This systematic review aims to report the current knowledge of retinoblastoma (Rb) and its implications in Mexico. We analyzed clinical and demographic data of patients with Rb at select hospitals with Rb programs or that treat and refer patients with Rb, and identified the gaps in practice. We propose solutions to improve diagnosis, provide adequate treatment, and improve patient uptake.
MethodsA general review was conducted on PubMed of peer-reviewed literature on Rb in Mexico. Ophthalmology Department Heads or Directors of Rb programs at seven hospitals in Mexico were contacted for data available on their patients with Rb.
ResultsFive hospitals provided clinical data on 777 patients with Rb in a period spanning 2000-2015. Of the 122 patients with treatment, 83.4% underwent enucleation. From 33 to 45.3% of Rb tumors in Mexico reach an advanced intraocular stage of development. Knowledge of the disease is limited, despite the fact that the Mexican Retinoblastoma Group has elaborated Rb treatment guidelines and is developing a national Rb registry. Especially in the Southern states, prevalence and outcomes are comparable to African and Asian countries, and only few patients are referred to national treatment centers. Only three institutions have comprehensive Rb programs.
ConclusionsThere is an immediate need in Mexico to expand primary care providers’ knowledge of Rb and to expand and upgrade current Rb programs to meet the needs of the population adequately. Diagnosis and care of Rb patients in Mexico can also be improved by the establishment of a national Rb registry and a national early detection program, and by increased use of the national treatment protocol.
Esta es una revisión sistemática de los conocimientos actuales del retinoblastoma (Rb) y sus implicaciones en los centros de referencia más importantes del país. Se presenta un análisis situacional de los programas de Rb en México, se identificaron las brechas en la práctica, y se proponen soluciones para mejorar el diagnóstico, tratamiento y referencia oportuna de pacientes.
MétodosSe realizó una revisión general de la literatura publicada sobre Rb en México a través de PubMed. Los datos sociodemográficos de pacientes con Rb fueron obtenidos a través de los directores de programas de retinoblastoma en siete hospitales.
ResultadosCasi una tercera parte de los casos Rb se diagnostican en estadios avanzados. A pesar de la existencia del Grupo Mexicano de Retinoblastoma, el conocimiento de esta patología entre los médicos es limitado. Las diferencias en el tratamiento son notorias en el sur del país, donde la prevalencia y los resultados son comparables con África y Asia. Solamente tres instituciones a nivel nacional tienen un programa establecido de Rb.
ConclusionesExiste la necesidad inmediata de consolidar los programas de Rb para cubrir las necesidades reales de la población. Se requiere mejorar la educación del médico de primer contacto, establecer el registro nacional de casos y el programa de detección temprana, establecer los programas de salvamento ocular nacional, y reforzar las instituciones que brindan tratamiento.
This systematic review aims to report the current knowledge of retinoblastoma and its implications in Mexico. Retinoblastoma (Rb) is the most common intraocular malignancy in Mexican children, occurring in 24 persons per million population.1–3 The incidence of Rb among children in an upper middle-income country (UMIC)4 as Mexico, has been estimated to be as high as 56 children per million, which parallels North American-European trends in cancer.5,6 However, the incidence rate in the poorest Mexican state of Chiapas would be comparable to rates in Africa and India.5–7 It has been suggested that the incidence of Rb is underestimated in Mexico and higher than the global incidence, but due to the limited knowledge of cancer in Mexico and the lack of a national registry for pediatric cancer, it is difficult to understand the burden and impact of the disease clearly.1,5,6
Rb is a curable type of cancer when treatment is provided promptly following early diagnosis, with survival rates ranging from 90-95% in developed countries.8–10 However, survival rates are as low as 20% in developing countries,10 where poorer outcomes have been associated with late diagnosis and treatment, lower education levels of the mother, a lack of access to health services, and treatment abandonment by families of the patient.1,11,12 Abandonment rates for pediatric cancer, in general, have been found to range from 10 to 24% in Latin America, regardless of the availability of financial coverage.12 The lack of treatment provided to children with cancer in the region results in dire consequences. In Mexico, cancer is the leading cause of death in 5-9-year-old children and the fourth leading cause of death in children < 20 years of age.13
In a previous article,14 we presented a general review of the current knowledge and advances in Rb diagnosis and management. Provided that early detection and referral of patients with Rb be performed, it was explained that cancer is curable, with the primary treatment outcome being ocular salvage and sight preservation. Today, even eyes with more advanced intraocular tumors can be salvaged using advanced chemotherapeutic options, such as intra-arterial chemotherapy (IAC) and intravitreal chemotherapy (IVC). Unfortunately, developing countries are still lagging behind in early diagnosis and referral rates, adequate Rb management programs, and ocular salvage and survival rates.14 In this systemic review, we aim to report the current situation of Rb in Mexico, including the authors’ experience at the country's leading Rb centers. We will analyze demographic, and clinical data of patients with Rb at select hospitals with Rb programs or that treat and refer patients with Rb, and we will identify the gaps in practice and propose solutions to improve diagnosis, provide adequate treatment, and improve patient uptake. Results will be presented within the context of our key findings from our previous review14 on the general, universal knowledge of Rb, as they relate to the importance of an early diagnosis, conservative management, and advanced therapies for Rb.
2MethodsWe conducted a Pubmed search for peer-reviewed journal articles on Rb in Mexico using the terms “retinoblastoma” and “Mexico.” The initial search found 69 results on Rb in Mexico through September 2015. After reviewing the abstracts, the literature search was narrowed down to 19 articles1,3,5–7,15–27 that specifically addressed early detection, diagnosis, treatment, and disease management of Rb in Mexican patients. Two additional articles were included from Mexican journals not listed on PubMed.28,29
There are three hospitals with formal, comprehensive Rb management programs in Mexico: Hospital Infantil de México Federico Gómez (HIMFG) in Mexico City; Instituto Nacional de Pediatría (INP) in Mexico City; and Hospital Civil de Guadalajara Fray Antonio Alcalde (Hospital Civil) in Guadalajara, Jalisco. There are four additional hospitals known to the authors that treat and refer patients with Rb (based on our experiences with patients referred to the leading national referral Rb centers), but they do not have formal Rb programs. These hospitals are Hospital General “Dr. Gaudencio González” Centro Médico de la Raza (La Raza) in Mexico City; Centro Médico Nacional Siglo XXI in Mexico City; Hospital General in Tuxtla Gutierrez, Chiapas; and Hospital Infantil Teletón de Oncología (HITO) in Queretaro, Queretaro.
From July 2015 through September 2015, the authors requested that ophthalmologists at each facility provide demographic data on the number of patients with Rb. These data included sex, age at diagnosis, initial symptoms, duration of the disease, and stage of tumors according to International Classification of Intraocular Retinoblastoma (ICIR)30, as well as patient distribution by state for the past ten years. Data were collected through November 11, 2015.
Patient data on Rb in Mexico and the results of the literature research are presented in the context of the key findings from our previous review14, and the implications in Mexico for each finding are discussed.
3Results3.1Patient data on RbOphthalmologists from five hospitals provided patient clinical and demographic data on Rb: HIMFG, INP, Hospital Civil, La Raza, and HITO (Tables 1–5) (Ramirez-Ortiz MA, e-mail communication, August 15, 2015; Bosch-Canto V, e-mail communication, August 11, 2015; Gonzalez-Perez G, e-mail communication, October 25, 2015; Villavicencio Torres A, e-mail communication, October 22, 2015; Etulain Gonzalez A, e-mail communication, November 11, 2015). The geographic distribution of the patients with Rb by state at each hospital is also summarized in Table 6. The types of data, data completeness (versus missing data), and the years provided varied among hospitals, as shown in Tables 1–6. For example, clinical data were reported for different years at each hospital from January 2000 through November 2015. HIMFG reported clinical data for 179 patients over ten years spanning 2000-2010 (Table 1), but geographic distribution data were reported for 318 patients for 1997-2014 (Table 6). HITO only reported all patient data for October 2013 through November 2015 (Tables 5 and 6). Clinical data for 777 patients and 782 eyes were provided by the five hospitals, and geographic data for 880 patients were provided.
Patient data for retinoblastoma cases at the Hospital Infantil de México Federico Gómez in Mexico City, from 2000 to 2010 (Ramirez-Ortiz MA, e-mail, August 15, 2015).
| Clinical characteristic | Unilateral | Bilateral | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| n (%) | 109 | 60.9 | 70 | 39.1 | 179 | 100 |
| Sex, n=179 | ||||||
| Female | 61 | 56.0 | 37 | 52.9 | 98 | 54.7 |
| Male | 48 | 44.0 | 33 | 47.1 | 81 | 45.3 |
| Symptoms, n=174 | ||||||
| Strabismus | 54 | 31.0 | 33 | 19.0 | 87 | 50.0 |
| Leukocoria | 86 | 49.4 | 52 | 29.9 | 138 | 79.3 |
| ICIR Classification, n=178 eyes | ||||||
| Group A | 0 | 0.0 | 1 | 1.4 | 1 | 0.6 |
| Group B | 0 | 0.0 | 1 | 1.4 | 1 | 0.6 |
| Group C | 3 | 2.8 | 2 | 2.9 | 5 | 2.8 |
| Group D | 43 | 39.8 | 28 | 40.0 | 71 | 39.9 |
| Group E | 62 | 57.4 | 38 | 54.3 | 100 | 56.2 |
| Total | 108 | 100.0 | 70 | 100.0 | 178 | 100.0 |
| Mean age at diagnosis (months), n=174 | 27.7±19.6 (n=107) | N/A | 14.6±9.6 (n=67) | N/A | (-) | N/A |
| Mean lag time (months), n=174 | 6.7±9.2 (n=107) | N/A | 7.5±6.4 (n=67) | N/A | (-) | N/A |
| Mean follow-up time (months), n=174 | 5.0±3.4 (n=105) | N/A | 5.0±3.1 (n=69) | N/A | (-) | N/A |
| Deceased during follow-upa | 12 | 11.7 | 8 | 12.1 | 20 | 11.8 |
ICIR: International Classification of Intraocular Retinoblastoma.30
Patient data for retinoblastoma cases at the Instituto Nacional de Pediatría in Mexico City, from January 2005 through August 2015 (Bosch-Canto V, e-mail, August 11, 2015).
| Clinical characteristic | N | Percentage (%) |
|---|---|---|
| Total cases | 457 | 100 |
| Unilateral | 265 | 58.0 |
| Bilateral | 183 | 40.0 |
| Trilateral | 9 | 2.0 |
| Sex, n=351 | ||
| Female | 158 | 45.0 |
| Male | 193 | 55.0 |
| Symptoms | ||
| Strabismus | 48 | 10.5 |
| Leukocoria | 341 | 74.6 |
| Palpebral edema | 1 | 0.2 |
| Glaucoma | 3 | 0.7 |
| Proptosis | 15 | 3.3 |
| ICIR Classification, n=456 eyes | ||
| Group A | 1 | 0.2 |
| Group B | 59 | 12.9 |
| Group C | 106 | 23.2 |
| Group D | 109 | 23.9 |
| Group E | 181 | 39.7 |
| Mean age at diagnosis (months), n=457 | 23.1 (range: 1 – 159) | N/A |
| Mean follow-up time (months), n=334a | 19.5 (range: 1 – 144) | N/A |
ICIR: International Classification of Intraocular Retinoblastoma.30
Patient data for retinoblastoma cases at the Hospital Civil de Guadalajara Fray Antonio Alcalde in Guadalajara, Jalisco, from October 2001 through August 2015 (Gonzalez Perez G, e-mail, October 25, 2015).
| Clinical characteristic | Unilateral | Bilateral | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| n (%) | 61 | 64.9 | 27 | 28.7 | 94 | 100 |
| Sex, n=93 | ||||||
| Female | 32 | 52.5 | 10 | 37.0 | 44 | 46.8 |
| Male | 29 | 47.5 | 17 | 63.0 | 49 | 52.1 |
| Symptoms, n=89 | ||||||
| Strabismus | 10 | 60.7 | 1 | 37.1 | 11 | 11.7 |
| Leukocoria | 47 | 96.6 | 21 | 58.4 | 69 | 73.4 |
| Low vision | 1 | 1.1 | 0 | 0.0 | 1 | 1.1 |
| Glaucoma | 1 | 1.1 | 1 | 1.1 | 2 | 2.1 |
| Orbital cellulitis | 1 | 1.1 | 0 | 0.0 | 1 | 1.1 |
| Exotropia | 4 | 4.5 | 0 | 0.0 | 4 | 4.3 |
| Uveitis | 1 | 1.1 | 0 | 0.0 | 1 | 1.1 |
| Headache | 1 | 1.1 | 0 | 0.0 | 1 | 1.1 |
| Corneal edema | 0 | 0.0 | 1 | 1.1 | 1 | 1.1 |
| Extraocular nodule | 0 | 0.0 | 1 | 1.1 | 1 | 1.1 |
| Proptosis | 1 | 1.1 | 1 | 1.1 | 3 | 3.2 |
| Preventive exam | 1 | 1.1 | 1 | 1.1 | 2 | 2.1 |
| None | 0 | 0.0 | 0 | 0.0 | 1 | 1.1 |
| ICIR Classification, n=88 eyes | ||||||
| Group A | 1 | 1.7 | 12 | 27.3 | 13 | 12.6 |
| Group B | 4 | 6.8 | 2 | 4.5 | 6 | 5.8 |
| Group C | 5 | 8.5 | 6 | 13.6 | 11 | 10.7 |
| Group D | 16 | 27.1 | 10 | 22.7 | 26 | 25.2 |
| Group E | 33 | 55.9 | 14 | 31.8 | 47 | 45.6 |
| Total | 59 | 100.0 | 44 | 100.0 | 103 | 100.0 |
| Mean age at diagnosis (months), n=91 | 20.8 (range 1 - 108) | N/A | 6.2 (range 0.5-36) | N/A | 16 (range 0.5- 108) | N/A |
| Treatment, n=82 | ||||||
| Chemotherapy | 20 | 35.7 | 17 | 65.4 | 37 | 45.1 |
| Chemoreduction | 12 | 21.4 | 9 | 34.6 | 21 | 25.6 |
| Radiation | 1 | 1.8 | 0 | 0.0 | 1 | 1.2 |
| Enucleation | 49 | 87.5 | 15 | 57.7 | 64 | 78.0 |
| Laser/Thermotherapy | 5 | 8.9 | 13 | 50.0 | 18 | 22.0 |
| Cryotherapy | 3 | 5.4 | 8 | 30.8 | 11 | 13.4 |
ICIR: International Classification of Intraocular Retinoblastoma.30
Patient data for retinoblastoma cases at the Hospital General “Dr. Guadencio González Garza” del Centro Médico La Raza in Mexico City, from January 2012 through December 2014 (Villavicencio Torres A, e-mail, October 22, 2015).
| Clinical Characteristic | Unilateral | Bilateral | Trilateral | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| n (%) | 19 | 52.8 | 9 | 25.0 | 1 | 2.8 | 36 | 100 |
| Sex, n=33 | ||||||||
| Female | 9.0 | 47.4 | 4.0 | 44.4 | 1.0 | 100.0 | 15.0 | 45.5 |
| Male | 10.0 | 52.6 | 5.0 | 55.6 | 0.0 | 0.0 | 18.0 | 54.5 |
| Symptoms, n=26 | ||||||||
| Strabismus | 4.0 | 22.2 | 2.0 | 28.6 | 0.0 | 0.0 | 6.0 | 23.1 |
| Leukocoria | 13.0 | 72.2 | 7.0 | 100.0 | 1.0 | 100.0 | 21.0 | 80.8 |
| Palpebral edema | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| Low vision | 2.0 | 11.1 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 7.7 |
| Proptosis | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| Hypopyon | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| Buphthalmos | 1.0 | 5.6 | 1.0 | 14.3 | 0.0 | 0.0 | 2.0 | 7.7 |
| Mydriasis | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| Perelimbal staphyloma | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| Eye pain | 1.0 | 5.6 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 3.8 |
| ICIR Classification, n=26 eyes | ||||||||
| Group A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Group B | 0.0 | 0.0 | 2.0 | 13.3 | 0.0 | 0.0 | 2.0 | 5.9 |
| Group C | 0.0 | 0.0 | 4.0 | 26.7 | 0.0 | 0.0 | 4.0 | 11.8 |
| Group D | 9.0 | 52.9 | 5.0 | 33.3 | 1.0 | 50.0 | 15.0 | 44.1 |
| Group E | 8.0 | 47.1 | 4.0 | 26.7 | 1.0 | 50.0 | 13.0 | 38.2 |
| Total | 17.0 | 100.0 | 15.0 | 100.0 | 2.0 | 100.0 | 34.0 | 100.0 |
| Mean age at diagnosis (months), n=26 | 43.5 (range 1-120) | N/A | 7 (range 2-16) | N/A | 5.0 | N/A | 28.5 (range 1-120) | N/A |
| Mean follow-up time (months), n=22 | 20.2 (range: 1-58.5) | N/A | 19.0 (range: 0.03-79) | N/A | 40.0 | N/A | 20.8 (range: 0.03-58.5) | N/A |
| Deceased during follow-up, n=22 | 2 | 16.7 | 0 | 0.0 | 0 | 0.0 | 2 | 9.1 |
| Treatment, n=30 | ||||||||
| Enucleation | 18 | 94.7 | 9 | 100.0 | 1 | 100.0 | 28 | 93.3 |
| Cryotherapy | 0 | 0.0 | 3 | 33.3 | 0 | 0.0 | 3 | 10.0 |
| Laser | 0 | 0.0 | 3 | 33.3 | 0 | 0.0 | 3 | 10.0 |
| None | 1 | 5.3 | 0 | 0.0 | 0 | 0.0 | 1 | 3.3 |
ICIR: International Classification of Intraocular Retinoblastoma.30
Patient data for retinoblastoma cases at the Hospital Infantil Teletón de Oncología in Queretaro, Queretaro, from October 2013 through November 2015 (Etulain Gonzalez A, e-mail, November 11, 2015).
| Clinical characteristic | Unilateral | Bilateral | Trilateral | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| n (%) | 7 | 63.6 | 4 | 36.4 | 0 | 0.0 | 11 | 100 |
| Sex, n=11 | ||||||||
| Female | 3.0 | 42.9 | 2.0 | 50.0 | 0.0 | 0.0 | 5.0 | 45.5 |
| Male | 4.0 | 57.1 | 2.0 | 50.0 | 0.0 | 0.0 | 6.0 | 54.5 |
| Symptoms, n=11 | ||||||||
| Leukocoria | 7.0 | 100.0 | 2.0 | 50.0 | 0.0 | 0.0 | 9.0 | 81.8 |
| Orbital symptoms | 0.0 | 0.0 | 1.0 | 25.0 | 0.0 | 0.0 | 1.0 | 9.1 |
| None | 0.0 | 0.0 | 1.0 | 25.0 | 0.0 | 0.0 | 1.0 | 9.1 |
| ICIR Classification, n=11 eyes | ||||||||
| Group A | 0.0 | 0.0 | 1.0 | 25.0 | 0.0 | 0.0 | 1.0 | 9.1 |
| Group B | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Group C | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Group D | 5.0 | 71.4 | 2.0 | 50.0 | 0.0 | 0.0 | 7.0 | 63.6 |
| Group E | 2.0 | 28.6 | 1.0 | 25.0 | 0.0 | 0.0 | 3.0 | 27.3 |
| Total | 7.0 | 100.0 | 4.0 | 100.0 | 0.0 | 0.0 | 11.0 | 100.0 |
| Mean age at diagnosis (months), n=11 | 21.8 (range: 1.1-51.7) | N/A | 7.8 (range: 5-11.4) | N/A | N/A | N/A | 16.7 (range: 1.1-51.7) | N/A |
| Mean follow-up time (months), n=10 | 7.7 (range 0.2-20.4) | N/A | 11.5 (range: 2.3-21.8) | N/A | N/A | N/A | 8.8 (range: 0.2-21.8) | N/A |
| Deceased during follow-up, n=10 | 0 | 0.0 | 1 | 33.3 | N/A | N/A | 1.0 | 10.0 |
| Treatment, n=10 | ||||||||
| Enucleation | 7 | 100.0 | 3 | 100.0 | 0.0 | 0.0 | 10.0 | 90.9 |
ICIR, International Classification of Intraocular Retinoblastoma.30
Geographic distribution of patients (n=880) with retinoblastoma by state in Mexico.
| State | HIMFG Jan. 1997-Dec. 2014a | INP Jan. 2005-Aug. 2015b | Hospital Civil Oct. 2001-Aug. 2015c | La Raza Jan. 2012-Dec. 2014d | HITO Oct. 2013-Nov. 2015e | State totals | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Mexico City | 33 | 10.4 | 66 | 15.0 | 0 | 0.0 | 3 | 12.5 | 0 | 0 | 102 | 11.6 |
| Chihuahua | 0 | 0.0 | 5 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 5 | 0.6 |
| Sonora | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1 | 0.1 |
| Coahuila | 0 | 0.0 | 2 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 2 | 0.2 |
| Durango | 0 | 0.0 | 2 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 2 | 0.2 |
| Oaxaca | 12 | 3.8 | 21 | 4.8 | 0 | 0.0 | 0 | 0.0 | 1 | 9 | 34 | 3.9 |
| Tamaulipas | 0 | 0.0 | 4 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 4 | 0.5 |
| Jalisco | 0 | 0.0 | 2 | 0.5 | 67 | 77.0 | 0 | 0.0 | 0 | 0 | 69 | 7.8 |
| Zacatecas | 4 | 1.3 | 3 | 0.7 | 4 | 4.6 | 0 | 0.0 | 0 | 0 | 11 | 1.3 |
| Baja California Sur | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1.0 | 0.1 |
| Chiapas | 6 | 1.9 | 20 | 4.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 26 | 3.0 |
| Veracruz | 31 | 9.7 | 39 | 8.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 70 | 8.0 |
| Baja California | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1 | 0.1 |
| Nuevo Leon | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1 | 0.1 |
| Guerrero | 16 | 5.0 | 34 | 7.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 50 | 5.7 |
| San Luis Potosi | 0 | 0.0 | 8 | 2.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 8 | 0.9 |
| Michoacan | 22 | 6.9 | 27 | 6.1 | 5 | 5.7 | 0 | 0.0 | 0 | 0 | 54 | 6.1 |
| Campeche | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1 | 0.1 |
| Sinaloa | 0 | 0.0 | 1 | 0.2 | 1 | 1.1 | 0 | 0.0 | 0 | 0 | 2 | 0.2 |
| Quintana Roo | 0 | 0.0 | 4 | 0.9 | 0 | 0.0 | 4 | 16.7 | 2 | 18 | 10 | 1.1 |
| Yucatan | 0 | 0.0 | 3 | 0.7 | 0 | 0.0 | 1 | 4.2 | 1 | 9 | 5 | 0.6 |
| Puebla | 25 | 7.9 | 26 | 5.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 51 | 5.8 |
| Guanajuato | 13 | 4.1 | 30 | 6.8 | 0 | 0.0 | 0 | 0.0 | 3 | 27 | 46 | 5.2 |
| Nayarit | 0 | 0.0 | 0 | 0.0 | 8 | 9.2 | 0 | 0.0 | 0 | 0 | 8 | 0.9 |
| Tabasco | 0 | 0.0 | 5 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 5 | 0.6 |
| State of Mexico | 109 | 34.3 | 87 | 19.8 | 0 | 0.0 | 15 | 62.5 | 0 | 0 | 211 | 24.0 |
| Hidalgo | 33 | 10.4 | 16 | 3.6 | 0 | 0.0 | 1 | 4.2 | 0 | 0 | 50 | 5.7 |
| Queretaro | 3 | 0.9 | 10 | 2.3 | 0 | 0.0 | 0 | 0.0 | 4 | 36 | 17 | 1.9 |
| Colima | 0 | 0.0 | 0 | 0.0 | 2 | 2.3 | 0 | 0.0 | 0 | 0 | 2 | 0.2 |
| Aguascalientes | 0 | 0.0 | 2 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 2 | 0.2 |
| Morelos | 0 | 0.0 | 13 | 3.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 13 | 1.5 |
| Tlaxcala | 11 | 3.5 | 5 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 16 | 1.8 |
| Hospital totals | 318 | 100 | 440 | 100 | 87 | 100 | 24 | 100 | 11 | 100 | 880 | 100 |
HIMFG, Hospital Infantil de México Federico Gómez; INP, Instituto Nacional de Pediatría; Hospital Civil, Hospital Civil de Guadalajara Fray Antonio Alcalde; La Raza, Hospital General “Dr Gaudencio González Garza” del Centro Médico; HITO, Hospital Infantil Teletón de Oncología.
The three states home to the most number of patients with Rb were the State of Mexico (n=211; 24%), Mexico City (n=102; 11.6%), and Veracruz (n=70, 8.0%) (Table 6). Only 26 patients were from Chiapas, a state with a very high incidence rate of Rb at 22 patients per million children/year.5
Gender was known for 667 patients: 320 females (48%) and 347 males (52%) (Tables 1–5). According to the patient data collected from the five hospitals in this study, 45.3% of eyes diagnosed with Rb were advanced intraocular stages (Groups D and E). The distribution of the ICIR staging among 782 eyes was as follows: 16 eyes in Group A (2%), 68 eyes in Group B (8.7%), 126 eyes in Group C (16.1%), 228 eyes in Group D (29.2%), and 344 eyes in Group E (44%).
Additional patient data analysis from Tables 1–5 are discussed below in the context of our previous findings14 and from the review of Mexican literature.
4Discussion4.1Rb genetic analysis in MexicoIn Mexico, Rb screening focuses on the two traditional hereditary and non-hereditary forms of cancer through the detection of the RB1 mutation.18 However, a genetic sequencing study of 48 Mexican patients found that there were a lower detection rate and earlier age at diagnosis, suggesting that other RB1-inactive mechanisms may be involved in cancer development. This finding should be analyzed from the perspective of the technique used in this analysis. RB1 mutation analysis did not distinguish the hereditary form of Rb. The authors concluded that improvements in molecular diagnosis of the disease are needed.18
The adjunct diagnostic use of molecular markers to stage tumors and help determine therapy has been explored in Mexico using tissue from 86 patients with the non-hereditary form of the disease.17 The expression of pRB, P16/INK4A, and E2F1 and the relationship of these phenotypes to the proliferative index, using analysis of the Ki67 antigen expression, were analyzed. The cell cycle markers were expressed differently in unilateral versus bilateral cases, suggesting that cell cycle regulation has a different capacity in each form (localized vs. metastasized) of Rb. For example, among unilateral cases, the mean Ki67 proliferative index was significantly higher in patients with advanced stages of Rb than in those with earlier staged Rb (81.25 vs. 69.50, p=0.0001), a trend that was not significant in bilateral cases. The presence of detectable pRB was associated with a lower percentage of cells expressing E2F1 (p=0.05) in unilateral cases only.17
4.2Pediatric eye cancer early detection and delayed diagnosisEarly detection of Rb in Group A through C eyes is essential for a timely referral to treat and potentially cure the patient without risking vision loss. According to the literature, nearly one-third of Rb cases are diagnosed at advanced intraocular stages in Mexico.5,14
Unfortunately, Rb (along with Hodgkin disease) has the longest time to diagnosis among all pediatric cancers in Mexico with a median of five months from cancer onset.16 During our study, HIM reported a mean lag time of 6.7±9.2 months (n=107) for unilateral cases and 7.5±6.4 months (n=67) for bilateral cases (Table 1). Hospital Civil reported the youngest median age at diagnosis for all patients at 16 months while La Raza had the oldest mean age at 28.5 months. For bilateral cases, La Raza reported the youngest median age at diagnosis at seven months, but it had the oldest median age at diagnosis for unilateral cases at 43.5 months. HIM reported the oldest age at diagnosis for bilateral cases at 14.6 months.
The clinical characteristics of the disease have been studied retrospectively in Mexico.15,19 In the first study, data were analyzed from 86 patients over an 11-year period.19 The average patient age was 24.5 months, and 51% were female patients. Approximately 75% of patients had unilateral cancer, and 25% had bilateral cancer. Leukocoria, strabismus, and glaucoma were present. Cancer metastasized to the central nervous system and bones in 20% of patients.19
In a more recent study,15 the data of 108 patients, all of whom were diagnosed in the first year and were followed up on for up to 19 years (from 1995 through 2014), were analyzed. The mean age at diagnosis was 7.7 months, and 15.7% had a familial history of Rb. The majority of patients had bilateral Rb (55%) and advanced stage (84.1%). Leukocoria was the main manifestation, and most patients were underweight.15 Advanced age and histopathologic evidence have been associated with moderately differentiated tumors in Mexican patients.20
We found a rate of bilateral cases among the patient data reported by the five hospitals in this current study that was in between the previous rates reported,15,19 with 293 bilateral cases reported (37.7%) (Tables 1–5). In our study, leukocoria was the most common presenting symptom in the diagnosis and found n 578 patients (74.4%) (Tables 1–5). Strabismus was present in 152 patients (19.6%). Only five patients presented with glaucoma (0.64%), and two patients showed no symptoms at diagnosis (0.3%). There were also ten trilateral cases (1.3%) reported. About the family history of Rb, there were six patients (one patient with unilateral Rb and five patients with bilateral Rb) with a family history noted at Hospital Civil (Gonzalez-Perez G, e-mail communication, October 25, 2015). No patients had a family history of Rb at La Raza and HITO (Villavicencio Torres A, e-mail communication, October 22, 2015; Etulain Gonzalez A, e-mail communication, November 11, 2015). HIM reported 16/179 (8.9%) familial Rb cases.
In the literature, the relationship between the delayed diagnosis and socio-demographic factors has been studied on the extent of the disease at diagnosis, by interviewing parents of 179 patients about their children's symptoms and their household characteristics.21 In other UMICs of Latin America, an increased delay in diagnosis was associated with more advanced Rb in Argentina31 and Brazil.32 In central Mexico, delayed diagnosis was found to be a predictive factor in staging of the disease at diagnosis in bilateral cases only,21 which is consistent with the suggestion made in the molecular analysis of cell cycle regulators17 that there are underlying differences between localized and metastatic Rb. Prenatal poverty, lower education of the mother, and the presence of dirt flooring in the home predicted an advanced stage of Rb and overall survival for both unilateral and bilateral cases.21
Risk factors in Mexico vary across geographic regions. In Chiapas, where the burden of Rb is as high as in the least developed countries of the world, poor parental nutrition has been found to be a significant risk factor.22 Vegetable-derived folate that was consumed during pregnancy was found to be protective against having a child with unilateral Rb. However, since this study was published in 2005, folic acid-fortified flour has been mandated in Mexico, and there is contradictory evidence that too much consumption of folic acid may risk cancer.23 A recently published case-control study from central Mexico suggests that maternal metabolism of folic acid may be a risk factor for Rb and that the maternal genotype for dihydrofolate reductase (DHFR), the gene needed to convert synthetic folic acid into biologic folates, is predictive for unilateral Rb.23 A California-based study found that children born to Mexican-born mothers versus U.S.-born Mexican mothers had a lower risk of developing Rb, and again, poor nutrition on the part of the U.S.-born Mexican mothers may have been a factor.24 A U.S. survey of Rb incidence patterns from 2000 to 2009 revealed that Rb is significantly more common in white Hispanic boys compared with white non-Hispanic boys and white Hispanic girls, suggesting a potential effect of male sex on Rb origin.33 However, in our study, there were only slightly more male patients than female patients (347 vs. 320). In general, the risk factors of Rb in Mexican-born patients demonstrate the effect of poverty and diet on the extent and burden of Rb; however, more research is needed to confirm these results.21
The main barrier to early detection in Mexico is the lack of knowledge of Rb.25 In a questionnaire distributed to 791 final-year medical students, only 3% were proficient in Rb, and fewer than half could diagnose the disease upon viewing an image. Continuing education of primary care physicians is essential to improve early detection and timely referral.25 The Mexican Retinoblastoma Group (MRG) has produced guidelines for the diagnosis and management of Rb, which have been adopted by the government as national guidelines and are currently used in practice in many pediatric hospitals in Mexico.34 However, more efforts should be made to encourage widespread use of the guidelines among medical professionals further.
A national early detection program is highly recommended to reduce the number of advanced stage tumors.34 In 2013, the Diario Oficial de la Federación (Official Gazette of the Federation), under the General Health Law in Chapter V Maternal-Child Care, article 61, section IV, published that the neonatal ophthalmological screening should be done four weeks after birth for the early detection and treatment of ocular problems, such as Rb, that could cause blindness.35 However, there is no technical guideline available that explains how to screen newborns.28 Juárez-Echenique28 recommends health professionals to use portable cameras that take high-quality photographs of the back of the eye, including the retina, optic nerve, and anterior segment. Such cameras are easy to use by non-ophthalmologists, and the images can be sent to specialists for their review, which allows for greater coverage of newborns at a reduced cost. Telemedicine might be a viable solution to increasing early detection of ocular diseases like Rb in newborns in Mexico.28 However, neonatal Rb represents a small percentage of all Rb new cases, and retinal tumors are not always present during the first two months of life. Therefore, the authors recommend pediatricians to screen with the red reflex technique during all outpatient appointments for the first three years of life.
A national registry of Rb is also needed to analyze the cancer impact in Mexico.1 An accurate number of new Rb cases per year is not yet known in Mexico. Rivera-Luna et al.29 recently published that the Fund for Protection Against Catastrophic Expenditures (FPGC, for the Spanish acronym) covered 100 Rb cases/year for 2007-2012, or a total of 539 new cases. From our data collection in this study, the great variations, inconsistencies, and a high number of missing data are observed in Tables 1–5. A national Rb cancer registry (one that is preferably online and linked to de-identified electronic health records of patients) would be a valuable asset to improve data collection, reporting, and analysis.
4.3Current treatment of Rb in MexicoAlthough with systemic chemotherapy, ocular salvage and sight preservation are now the primary treatment outcomes for Rb (B2),14 there are gaps in practice and skill in conservative management in lesser developed countries, including UMICs.26,36 In 2004, the MRG produced the first multi-center study on pediatric oncology in Mexico, a retrospective analysis of Rb cases in 16 institutions during a 6-year period.1 This study revealed interesting and important trends in Rb treatment in Mexico. Treatment varied extensively from one center to the next. Although chemotherapy was used on nearly three-quarters of the patients, there were more than 15 different chemotherapy regimens used, and some were originally proposed as far back as 1972 by the Pediatric Oncology Group, suggesting that providers need to be updated on better chemotherapy options now available. Indications for chemotherapy also varied. External beam radiotherapy (EBRT) was applied to more than one of five patients as adjuvant therapy, and approximately 88% of patients underwent enucleation, suggesting the advanced progression of cancer. The overall survival rate at a 73-month follow-up was 85%, below the 90-95% survival rates observed in developed countries. Survival rates were even considerably lower in a retrospective analysis of 81 patients with metastatic extraocular Rb. Only 30.8% survived following chemotherapy, whereas 56 patients died due to tumor progression.26 However, in patients diagnosed during their first year, the survival rate was 92%,21 which is comparable with those of developed countries and suggests that there is a better prognosis with early diagnosis. Given the extent of variation in treatments and the lower survival rate, the MRG strongly proposed the establishment of a national early detection program,1 as well as the more widespread use of the national Rb guidelines that have been developed since, which would further improve patient care and outcomes.26 Since 2004, MRG has collaborated with the Ministry of Health and children's hospitals in Mexico on public awareness campaigns in order to improve early detection and treatment. However, more work still needs to be done.
In our study, treatment was reported for only 122 patients (15.7%) at Hospital Civil, La Raza, and HITO (Tables 3–5). Most patients (n=102, 83.6%) underwent enucleation, indicating that current Rb programming in Mexico is a long way off from achieving a primary outcome of ocular salvage. La Raza also reported that one patient abandoned treatment shortly after diagnosis was confirmed. HITO reported that ocular salvage occurred in one patient, but did not indicate the treatment provided. Survival rates are difficult to compare to the literature in this study, as only HIMFG, La Raza, and HITO reported data on deceased patients [20 patients (11.8%), two patients (9.1%), and one patient (10%), respectively] during their follow-up (which, for many, is ongoing).
In advanced intraocular Rb, conservative management may fail in the presence of chemo-resistant vitreous seeding.34 IAC and IVC are emerging therapies in developed countries for these cases, with the aim to salvage the eye with minimal toxic effects.37–40 IAC with melphalan/topotecan is a primary treatment for Group-C and Group-D eyes.41,42 In Mexico City, IAC is available at one hospital, the Instituto Nacional de Pediatría (INP), where adequate control of tumors has been observed.
Currently, there is no literature available on IAC or IVC in Rb management in Mexico, and hospitals that provided treatment data in our study did not specify IAC or IVC. In Latin America, Argentina has spearheaded research in IAC/IVC,14,43–50 which is discussed in great detail in our previous review. A Colombian study was recently published on using IAC as primary therapy on all Rb patients (rather than a select patient population of advanced cases): satisfactory outcomes were found, but follow-up was not carried out enough in this 2-year study to determine the long-term impact of IAC, delayed complications, and vision salvage.51
4.4Accessibility to pediatric cancer care in MexicoThe socio-economic impact caused by pediatric cancer in Mexico is that most families without insurance cannot afford care for their children.13 They are faced with catastrophic medical costs that make them vulnerable to debt, need to sacrifice other necessities or risk of treatment abandonment. Despite the limited knowledge of Rb, the limited treatment options, and the need to improve and update the overall diagnosis and management processes in Mexico, there have been government advances in recent years to improve accessibility to care among the population.
In 2005, the Mexican government issued a formal decree to establish the National Council for the Prevention and Treatment of Childhood and Adolescent Cancer as a permanent coordinating body of the public, social, and private sector activities in research, prevention, diagnosis, and comprehensive treatment of pediatric cancer.52 FPGC was launched by the government in 2006 to offset the cancer costs.27,53 The FPGC provides $5930 USD for the treatment of patients with Rb.27 However, this program does not finance ocular prostheses, medical equipment, or travel expenses for the parents of Rb patients.
A retrospective cohort was carried out in 47 public hospitals to analyze if FPGC improved cancer care coverage from 2006 to 2009 and to measure the survival rates.27 The 3-year survival rate for Rb was 59%, which was lower than those reported in other Mexican studies and varied between region, with higher mortality rates in the southeast.1,27 About one-half of pediatric cancer cases were funded by FPGC at this time, which suggests an increase in coverage. The overall abandonment rate was 6.2%, which was less than Latin American abandonment rates (10-24%).12,27
The other issues that influence accessibility to pediatric cancer are the infrastructure and human resources available to provide Rb care. Pediatric cancer care is treated in most major cities, and 108 pediatric oncologists are distributed throughout Mexico.54 However, most pediatric oncology units lack a Rb diagnosis and treatment program, which requires ophthalmologists specialized in ocular cancer diagnosis and treatment, as well as state-of-the-art ophthalmic equipment. At present, only two hospitals in Mexico have adequate, comprehensive Rb programs, defined as multidisciplinary Rb programs, in which ocular salvage is the primary treatment outcome. These hospitals are the INP and HIMFG, both of which are located in Mexico City. Both general directors are members of the National Council for the Prevention and Treatment of Childhood and Adolescent Cancer. The Hospital Civil in Guadalajara had a formal Rb clinic in operation until 2011, although the hospital still sees about 10 to 12 patients each year (Ramirez-Ortiz MA, e-mail communication, 20 March 2014 and Zepeda C, e-mail communication, 31 March 2014). The INP and HIMFG are non-profit, teaching hospitals affiliated with the Autonomous National University of Mexico and have well-established medical residency programs. Most of the pediatric cancer specialists and pediatric ophthalmologists in Mexico have been trained in these two hospitals. HIMFG and INP additionally serve as national referral centers.
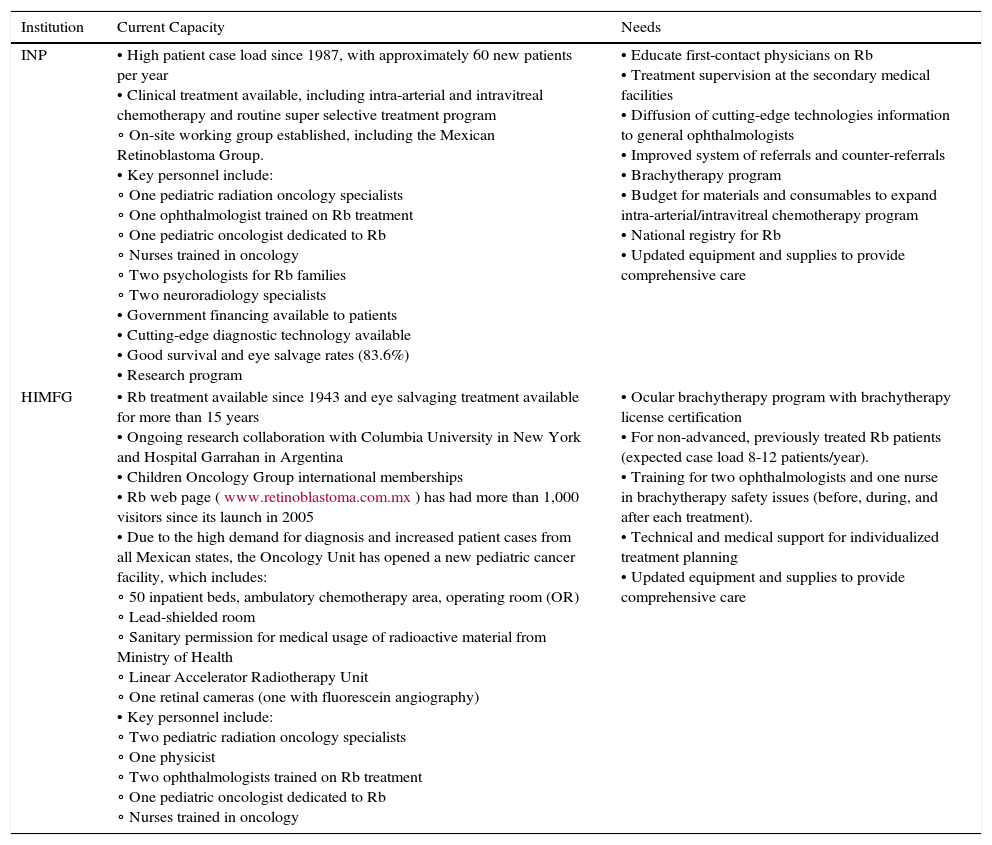
The current capacity, infrastructure, and human resources needs of INP and HIMFG are summarized by the authors, based on their experience working with their Rb programs (Table 7). Both institutions have the staffing, the historical experience with Rb, the patient case load, and some adequate technology and equipment to provide Rb diagnosis and management. INP has established the only IAC program in Mexico and Central America; however, given that the advanced treatment is not reimbursed by the government, the program struggles with the high cost of materials and consumables required to treat patients.
Current infrastructure, human resources capacity and needs of the retinoblastoma (Rb) programs at the Instituto Nacional de Pediatría (INP) in Mexico City, and the Hospital Infantil de México Federico Gomez (HIMFG) in Mexico City, Mexico, as reported by the authors.
| Institution | Current Capacity | Needs |
|---|---|---|
| INP | • High patient case load since 1987, with approximately 60 new patients per year • Clinical treatment available, including intra-arterial and intravitreal chemotherapy and routine super selective treatment program ∘ On-site working group established, including the Mexican Retinoblastoma Group. • Key personnel include: ∘ One pediatric radiation oncology specialists ∘ One ophthalmologist trained on Rb treatment ∘ One pediatric oncologist dedicated to Rb ∘ Nurses trained in oncology ∘ Two psychologists for Rb families ∘ Two neuroradiology specialists • Government financing available to patients • Cutting-edge diagnostic technology available • Good survival and eye salvage rates (83.6%) • Research program | • Educate first-contact physicians on Rb • Treatment supervision at the secondary medical facilities • Diffusion of cutting-edge technologies information to general ophthalmologists • Improved system of referrals and counter-referrals • Brachytherapy program • Budget for materials and consumables to expand intra-arterial/intravitreal chemotherapy program • National registry for Rb • Updated equipment and supplies to provide comprehensive care |
| HIMFG | • Rb treatment available since 1943 and eye salvaging treatment available for more than 15 years • Ongoing research collaboration with Columbia University in New York and Hospital Garrahan in Argentina • Children Oncology Group international memberships • Rb web page (www.retinoblastoma.com.mx) has had more than 1,000 visitors since its launch in 2005 • Due to the high demand for diagnosis and increased patient cases from all Mexican states, the Oncology Unit has opened a new pediatric cancer facility, which includes: ∘ 50 inpatient beds, ambulatory chemotherapy area, operating room (OR) ∘ Lead-shielded room ∘ Sanitary permission for medical usage of radioactive material from Ministry of Health ∘ Linear Accelerator Radiotherapy Unit ∘ One retinal cameras (one with fluorescein angiography) • Key personnel include: ∘ Two pediatric radiation oncology specialists ∘ One physicist ∘ Two ophthalmologists trained on Rb treatment ∘ One pediatric oncologist dedicated to Rb ∘ Nurses trained in oncology | • Ocular brachytherapy program with brachytherapy license certification • For non-advanced, previously treated Rb patients (expected case load 8-12 patients/year). • Training for two ophthalmologists and one nurse in brachytherapy safety issues (before, during, and after each treatment). • Technical and medical support for individualized treatment planning • Updated equipment and supplies to provide comprehensive care |
There is a great need to expand Rb programs at these institutions, primarily through the establishment of an ocular brachytherapy program which is currently unavailable in Mexico. For the past several years, for example, HIMFG has had to refer patients to the Institute of Cancer in Guatemala for eye brachytherapy. Furthermore, INP and HIMFG recognize the needs to strengthen the Rb referral system and educate primary care providers, as well as to establish a national registry for Rb and more widely adopt the national guidelines to improve patient care and outcomes.
Rb is a manageable and curable disease, where adequate resources, infrastructure, and training are available. Gaps in practice and skill exist in lesser developed countries, where Rb is often diagnosed at a metastatic stage.20,34,39,55,56 We reported here clinical data from 777 patients in five hospitals in Mexico, nearly half of which had advanced tumors (45.3%). Of the 122 patients with treatment reported, 83.6% underwent enucleation, indicating that most patients were left blind in one eye or both. UMICs, such as Mexico, may have adequate technology and skilled, highly specialized medical professionals to manage Rb successfully, but these services are often not accessible to the population living outside urban areas,34,55 and enucleation (with vision loss) is the main treatment in Mexico.
In Mexico, Rb is a cancer of poverty, with the burden, impact, and prognosis more severe and grave in lesser-developed and lower-income areas. It concerns that in Chiapas, the poorest state of Mexico, the incidence rate of Rb is very high at 22%,5 but only 3% of the patients treated at the five hospitals in this study were from Chiapas (Table 6). However, we did not receive data from the Hospital General in the state capital of Tuxtla Gutierrez, which is known by the authors to treat and refer patients with Rb. Nevertheless, the need to increase Rb programming coverage to southern Mexico and poorer states is demonstrated, as most patients were from central Mexico in this study (Table 6).
The knowledge of Rb is quite limited in Mexico, where published evidence is sparse, advanced cases are common, and care is limited. Table 8 provides a summary of the key issues facing Rb management in Mexico in this literature review. The most important issue to be addressed is that, although conservative management following early detection results in high survival and eye salvage rates, about one-third to 45.3% of Rb cases are diagnosed at advanced stages in Mexico; thus, enucleation is common. Key actions should be taken to improve early detection, timely referral, and patient outcomes: educate first-contact physicians on the disease, establish a national early detection program and national registry exclusive for Rb, increase the use of the national Rb guidelines, and expand and update the current Rb programs at the INP and HIMFG with modern, advanced technology and the formation of a collaborative, multidisciplinary team of Rb professionals.
Key points facing retinoblastoma (Rb) programs in Mexico.
| Key points |
|---|
| • Early detection of Rb followed by good conservative management of the disease result in high survival and eye salvage rates. |
| • In Mexico, about one-third to 45.3% of Rb cases are diagnosed at advanced stages, and enucleation is common. |
| • Continuing education of primary care/first-contact physicians in Mexico is essential to improve early detection and timely referral. |
| • The establishment of a national early detection program and a national registry for Rb is key to improve diagnosis, patient care, and outcomes. • The widespread and increased use of the national treatment protocol is also key to improve patient care and outcomes. |
| • Only two hospitals in Mexico currently have adequate, comprehensive Rb programs: Instituto Nacional de Pediatría (INP) in Mexico City and Hospital Infantil de México Federico Gomez (HIMFG) in Mexico City. |
| • The expansion and upgrade of Rb programs are necessary for Mexico to improve access to care, especially in impoverished Southern states, and should include: comprehensive training of multi-disciplinary health professionals, the provision of adequate equipment to carry out up-to-date, conservative therapies and improve outcomes, and strengthening of the referral system, including the education of primary care providers on how to detect Rb. |
The expansion of Rb programming has been successfully implemented by some of Mexico's Central American neighbors. St. Jude Children's Research Hospital and the University of Tennessee partnered with pediatric oncology centers in El Salvador, Guatemala, and Honduras to develop Rb programs that emphasized early detection and referral.11 A local protocol for individualized patient treatment was developed based on the Association of Central American Pediatric Hematologists/Oncologists protocol, which uses a multidisciplinary treatment approach to chemotherapy, surgery, and radiation therapy for children with Rb. The Central American programs used telemedicine for virtual consultations between local pediatric oncologists and ophthalmologists and practitioners in the United States. The education of local health care professionals and the establishment of satellite eye clinics were key to strengthening the national referral programs. Local non-profit foundations also helped finance patients’ treatments, which were crucial to improving patient uptake and accessibility of care. The results have thus far been impressive, with the best outcomes in El Salvador (survival rate 83%), which is the most homogenous and smallest country. From 2000 to 2007, abandonment rates decreased from 6% to 3% in El Salvador, from 21% to 11% in Guatemala, and from 35% to 19% in Honduras. Although the abandonment rate specific to Rb has not yet been studied in Mexico (La Raza only reported treatment abandonment in one patient in our study), the FPGC retrospective cohort did find an overall cancer abandonment rate of 6.2%,27 similar to El Salvador's earlier Rb rate, which suggests that, with proper intervention, the abandonment rate can also be decreased in Mexico. Central American survival rates increased from 29% to 56% in Guatemala and from 32% to 56% in Honduras, indicating a great improvement in care; however, there is a need to improve outcomes to those of developed countries. Nevertheless, as the Central American programs improved, the need for more advanced ocular salvage treatment options now requires additional resources to continue to develop Rb management in the region.11 Perhaps the collaborative Rb model in Central America could be a starting point to establish similar programs, particularly in Southern Mexican states, which lack tertiary-level Rb facilities, trained specialists, and adequate care and which have the greatest burden of the disease.
Two tertiary-level hospitals, INP and HIMFG, handle 50% of the new Rb case load in Mexico. These hospitals also train both ophthalmologists and pediatric oncologists to treat Rb in new children's’ hospitals located outside of Mexico City and Guadalajara. It is very probable that HIMFG and INP will decrease the number of new annual Rb cases as the new children's hospitals gain expertise and experience treating uncomplicated Rb. Meanwhile, there is a great need for state-of-the-art technology for eye-salvaging programs in Mexico. HIMFG and INP have a great challenge in the near future because new therapies, such as super selective IAC, and already proven treatments, such as ocular brachytherapy, should be incorporated in their medical arsenal to salvage eyes that otherwise would be enucleated. Due to Mexico's large population and geography, potential collaborative programs with internationally renowned cancer centers should be focused on promoting early detection and increase community awareness of the disease, medical research, training doctors, and sponsoring unavailable treatments. As governmental financing is very limited in providing these needs to all new cancer centers, existing programs could work together with international collaborators to merge their resources and accomplish this goal.
This study has its limitations. Although we requested the same data from all hospitals for the past ten years, the data collection and reporting processes were very limited and inconsistent, with hospitals only reporting some data for some or different years, many patients missing complete data, and some hospitals reporting a summary of data versus a list of patient data. We are limited in our statistical analysis by these inconsistencies, and we cannot make generalizations applicable to all of Mexico when we know there are more hospitals that treat and refer Rb patients to the national centers. However, our findings reveal a great need to standardize data collection and reporting, ideally through a national Rb registry with a government mandate that all patients be captured in that registry. Ideally, such a national registry would be electronic and linked directly to de-identified patient medical records, preferably via electronic health records. This would allow investigators to a better study of the disease epidemiology, treatment efficacy, and patient outcomes, as well as to review benchmarking reports and quality of care provided by each facility, both regionally and nationally.57 Realistically, this would require funding, trained database managers and statisticians, patient privacy and protection mechanisms, and the buy-in of the participating hospitals (i.e., incentives to report data), particularly because our data collection results show how difficult it is to obtain data.
There is an immediate need in Mexico to expand primary care providers’ knowledge of Rb and to expand and upgrade current Rb programs to meet the needs of the population adequately. Diagnosis and care of Rb patients in Mexico can also be improved by the establishment of a national Rb registry and a national early detection program, and by increased use of the national treatment protocol.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingInternational Agency for the Prevention of Blindness/Orbis and the Hamilton Eye Institute of the University of Tennessee.
Conflict of interestVCL: paid employee of HelpMeSee, ad-honorem employee of the Instituto Mexicano de Oftalmología and the University of Tennessee, former employee of the International Agency for the Prevention of Blindness; KAE: independent consultant to International Agency for the Prevention of Blindness and Strategic Solutions, Inc; BGH: employed by University of Tennessee; BXP: employed by University of Tennessee; MARO: employed by Secretaría de Salud Pública (México); VBC: employed by Secretaría de Salud Pública (México); GGP: employed by Hospital Civil de Guadalajara Fray Antonio Alcalde; AVT: employed by Hospital General “Dr. Gaudencio González Garza” del Centro Médico Nacional La Raza; AEG: employed by Hospital Infantil Teletón de Oncología.