% at the superior vena cava and 86% at the inferior vena cava, due to the right pulmonary drainage (this saturation was maintained up to the pulmonary artery). The mean pressure in both atria was 4 mmHg as well as in the right inferior pulmonary vein probed from the inferior vena cava; oxygen saturation in left cavities remained stable at 95%. With these data, we obtained a vascular pulmonary resistance of 1.95 μW, vascular systemic resistance of 12.4 μW, pulmonary output of 8.09 l/min and a systemic output of 2.32 l/min (QPQS=3.4:1), which indicated a great left to right short-cut through the ductus arteriosus and low vascular pulmonary resistance. Angiographies showed the frontal projection with contrast injection in the pulmonary stem; confluent pulmonary branches (Figure 1A), discrete diffuse hypoplasia of the right branch (diameter of 8.1 mm with Z of -2 vs a diameter of 14.4 mm in the left branch with a Z of +2) in the levo-phase (Figure 1B). It is observed that left pulmonary veins and the right superior drain to the left atrium (*) and that the right inferior pulmonary vein drains directly to the inferior vena cava with scarce flux, without obstruction evidence. Descending aorta frontal angiography shows scarce blood flow to the right lung base (Figure 2), particularly at segments 4, 5 and 10.
A. Frontal projection with contrast injection in the pulmonary stem; it is shown that pulmonary branches are confluent, discrete diffuse hypoplasia of the right branch (arrow). B. In the levo-phase pulmonary angiography, it is observed that left pulmonary veins and the right superior drain to the left atrium (*) and that the right inferior pulmonary vein drains directly to the inferior vena cava with scarce flux, without obstruction evidence (arrow).
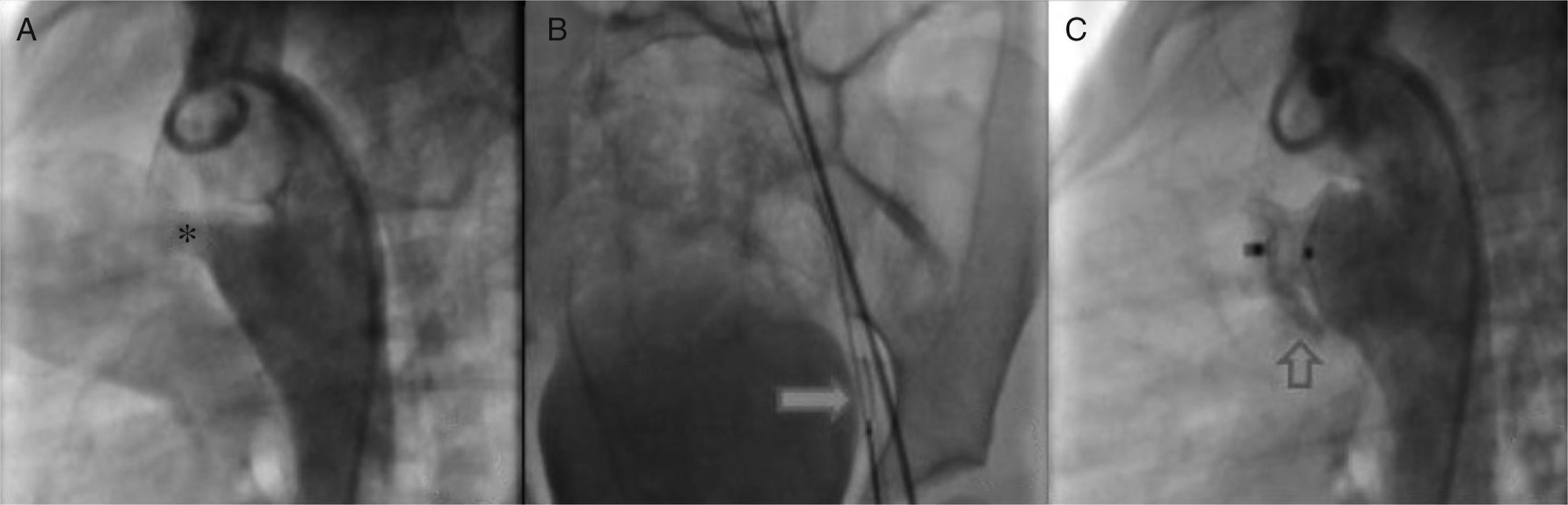
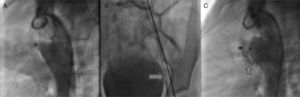
Once we determined that associated lesions were insignificant, we practiced a lateral angiography at the descending aorta in which a conical persistent ductus arteriosus with a 4.6 mm diameter and the pulmonary end, 19.49 mm at the aorta and length of 16.04 mm (Figure 3A) was detected. Subsequently, it was decided to block the PDA with an Amplatzer® device PDA 12/10.
A. Through the femoral artery, a MPB catheter was introduced to the ductus arteriosus ampulla. B. Through it, a 0.025″X260cm change guide was placed in the pulmonary stem, where it was placed with a 10mm Amplatzer® system Gooseneck Snare and was exteriorized through the vein, establishing the arteriovenous loop. C. Angiography to confirm the correct position of the device.
According to the persistent ductus arteriosus protocol, a 5Fr MPB catheter was introduced up to the pulmonary stem; using a hydrophilic 0.032″ guide we tried to access the ductus arteriosus without success. We obtained similar results with multipurpose and right Judkins catheters; thus, we decided to settle an arteriovenous loop. Through the femoral artery, an MPB was introduced up to the ductus arteriosus ampulla and, through it, a 0.025″X260 cm change guide was placed in the pulmonary stem, where it was placed with a 10 mm Amplatzer® system Gooseneck Snare and was exteriorized through the vein, establishing the arteriovenous loop (Figure 3B). This condition provided enough stability to introduce an 8 Fr Mullins sheath, achieving the successful closure of the ductus arteriosus.
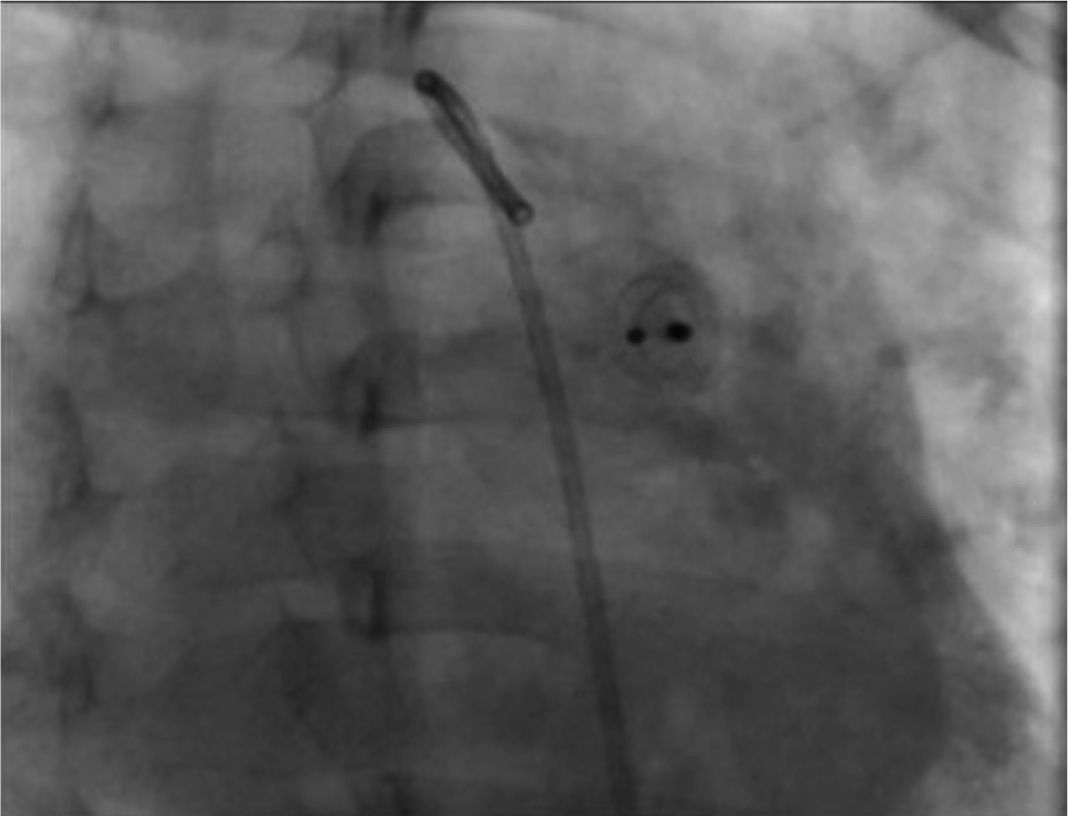
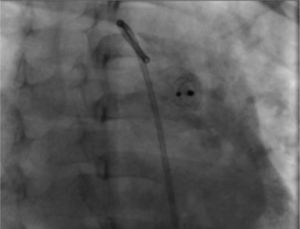
Ten minutes after the release of the device, we practiced an angiography in which we confirmed its correct position as well as a minimal residual short-cut (Figure 3C), which disappeared 24 hours after the intervention (corroborated by echocardiography). The pulmonary pressure after the closure of the ductus arteriosus decreased to 30 mmHg.
During the radiographic and echocardiographic follow-up at 24 hours and 1, 3 and 6 months, the device has been found in its original position and without residual short-cut (Figure 4).
3DiscussionClosure of the ductus arteriosus by catheterism has been an alternative to surgery since 1967, when Portsmann et al. achieved the closure of the ductus arteriosus using a polyvinyl alcohol plug (Ivalon®).4 This established the beginning of research with different devices that currently enable a high grade of success in the treatment of PDA.
In 1998, Masura et al. marked the begging of the clinical experience with the Amplatzer® device, made of an alloy of nickel and titanium (nitinol), which has demonstrated to be successful and versatile in lesions of moderate and great dimensions.5 At the beginning of this experience, the devices were prescribed for small and relatively large ductus arteriosus.6 However, modifications made to current devices allow their use in large and short structures and even in patients with pulmonary hypertension, widening their prescription to elders with calcified conducts or in patients with high risk for thoracotomy.
Current repertoire of devices is extensive and enables an appropriate selection according to morphology, particularly when they are conical. Surgical prescription has gradually diminished and currently is limited to premature patients, very small patients with severe symptoms, when it is difficult to use sheaths and extra-rigid guides or tubular very short conducts because, under these conditions, these devices do not have a structure that provides support or they may protrude and obstruct the descending aorta.7
Furthermore, the clinical presentation of the SS is variable and depends on the anomalies that integrate it. In 1976, Folger reported many extra-cardiac anomalies,8 such as horseshoe lung and right bronchial anatomical anomalies, which include diverse grades of hypoplasia and may result in surgical limitations, particularly in cases with pulmonary hypertension. At the beginning of the 90s, Dupuis et al. published two articles9,10 in which they differentiated the clinical manifestations of the pediatric and adult SS types. From 147 pediatric patients, the principal reported difference was that in the pediatric group (25 cases) pulmonary hypertension predominated secondary to stenosis of the anomalous connection, pulmonary kidnapping, right lung hypoplasia or to the associated anomalies (mainly left obstructions) that undermined prognosis. In contrast, in 122 of adult SS, clinical manifestations were minimal, with normal pulmonary pressure in 94 patients, slightly elevated in 28 and long term good prognosis.
- To take an appropriate therapeutic decision in patients with SS, some pathophysiological aspects must be considered:
- a)
The type and severity of the associated anomalies; in the present case, moderate PDA, which was responsible for the slight pulmonary pressure elevation
- b)
The amount of flow through the anomalous connection to the inferior vena cava; in this patient, there was scarce volume and did not cause hemodynamic alterations
- c)
The presence of an obstruction in the anomalous connection, which was ruled out in this patient
- d)
The magnitude of the vicariant circulation between the descending aorta and the right lung base; in this case, was minimal and did not cause clinical manifestations such as cardiac insufficiency, infection or hemoptysis
- e)
Right bronchial alterations, which were ruled out in this patient
It is evident that clinical symptoms were secondary exclusively to the PDA. Therefore, closure with an Amplatzer device was performed.
In the present case, the patient had right lung hypoplasia and extrinsic dextrocardia that caused an exaggerated angulation of the ductus arteriosus with respect to the pulmonary artery, which made impossible the anterograde cannulation of the descending aorta through the ductus arteriosus. For this reason, we decided to settle an arteriovenous loop that would enable us to place a sheath for the placement of a device in the ductus arteriosus.
Reports of this technique in the literature11,12 mention that the principal indications are tortuosity and calcification of the ductus arteriosus in adult patients, in whom excessive manipulation may cause rupture of the ductus arteriosus (the placement of the arteriovenous loop minimizes this risk); very small pulmonary end conducts that difficult the passage of a guide through the ductus arteriosus in an anterograde direction and excessive angulation of the ductus arteriosus, as in this patient.
Within the experience developed in the hemodynamic lab at the HIMFG, the case we report is the first in which the ductus arteriosus is closed by an arteriovenous loop in more than 200 PDA percutaneously treated cases. The majority of our cases occur in toddlers, in school-aged children and in adolescents in whom there has not been the calcification described in elders. In the cases with minuscule pulmonary ends, we opt for the utilization of Gianturco's spiral to the occlusion and, to this date, we have had excellent results.
In our experience, we have noted that the utilization of hydrophilic guides and multipurpose catheters facilitates the anterograde access to the ductus arteriosus and the descending aorta for the posterior exchange for extra-rigid guides and wide sheaths for the definitive placement of devices.
In conclusion, we report a pediatric patient with adult type SS, whose clinical manifestations were secondary to PDA. After six months of follow-up, the patient was found asymptomatic, with a complete occlusion of the ductus arteriosus, a 6 mm interatrial communication with a discrete left to the right short-cut and pulmonary pressure of 24 mmHg (measured with echocardiography). The patient continues her follow-up to determine the necessity of further transcatheter closure of the interatrial communication.
Finally, it is suggested that the therapeutic plan for SS cases must be individualized and the required procedure must be precisely determined. The procedures include from the correction of the anomalous partial connection of the pulmonary veins, the closure of the interatrial communication that is typically associated with the syndrome, the embolization of the vicariant aortopulmonary circulation, to the extreme cases of pneumonectomy when the SS is associated with bronchial alterations, or to simply provide palliative treatment for pulmonary hypertension.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare no conflict of interests.
Please cite this article as: Arévalo SLA, Solano FL, Villatoro FJL. Cierre percutáneo de conducto arterioso mediante un asa arteriovenosa en un paciente con síndrome de la cimitarra. Bol Med Hosp Infant Mex. 2017;74:55–59.