The first urinary tract infection can be a marker of a urinary tract anomaly, mainly vesicoureteral reflux. The aim of this work was to determine the association between isolated Enterobacteriaceae with the presence and grade of vesicoureteral reflux in neonatal patients at their first urinary tract infection.
MethodsA retrospective, observational and analytic study of newborns, who were admitted to the Neonatal Department, of the University Pediatric Hospital Juan Manuel Márquez, in Havana, Cuba, from 1992 to 2013, was conducted. The causal microorganism of urinary tract infection was from the Enterobacteriaceae family. They were evaluated by radio imaging. The association between the presence and grade of vesicoureteral reflux with the causal microorganism of the urinary tract infection was analyzed.
ResultsNewborn infants with urinary tract infection (450) were studied. Bacterial isolations in the urine cultures corresponded to E. coli in 316 cases (70.2%). The prevalence of vesicoureteral reflux was 18.2%. The presence of bacteria corresponding to the Enterobacteriaceae family (other than E. coli) had a significant risk association with vesicoureteral reflux (OR: 2.02; p < 0.01) and vesicoureteral reflux classification (for higher grades, p < 0.01).
ConclusionsE. coli is the most common causal microorganism in neonatal urinary tract infection. However, an association between the isolation of a microorganism of the Enterobacteriaceae family different to E. coli with the presence of vesicoureteral reflux and mainly with higher grades of vesicoureteral reflux exists.
La primera infección del tracto urinario puede ser un marcador de una anomalía del tracto urinario, principalmente de reflujo vésico-ureteral. El objetivo de este trabajo fue determinar la asociación entre microorganismos de la familia Enterobacteriaceae con la presencia y grado de reflujo vésico-ureteral en pacientes neonatales quienes debutaron con infección del tracto urinario.
MétodosSe realizó un estudio retrospectivo, observacional y analítico de recién nacidos con infección del tracto urinario, quienes ingresaron en el Servicio de Neonatología del Hospital Pediátrico Universitario “Juan Manuel Márquez”, La Habana, Cuba, desde 1992 hasta 2013, y en quienes el microorganismo causal era de la familia Enterobacteriaceae. Se realizaron estudios por imagen y se analizó la asociación entre la presencia y grado de reflujo vésico-ureteral con el microorganismo causal de la infección del tracto urinario.
ResultadosSe estudiaron 450 recién nacidos. Los aislamientos bacterianos en los urocultivos correspondieron a E. coli en 316 casos (70.2%). La prevalencia de reflujo vésico-ureteral resultó del 18.2%. Se comprobó que el microorganismo causal —otras bacterias diferentes a E. coli correspondientes a la familia Enterobacteriaceae— se asoció significativamente con el riesgo (RM: 2.02; p < 0.01) y el grado de reflujo vésico-ureteral (para los de más alto grado, p < 0.01).
ConclusionesE. coli es el agente causal más frecuente de la infección del tracto urinario neonatal. Sin embargo, existe una asociación entre la presencia de un microorganismo de la familia Enterobacteriaceae diferente a E. coli y el reflujo vésico-ureteral, principalmente los de mayor grado.
Urinary tract infection (UTI) is the most common bacterial infection involving this system in the first year of life.1 The causative organisms most frequently involved in this infection are those typical of the colonic bacterial flora, predominantly E. coli; however, other microorganisms from the Enterobacteriaceae family are also common.2–5 It is recognized that in order to produce high UTI, the main pathway is the canaliculi. E. coli has many features which give it the ability to adhere to the uroepithelium, ascend and cause pyelonephritis, such as virulence factors (P fimbriae and MR Adhesins).6–10
Moreover, when a child has a UTI early in life, the presence of an abnormality of the urinary tract (AUT) is found in many cases, mainly vesicoureteral reflux (VUR), which is found between 14 - 39.2% of the cases.2,11–14 Previous studies in patients with UTI have reported a significant association of VUR and microorganisms other than E. coli as causative agents. These microorganisms englobe a variety of agents from different families.2,13,15–19 However, the degree of VUR has not been considered, making, if true, this relationship relevant for clinical practice. The aim of this study was to determine the association between microorganisms of the Enterobacteriaceae family with the presence and degree of VUR in neonatal patients who debuted with UTI. To our knowledge, this is the first report of this type in Latin America that focuses on a particular population: newborns (NB).
2MethodsA retrospective, observational and analytical study of NB from this community and with their first high UTI was conducted.
Patients were admitted to the Neonatology Service at the Pediatrics University Hospital Juan Manuel Márquez from February 1992 to May 2013. Only patients whose causative organism was from the Enterobacteriaceae family with performed ultrasound (US) and renal VCUG (UCGM) studies were included. Patients who did not meet these conditions were excluded, so the studied population was 450 NB. This research was approved by the Ethics Committee and Scientific Council of the hospital.
The variables studied were gender, chronological age, gestational age, birth weight, method of urine collection, age at UCGM, causative organism, VUR (primary or secondary to another AUT) and degree of VUR.
Kidney ultrasonography was performed during the first three days of the diagnosis of UTI to detect structural abnormalities of the urinary tract and pyelocaliceal dilations. The UCGM was performed after the remission of the UTI. A standard technique was used, with a full bladder and observing the filling phase and spontaneous urination. The degree of VUR was based on the classification of the International Committee for the Study of VUR;20 and for patients with bilateral VUR, the higher grade was considered.
The UTI was diagnosed based on the presence of clinical manifestations, and laboratory tests compatible with this condition (fever, pyuria> 10,000 / ml in non-centrifuged urine and some positive acute phase reactant, such as erythrocyte sedimentation rate ≥ 20mm / h, positive qualitative C-reactive protein, total blood leukocyte count < 5 or ≥ 15 x 109 / l) in addition to a single organism growth in urine in any number of colony forming units (CFU) / ml in samples taken by suprapubic bladder puncture (SBP), or > 10,000 CFU / ml where it was by bladder catheterization, or> 100,000 CFU / ml if obtained by other methods of urine collection. For methods of SBP and catheterization, only one urine sample was required; for other techniques, two urine samples obtained at different times and having the same microorganism were required.
For statistical analysis, absolute and relative frequencies summary measures (mean and median) and dispersion (standard deviation, and range quartiles) were calculated. For analyzing the association between VUR and the causative organism of the UTI, the value of the odds ratio (OR) and confidence intervals (CI) at 95% for categorical variables, was estimated by 2 x 2 tables, as well as 2 x n tables for the association of the different degrees of VUR. The assumed significance level for the value of p was < 0.05. All analysis were computed using the statistical software SPSS, version 12.0 and the program Epidat version 3.1.
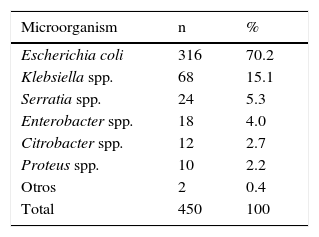
3ResultsA total of 450 infants with a diagnosis of UTI met the inclusion criteria for this research. The mean and standard deviation of patients to age, birth weight and gestational age were 16 ± 7.1 days, 3,458 ± 496g (range 2,010-5,100g) and 39 ± 1.4 weeks (range 32 to 43 weeks), respectively. Of these, only 16 NB (3.5%) were preterm and 412 NB were uncircumcised males (91.5%). The recollection of urine for the diagnosis of UTI was performed by SBP in 431 patients (95.8%). Bacterial isolates in urine cultures corresponded to E.coli 316 patients (70.2%) (Table 1). Klebsiella spp. was the second most frequent causative agent with a frequency in NB of 68 (15.1%).
All NB included in the study had undergone a kidney US within the first 3 days of diagnosis, as well as a UCGM after remission. During the latter imaging study, the median age was 4 months, and the first and third interquartile were 3 and 6 months, respectively.
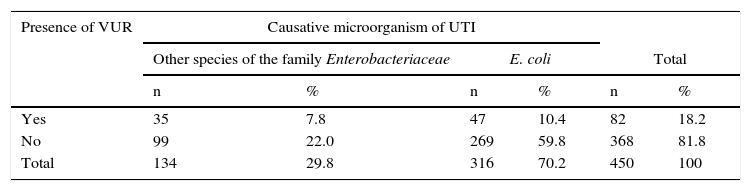
Different types VUR in 82 patients (18.2%; 95% CI 14.5-22.6) were observed, predominantly primary (15.8%) and secondary (2.4%) VUR of patients with UTI respectively (Table 2).
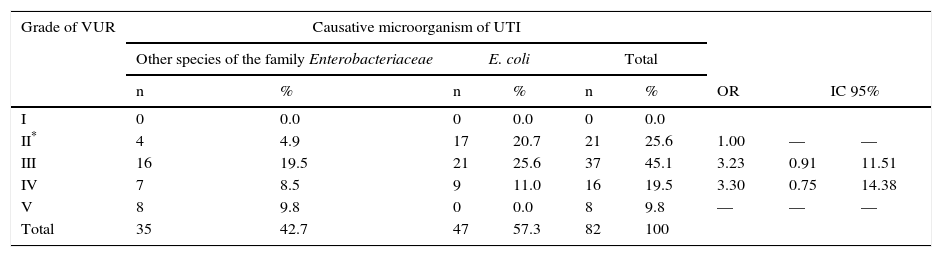
VUR with the causative organism was compared. Two groups were formed: the first group corresponded to those cases in which other species corresponding to the Enterobacteriaceae family were isolated; the second group was composed of cases in which E. coli (Table 3) was isolated. It was found that VUR presents a statistically significant risk association with other than E. coli causative organisms from the Enterobacteriaceae family (OR 2 02, p. < 0.01). Patients were distributed according to the degree of VUR and in relation to the causative organism (Table 4). Comparing VUR grade III with grade II denoted an OR of 3. 23, while after comparing grade IV with grade II an OR 3.30 was estimated. It was impossible to calculate the OR between grades V and II because there were zero cases in a square case; however, the homogeneity test between levels revealed a significant association between these variables (p < 0. 01), demonstrating that causative organisms other than E. coli from the Enterobacteriaceae family are associated with a higher grade of VUR.
Presence of VUR regarding causative organism in patients with UTIs in the neonatal period.
| Presence of VUR | Causative microorganism of UTI | |||||
|---|---|---|---|---|---|---|
| Other species of the family Enterobacteriaceae | E. coli | Total | ||||
| n | % | n | % | n | % | |
| Yes | 35 | 7.8 | 47 | 10.4 | 82 | 18.2 |
| No | 99 | 22.0 | 269 | 59.8 | 368 | 81.8 |
| Total | 134 | 29.8 | 316 | 70.2 | 450 | 100 |
VUR, vesicoureteral reflux; UTI, urinary tract infection.
OR 2.02 (IC 95% 1.23-3.31), p < 0.01.
Degree of VUR regarding the causative organism in patients with UTIs in the neonatal period.
| Grade of VUR | Causative microorganism of UTI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Other species of the family Enterobacteriaceae | E. coli | Total | |||||||
| n | % | n | % | n | % | OR | IC 95% | ||
| I | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |||
| II* | 4 | 4.9 | 17 | 20.7 | 21 | 25.6 | 1.00 | — | — |
| III | 16 | 19.5 | 21 | 25.6 | 37 | 45.1 | 3.23 | 0.91 | 11.51 |
| IV | 7 | 8.5 | 9 | 11.0 | 16 | 19.5 | 3.30 | 0.75 | 14.38 |
| V | 8 | 9.8 | 0 | 0.0 | 8 | 9.8 | — | — | — |
| Total | 35 | 42.7 | 47 | 57.3 | 82 | 100 | |||
VUR, vesicoureteral reflux; UTI, urinary tract infection.
The observed results showed that comparing the causative organism of UTI with VUR has a statistically significant association between these two variables: when a microorganism of the Enterobacteriaceae family different than E. coli is isolated, it is likely that the patient has VUR and a high grade.
The studied population has the characteristic of being preponderantly non-premature NB with healthy birth weight, who acquired the UTI in the community after leaving the maternity facilities where they were born. Only 16 cases were preterm infants who, although they are more susceptible to infections, had little representation in this population. Another characteristic is that the UTI primarily affected male NB, and was predominantly caused by E. coli, which has been widely described in the literature.2,5
When a UTI presents at early stages of life, the possibility of an underlying AUT should be considered. Excluding transient and idiopathic hydronephrosis, prevalence rates of VUR range from 14-39.2%;2,11–14 in this study, we observed a rate within this range (24.2%). The most common anomaly among our patients was primary VUR, which also agrees with internationally reported prevalence rates, although most studies report rates of less than 24%.3,21,22
Most research has englobed under the term “not E.coli agents” microorganisms from both Enterobacteriaceae family and those belonging to the genus Streptococcus, Enterococcus, and other bacteria corresponding to non-fermenting bacilli.13,15,17,18,23,24 This spectrum of microorganisms is very dissimilar, so it was interesting to explore whether the association of the isolated microorganism with VUR also occurs in neonatal patients with UTI specifically caused by microorganisms from the Enterobacteriaceae family, comparing E. coli with other causative agents of the same family.
A significant association between VUR with the isolation of Enterobacteriaceae other than E. coli in infants with UTI was demonstrated, although E. coli was the most frequently isolated causal organism in both patients affected by VUR and those who had no obvious abnormalities. This finding has been previously reported in the medical literature, but it is the first time observed and reported in a Latin American context. Cleper et al.16 compared the isolation of E. coli vs. Klebsiella spp. in a study of NB affected by UTI, which demonstrated that VUR was four times more diagnosed in patients with UTI caused by Klebsiella spp. than in those in where E.coli was isolated. A retrospective study of 62 NB with their first UTI by Kanellopoulos et al. found that infants with VUR were more affected by Gram-negative bacteria other than E. coli than those without this abnormality, in whom E. coli was the predominant bacteria (p = 0.0008). However, this association was not significant for the presence of AUT other than VUR. The authors described that the other than E. coli bacteria isolated in nine infants with VUR were K. pneumoniae (six cases) and Proteus (three cases).2
Also, it was shown that among the different strains of E. coli causing UTI in children, those E. coli isolates without virulence factors were significantly related to the presence of some AUT. In this sense, a study reported that E. coli strains without the virulence factor papGII were more common among patients with AUT than in those who had a normal urinary tract (25% vs. 5%, p = 0043); it was also more common the isolation of strains from the non-virulent phylogenetic group A (58% vs. 10%, p = 0.0003) in those patients with AUT.19 Another study obtained similar results by finding that strains of E. coli without the factor pap had a greater incidence in the group of children suffering from VUR than in those without VUR (42.8% vs. 15.6%, p = 0.031). However, no significant differences between the group of patients with VUR and the group of patients without it were observed, for other virulence factors such as the O antigen and the production of hemolysin.25
In contrast to our results, Marcus et al.26 studied 158 children with UTI (median age 4 months) and verified that when the causative organism of the UTI was other than E. coli, a significant correlation with the presence of some underlying AUT was observed. However, when they analyzed VUR specifically, no statistically significant relationship was observed, although the organisms other than E. coli were mostly those that caused UTI in patients with VUR.
Primarily P fimbriae, and other adhesins, are surface virulence factors of uropathogenic E. coli. Therefore, the presence of these is typically associated with non - obstructive pyelonephritis.6,10 It has been noted that type 1 fimbriae play a major role in cystitis; however, more recent findings have found that P and type 1 fimbriae act synergistically to facilitate colonization, and thus face obstacles such as urine flow. Thus, they also play a role in kidney infection.27 Other microorganisms other than E. coli, or the same E.coli strains which do not exhibit adherence the mentioned factors, require the presence of certain anomalies to reach and produce high UTI compared with the uropathogenic E. coli that affect children with normal urinary tracts. This reasoning is based on the work of Man et al.28 who studied 241 children in their first UTI and determined adhesins in E. coli strains. They identified renal sequelae in most children in whom adhesins negative bacteria were isolated (RR = 8.3; 95% CI: 3. 3-20. 4, p < 0. 001); however, those children infected with adhesins negative E. coli more frequently presented VUR.
Klebsiella, Proteus, and Enterobacter, although they share some characteristics with the E. coli and they are classified into the Enterobacteriaceae family, do not necessarily possess the same virulence factors that facilitate the advancement through the urinary tract.
However, it has been noted that Proteus mirabilis has virulence factors, such as adhesins, that facilitate the colonization of the urinary tract.29
In 2007, the National Institute for Clinical Excellence (NICE) guidelines for the diagnosis and management of UTI in children were published.30 In these guidelines, the characteristics of an atypical UTI are mentioned. In 2011, the American Academy of Pediatrics issued the “Guidelines for the diagnosis and management of initial UTI in febrile infants and children between 2 to 24 months old”.31 One of the recommendations for performing a UCGM after the first UTI is an atypical UTI. Thus, isolating a different microorganism than E. coli as a causative agent of a UTI sets this diagnosis, which is not specifically addressed for NB in the international literature. These same guidelines only state these recommendations from two months of age. It is obvious that these guidelines are also compatible for NB who debut with UTI. In fact, this research reinforces one of the aspects that characterize an atypical UTI: when the causative organism is other than E. coli (for its proven association with VUR).
A novel finding is that a UTI is most likely caused by a microorganism of the Enterobacteriaceae family different than E. coli with a greater degree of VUR. To date, this has only been confirmed in the literature by Shaikh et al.,32 who determined in 769 children of different ages with UTI that patients with VUR grades III and IV were more prone to be infected by microorganisms other than E. coli.
This research is limited by the intrinsic aspects associated with retrospective study designs. Moreover, we could not determine the risk factors of the isolates, particularly adhesins, or other factors that could shed more light on the proven the association of AUT with the type of infecting organism.
On the other hand, we analyzed a large number of NB with similar characteristics and a close follow-up. In addition, during the research, the microbiological procedures and clinical management remained the same.
5ConclusionWe conclude that E. coli is the most frequent causative agent of neonatal UTI. However, there is an association between the isolation of a microorganism of the Enterobacteriaceae family other than E. coli with VUR, and also, with higher degrees of VUR. Facing this circumstance, underlying AUT should be ruled out.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe costs of the investigation were assumed by the authors themselves.
Conflict of interestThe authors declare they do not have conflicts of interest with the content of this article or any particular benefit aside from the fact of having the opportunity to disseminate new scientific knowledge through the publication of these results.
To the doctors and nurses of the Neonatology Service, as well as the physicians and technicians of Microbiology Laboratory and Radio Imaging Department of the Pediatric University Hospital Juan M. Márquez.
Please cite this article as: Díaz Álvarez M, Acosta Batista B, Pérez Córdova R, Hernández Robledo E. Infección del tracto urinario causada por Enterobacteriaceae y su relación con reflujo vésico-ureteral en recién nacidos. Bol Med Hosp Infant Mex. 2017;74:34–40.