Within the framework of the circular economy and waste recovery, this work deals with the possibility of manufacturing wall tiles whose bodies include waste in their formulation. The wastes selected are crushed scraps of defective fired tiles, marble dust residues and fly ash from thermal power plants. Fired tile waste is a material of interest for reuse due to its similarity to the product to be developed. Marble dust, from the manufacture and mechanical handling of marble blocks, is useful as a source of calcium oxide to replace the calcium carbonate which generates porosity. Fly ash, from the combustion of coal in thermal power stations, is a vitreous material that has undergone a thermal process at high temperature and can therefore be integrated into ceramic body compositions.
The obtained results show the correct formulation of sustainable porous bodies from these wastes in combination with clay in different proportions. In this way, it is possible to manufacture porous substrates for tiles using a percentage of waste higher than 50wt%, obtaining appropriate properties in the fired product: linear shrinkage, water absorption, mechanical strength and moisture adsorption.
En el marco de la economía circular y la valorización de residuos, el presente trabajo aborda la posibilidad de fabricar azulejos cuyos soportes incluyan residuos en su formulación. Los residuos seleccionados son tiesto triturado de baldosas cocidas defectuosas, residuos de polvo de mármol y ceniza volante de centrales térmicas. Los residuos de baldosas cocidas son materiales de interés para la reutilización por su semejanza con el producto a desarrollar. El polvo de mármol, procedente de la manufactura y manipulación mecánica de bloques de mármol, es útil como fuente de óxido de calcio para sustituir el carbonato de calcio que genera porosidad. La ceniza volante, proveniente de la combustión del carbón en centrales térmicas, es un material vítreo que ha sufrido un proceso térmico a alta temperatura y que, por tanto, puede integrarse en composiciones de soportes cerámicos.
Los resultados obtenidos ponen de manifiesto formulaciones optimizadas de soportes porosos sostenibles a partir de estos residuos en combinación con arcilla en diferentes proporciones. De este modo, es posible fabricar soportes porosos para baldosas utilizando un porcentaje de residuos superior al 50%, obteniendo unas propiedades en cocido adecuadas: contracción lineal, absorción de agua, resistencia mecánica y adsorción de humedad.
The ceramic manufacturing process has traditionally represented a good entry point for the recycling of all types of waste, both organic and inorganic in nature. This is based on the thermal treatment that takes place, which allows most of the waste to be eliminated or inertised [1].
On the one hand, organic substances undergo a thermal decomposition and oxidation process, leaving behind a residual porosity that has been the subject of research for its application in the production of porous ceramics. This is the case of the use of agro-industrial waste for the manufacture of ceramic membranes, for example [2]. However, although the reuse of organic waste in the manufacture of ceramic products is feasible, its industrial applications are scarce, as the function of the residual component is limited exclusively to the formation of porosity in the final ceramic product. For this reason, the utilisation of wastes of inorganic nature is of much greater interest, because of both the variety of wastes and the different functions they play in a given ceramic composition [3].
The recycling of inorganic waste in the manufacture of ceramic tiles began to be addressed by the scientific community in the 1980s [4–6]. At that time, industry's interest in waste reuse was low, as efforts were focused on quality and manufacturing costs, and there was little concern in society for the generation and/or use of waste. These works included wastes of a very diverse nature, which could come from mining, paper, oil, thermal power plants, incinerators, among other industries, as stated in an excellent review by Zanelli et al. [7]. As time went by, from the early years of this century onwards, social awareness of the environment and waste increased, generating greater scientific and industrial activity related to the recycling and reuse of waste. It was at this time that the complete recycling of wastewater and solid process waste was consolidated in the manufacturing process itself, being incorporated in the preparation stage of the spray-dried powder [8–10].
With the emergence of the principles of the circular economy at the beginning of the second decade of this century, research into the use of waste has reached its peak. The objectives of this intense activity are not only limited to assessing the technical feasibility of introducing a certain waste to a greater or lesser extent into the ceramic composition, but this reuse must also comply with the principle of economic and environmental sustainability while the function of the waste in the ceramic composition can be diverse [7]. On the one hand, one common function deals with low melting temperature materials, of vitreous or vitrocrystalline nature, such as waste glass [11], thermal power plant fly ash [12], mining waste [13], blast furnace slag [14], etc. These studies have demonstrated the feasibility of using varying proportions, generally up to 25wt% of waste in order to partially replace fluxing raw materials such as clay and/or feldspar. On the other hand, there are also very frequent studies that incorporate high melting temperature wastes whose role is that of filler material in the composition, partially replacing quartz which is the main filler material in ceramic tile compositions. In this line of action, works can be found addressing the feasibility of incorporating refractory waste [15], foundry sands [16], waste from melting furnaces [17], chamottes from other ceramic products such as sanitaryware [18] or bricks [19], and more recently, waste from the construction and demolition of buildings [20]. In these cases, the percentage of incorporation, depending on their nature, can be higher, reaching up to 30–40wt% on average. It is also possible to combine waste that performs the two functions described above, flux and filler, which leads, not without difficulty, to the incorporation of a higher proportion of waste in the final composition, reaching up to 50wt% or more of residual material [21,22].
Special mention should be made of the use of wastes that incorporate alkaline earth oxides, fundamentally calcium oxide, in porous wall tile compositions. These oxides are used as substitutes for calcium carbonate, the main raw material supplying calcium oxide because of its low price. Different studies have been directed towards the complete substitution of all calcium carbonate by marble dust residue, consisting almost exclusively of calcite, demonstrating the feasibility of substitution on a laboratory scale, although with the limitations imposed by the chromophoric impurities in this type of waste, fundamentally iron oxide [23]. Other sources of residual calcium oxide studied have been certain blast furnace or electric arc furnace slags [24,25]. In these cases, the substitution of calcium carbonate can only be partial as the residues incorporate other chromophoric or fluxing oxides that can alter the behaviour and properties of the final product. More recently, the use of kiln dust from the cement industry has been tested as a source of calcium oxide to replace calcite. Although the substitution of much of the calcite is possible, the sulphate and alkali ion content severely limit its industrial application [26].
The above highlights the intense research activity in relation to the reuse and recycling of various wastes in the manufacture of ceramic tiles. This activity has been accelerated in recent years by the sustainability challenges included in the different research programmes. However, the feasibility of designing compositions, scalable to industry, based primarily on waste material that performs the different functions of the components of a given ceramic composition, has hardly been investigated.
The wastes have been chosen to be used as secondary raw materials in this research following three basic criteria: (i) availability of use on an industrial scale, (ii) location in a geographical environment close to the area of consumption, in this case the Spanish ceramic industry located in the province of Castellón and (iii) the wastes used must fulfil relevant functions in the composition formulated. Based on these criteria, the wastes chosen were marble dust waste from the Region of Murcia, fly ash from the Andorra thermal power plant in the province of Teruel and fired ceramic tile waste from the Castellón ceramic tile industry itself.
Spain is one of the main European producers of marble, with most of the production concentrated in the southeast of the country (Alicante, Murcia and Almeria). During the extraction, cutting and treatment of marble, a significant amount of dust is produced at a rate of 170kg/m3 of block, which currently represents an accumulated quantity of sludge of around 75kt/year in dry basis. Although part of this quantity is employed as an aggregate in construction, its composition (practically made up of calcium carbonate) makes it of great interest for its use in the manufacture of ceramic wall tiles, as indicated above, which would significantly increase its added value. The literature also highlights the interest and feasibility of using thermal power plant fly ash as a fluxing raw material in various types of ceramic tiles [12,27]. In Spain, there are numerous thermal power stations that still burn coal, producing significant quantities of fly ash, which can account for up to 25% of the total mass of burnt coal. The availability in an area close to the Castellón ceramic industry would be around 50kt/year, and although they have a potential use in the manufacture of concrete, their current reuse is very limited. Finally, the Castellón ceramic industry itself generates around 2–3% of defective ceramic material that cannot be marketed. There are companies that crush and grind this material, making it suitable for being reused a priori without major problems by the ceramic industry. The current availability of this waste is 200kt/year and most of it is landfilled.
Based on the above, this research aims to go a step further by proposing a ceramic tile composition in which the basic functions of the ingredients are developed by waste available on an industrial scale. The research will carry out a comparative study, with respect to an industrial composition of ceramic wall tiles, on the processability and properties of fired products obtained from compositions incorporating large amounts of secondary raw materials provided by the three selected wastes (marble dust, fly ash and fired ceramic tile waste). These wastes currently represent a significant environmental impact that has not yet been resolved, contributing negatively to the carbon footprint of the industries from which they originate. The selection of porous wall tiles is based on the importance of this type of product in the Castellón ceramic industry and the possibility of incorporating marble dust as waste to replace calcite. Furthermore, the availability of these wastes in other European tile producing countries, such as Italy or Turkey, may facilitate the replication of the research results to these countries.
Experimental procedureMaterialsThree waste samples from Spanish industries were used for the research: residual dust from cutting and treatment of marble in Region of Murcia, fly ash from the Andorra thermal power plant in Teruel and fired tile scraps from the ceramic industry in Castellón. These samples were designated with the letters MD, FA and TS respectively. Together with these samples, a kaolinitic clay of the “ball-clay” type from Ukraine (hereinafter referred to as BC), commonly used in the manufacture of ceramic tiles due to its high plasticity, was used. Table 1 shows the chemical composition determined by X-ray fluorescence spectrometry (XRF; Axios, Panalytical) and the qualitative mineralogical composition determined by X-ray diffraction (XRD; D8 Advance diffractometer, Bruker Theta-theta). This table also includes the chemical and mineralogical composition of an industrial wall tile spray-dried powder used as a reference in the study (composition called STD), made up of a mixture of illitic-kaolinitic clays and a clayey marl that contributes a proportion of calcium carbonate of around 14% by weight to the composition. Loss on ignition (L.O.I.) was measured at 1000°C.
Chemical composition determined by XRF (in wt%) and qualitative mineralogical composition determined by XRD of MD, FA and TS residues, BC raw material and the STD composition used as reference.
| Compound | MD | FA | TS | BC | STD |
|---|---|---|---|---|---|
| SiO2 | 1.2 | 41.8 | 66.7 | 59.0 | 57.5 |
| Al2O3 | 0.2 | 24.5 | 17.6 | 26.7 | 15.6 |
| Fe2O3 | 0.1 | 20.0 | 1.5 | 1.0 | 5.3 |
| CaO | 52.2 | 6.1 | 7.2 | 0.4 | 6.3 |
| MgO | 2.5 | 1.2 | 1.1 | 0.5 | 1.8 |
| Na2O | 0.1 | 0.2 | 1.1 | 0.5 | 0.4 |
| K2O | 0.1 | 1.5 | 2.1 | 2.2 | 3.2 |
| L.O.I. | 43.4 | 0.9 | 0.5 | 7.9 | 9.2 |
| Minorities | 0.2 | 3.8 | 2.2 | 1.8 | 0.7 |
| Majority phases | Calcite | Mullite | Quartz | Quartz | Kaolinite |
| Dolomite | Quartz | Anorthoclase | Kaolinite | Quartz | |
| Quartz | Magnesium-ferrite | Mullite | Illite | Calcite |
With these four raw materials (MD, FA, TS and BC), compositions were prepared with the aim of maximising the content of reused residue, trying to preserve the processability of the composition, the industrial applicability and the final properties of the obtained tiles. Table 2 details the formulas used in the 3 compositions designed. As can be seen, in all cases the proportion of marble dust has been kept constant at 15%, in order to ensure the formation of crystalline phases in the SiO2–CaO–Al2O3 system and promote porosity during the heat treatment to which the composition is subjected.
For the preparation of each composition, the fired ceramic scraps were crushed in a jaw crusher. Subsequently, the different materials except the fly ash were ground in a laboratory hammer mill using a 500μm output sieve. These three ground samples, together with the fly ash sample, were further processed again in a ball mill, following the percentages described in Table 2, using water as liquid medium, simulating the grinding used in industrial practice. The suspensions were ground for 10minutes at a rotation speed of 410rpm until a residue on a 63μm sieve of less than 8wt% was obtained and the particle size distribution curves were obtained by laser diffraction (LD; Mastersizer 2000, Malvern). Together with these 3 compositions, a STD powder was processed. The real densities were measured with a helium pycnometer (AccuPyc II 1340, Micromeritics).
The obtained aqueous suspensions were dried in infrared lamps and then the dry powder was spray-conditioned with water to obtain pressing powders with a moisture content of about 0.055kg water/kg dry solid. Prior to the conditioning step, a simultaneous thermal analysis (STA 449 F5 Jupiter, Netzsch) was carried out with some of the dried powder samples. This thermal analysis includes differential scanning calorimetry (DSC) and thermogravimetry (TG) in order to evaluate, comparatively with the industrial STD composition, the temperatures at which the decomposition of the calcium carbonate and other crystalline phase reactions take place.
The conditioned powders were pressed at 250kg/cm2 in a laboratory press with a 4cm diameter die to obtain cylindrical specimens of approximately 8mm thickness. The pressed specimens were dried in a laboratory oven at 110°C for 24h and their bulk density was determined following the Archimedes method. Subsequently, the specimens were fired in an electric laboratory kiln (Fast kiln, Pirometrol) to maximum firing temperatures that varied between 1100°C and 1160°C, following a fast firing cycle with a heating ramp at 25°C/min that tried to simulate the one that takes place in industry.
The fired specimens were characterised in order to evaluate, comparatively with the STD composition, the suitability of the proposed compositions for its use in the manufacture of ceramic wall tiles. Thus, the linear shrinkage was determined by measuring the diameter of the pieces with a calliper before and after firing and the porosity of the fired pieces was achieved from the measurement of their water absorption following the method described in ISO 10545-3:2018 standard, using two pieces per condition. For some compositions, in order to investigate their behaviour during firing, sintering experiments were carried out at a constant heating rate of 10°C/min in a dilatometer (DIL 402 Expedis Classic, Netzsch) to monitor the dimensional variations experienced by the specimens with temperature, up to a maximum temperature of 1100°C (this temperature was not exceeded in order to avoid the possible deterioration of the dilatometer probe by the specimens at high temperature).
A microstructural characterisation of some of the fired pieces in cross-section was carried out by means of field-emission gun environmental scanning electron microscope (FEG-ESEM; Quanta 200 FEG, FEI Company). The specimens were additionally attacked with hydrofluoric acid to reveal the crystalline phases present as is commonly done in electron microscopy. Moreover, qualitative analyses were performed by energy-dispersive X-ray microanalysis (EDX; Genesis 7000 SUTW, EDAX) and the crystalline phases were identified by X-ray diffraction. For the latter, it should be noted that the samples were dry milled and the resulting powder was analysed by XRD. Finally, the diametral compression method, widely described in the literature [28], was used to evaluate the mechanical strength and the stability of the phases present in the fired bodies was evaluated by determining the moisture adsorption of these specimens subjected to a high water vapour pressure treatment in an autoclave, following the procedure described in the tile moisture expansion test in ISO 10545-10:1995 standard.
Results and discussionComposition characterisation before firingTable 3 shows the values of the dry bulk density of the cylindrical pieces pressed following the procedure described in the previous section at 250kg/cm2 pressure and 5.5% moisture content (dry basis). The value of the porosity of the specimens, calculated from the ratio between the bulk density and the true density measurement (2.65g/cm3), is included in the same table. As can be seen, the bulk density of the pressed specimens decreases (their porosity increases) with respect to the STD composition in all cases. The decrease is significant in relation to the STD even for composition S1 which incorporates less residue (45wt%), with a slight decrease with subsequent increases in residue in compositions S2 and S3.
Measured dry bulk density and total porosity of specimens of each composition pressed at 250kg/cm2 pressure and 5.5% humidity with parameters d90, d50 and d10.
| Composition | Bulk density (g/cm3) | Total porosity (%) | d90/d50/d10 (μm) |
|---|---|---|---|
| STD | 2.01 | 24.2 | 57.7/10.2/2.7 |
| S1 | 1.88 | 29.1 | 20.5/5.1/1.7 |
| S2 | 1.87 | 29.4 | 28.0/5.6/1.8 |
| S3 | 1.85 | 30.2 | 39.1/7.0/2.0 |
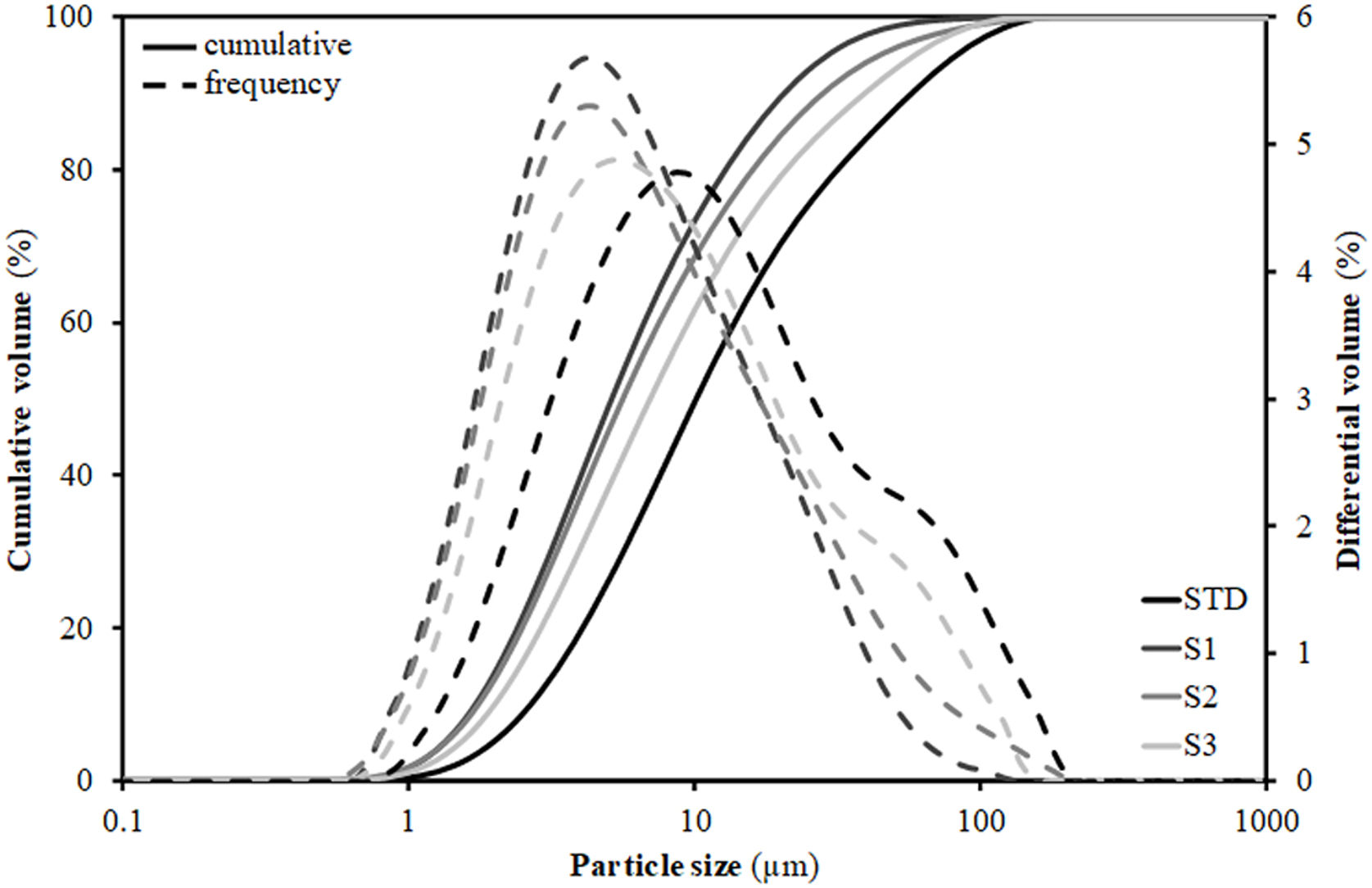
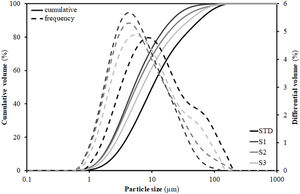
To understand this behaviour, Table 3 includes the particles’ diameters d10, d50 and d90 representative of the particle size distribution (PSD) of the compositions. These diameters were extracted from the PSD graphs shown in Fig. 1, which were obtained by laser diffraction, and are those that intercept respectively 10%, 50% and 90% of the accumulated volume of particle sizes. As can be seen, the introduction of residues results in a substantial modification of the width of the PSD, such that d50 and d90 decrease relative to the STD composition. As a result, the narrower PSD leads to a worsening of particle packing and consequently to a decrease in compactness as detailed in the literature [29]. However, this effect is not linear with the increment of the content of residues, as the greatest distortion of the distribution width occurs with the first incorporation of residues (composition S1), since the subsequent additions are made to the detriment of clay (colloid particles) proportion, so that when going from composition S1 to S2 and, above all, to S3, there is an enhancement in d90 (increase in the proportion of residue) associated with a decrease in the proportion of clay (enlargement in diameter d10). Although the values in Table 3 reflect a growth in the porosity of the pieces with respect to the STD composition of 5–6%, it should be noted that in all cases they are within the usual range of industrial practice for this type of product, where porosity values generally range between 20 and 30% [30].
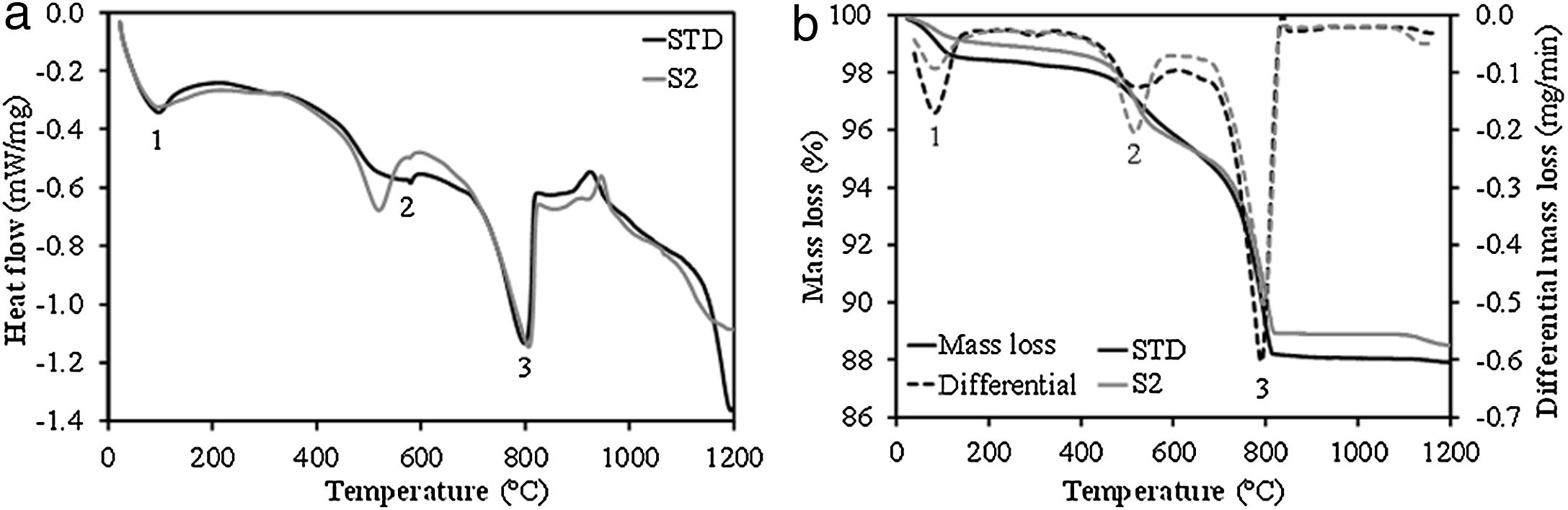
Behaviour of compositions during the firing stageFig. 2 shows the simultaneous thermal analyses (DSC-TG) of the STD composition and the intermediate composition S2. As can be seen, the profile of the DSC and TG curves of both compositions is very similar, presenting 3 endothermic peaks (marked as 1, 2 and 3 in the curves) associated with mass losses. Peak 1 relates to the loss of water adsorbed on the surface of the clay mineral. Peak 2 corresponds to the structural water of the clay mineral, this peak is much more defined and its area is larger in the case of composition S2, as a consequence of the greater kaolinitic character of the clay used (BC in Table 1). Finally, peak 3 describes the decomposition of the calcium carbonate, to which a greater mass loss is associated, as reflected in the TG curves. From this last peak, it should be noted that the decomposition temperature of calcium carbonate does not differ too much in both compositions, since, as can be observed, the peak corresponding to the maximum decomposition rate appears at very close temperatures (around 800°C). This occurs despite the presumably greater crystallinity of the calcium carbonate associated with the marble dust compared to that contributed by the clayey marl involved in the STD composition. This result can be considered of great relevance for the industrial viability of this composition incorporating marble dust, since the firing cycles used in the industry are very fast (less than 50min), which requires that the decompositions of the raw materials used occur relatively easily, so that the gaseous product of the decomposition does not interfere with the molten layer of glaze on the ceramic body.
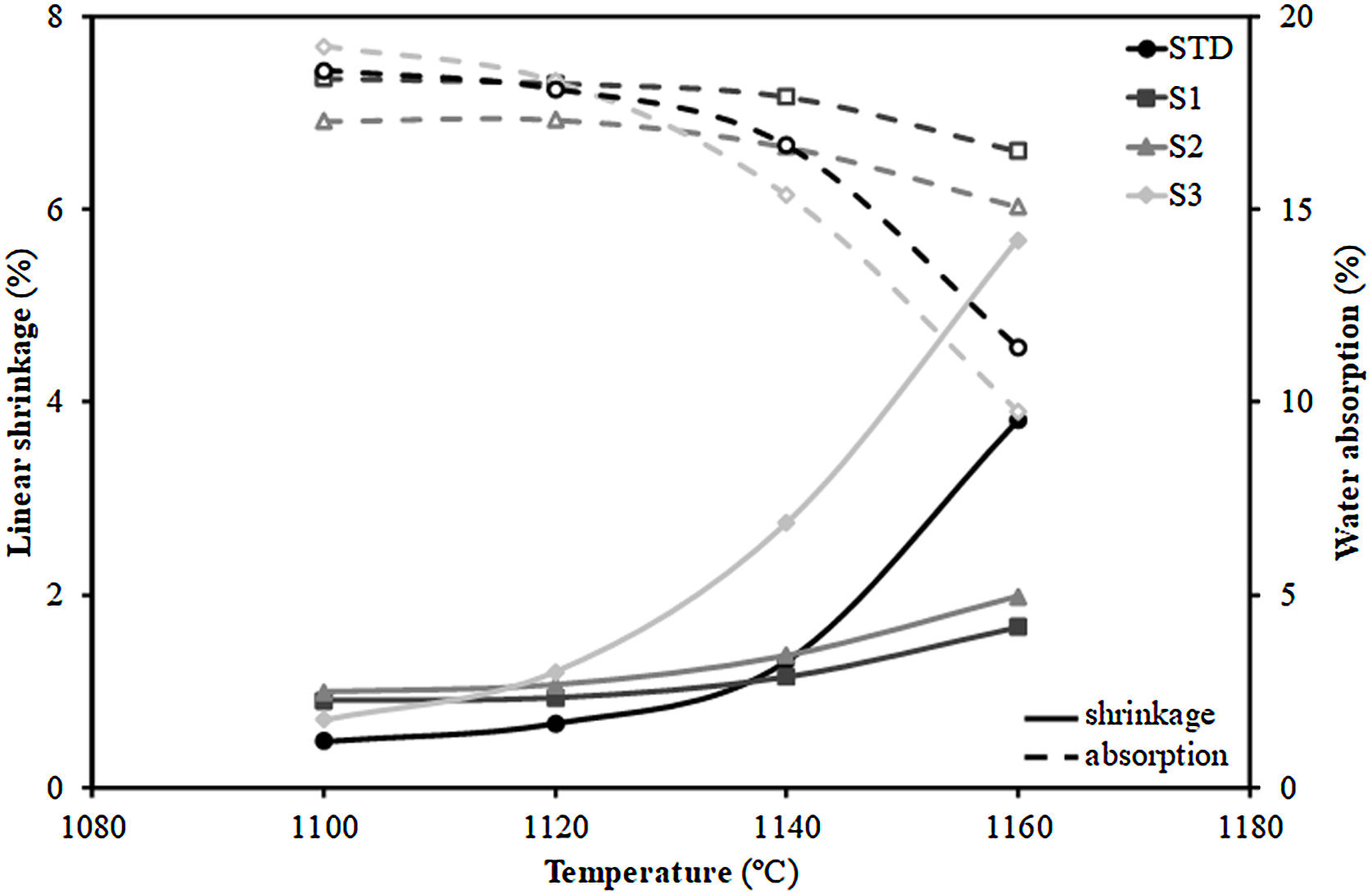
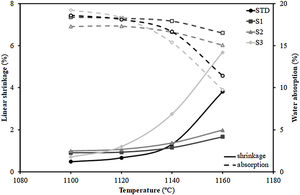
Additionally, Fig. 3 shows the firing diagrams (variation of linear shrinkage and water absorption of the pressed pieces with the maximum firing temperature). As can be observed, a typical composition used in the manufacture of porous wall tiles (STD composition) is characterised by a wide temperature range in which both the shrinkage of the tile and its porosity (water absorption) undergo little variation with temperature giving rise to great dimensional stability in the case of possible temperature variations that may occur in industrial processing. This behaviour is a consequence of the formulations used [29]. Indeed, the addition of alkaline earth oxide (generally calcium oxide in the form of calcium carbonate) facilitates the formation of crystalline phases (mainly wollastonite and anorthite) by reacting with the silica and alumina from the decomposition of the clay mineral, delaying the sintering process at higher temperatures. This reaction sintering mechanism has been widely described in the literature [31].
Concerning the compositions formulated with residues, the S1 and S2 not only maintain the profile of the curves, but also lengthen it, so that the range of dimensional stability (optimum firing range), which in the STD composition lies between 1100 and 1120°C, is increased to 1140°C, which is undoubtedly a very satisfactory technical result. In contrast, composition S3, which includes the highest proportion of residue, deviates widely from the profiles of compositions S1 and S2, showing a large variation of both linear shrinkage and water absorption with temperature and greatly reducing the temperature stability range with regard to the STD composition. The reason for the shortening of the firing range of the product is due to the significant increase in the proportion of fly ash from 15wt% (S1) to 30wt% (S3). Indeed, as described in the literature [32,33], the vitreous nature of fly ash, composed of an abundance of fluxing oxides (see Table 1), gives this material a role of liquid phase sintering promoter, which would explain the marked variation in linear shrinkage with temperature.
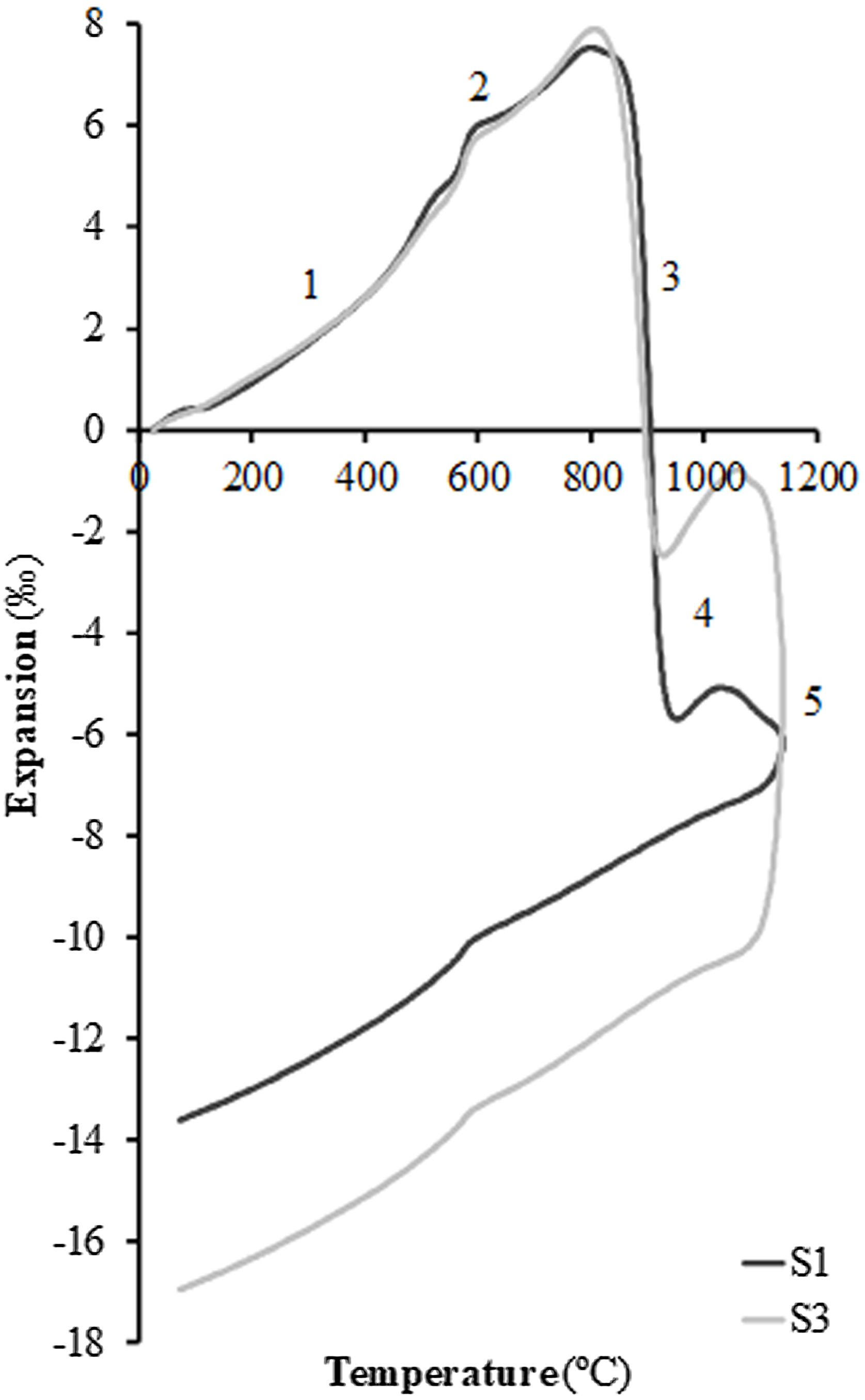
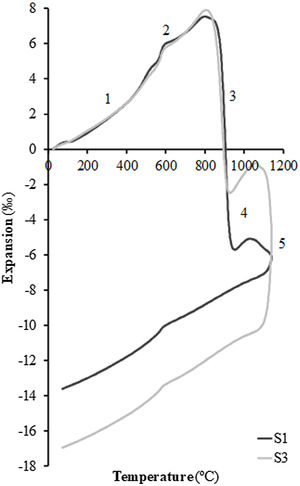
In order to test the effect of fly ash on the sintering of the composition, experiments were carried out in a dilatometer at a constant heating rate (10°C/min) starting from dry specimens of the S1 and S3 compositions up to the maximum temperature of 1100°C. Fig. 4 shows the curves obtained, whose profile is the typical one described in previous works on sintering of porous calcareous compositions for wall tiles [30]. As can be seen in the figure, the profile of the curves of the two samples is very similar, presenting the initial stretch of thermal expansion (stretch 1), followed by the allotropic expansion of the quartz (stretch 2) and, after the decomposition of the calcium carbonate, a stage of high sintering rate (stretch 3) associated with the sintering of the already decomposed clay mineral. This stage is greater in the case of composition S1, which contains a higher proportion of clay than composition S3 (45wt% compared to only 25wt%). This sintering stage is interrupted by the chemical reactions that take place for the formation of the crystalline phases based on the SiO2–Al2O3–CaO system, which are responsible, as mentioned above, for the dimensional stability of the piece over a wide range of firing temperatures (stretch 4 of the curves). When the proportion of fly ash in the composition increases, a second stage of sintering takes place above 1050°C (in this case interrupted by the maximum temperature of 1100°C at which the test was carried out, in order to avoid the deterioration of the measuring equipment's probe); this is a liquid phase sintering promoted by the greater presence of the molten glassy phase in the composition (stretch 5 of the dilatometric curves). As can be seen in Fig. 4, this liquid phase sintering section is much more pronounced in composition S3 than in S1 due to its higher fly ash content and is clearly visible despite the limitation of the maximum test temperature.
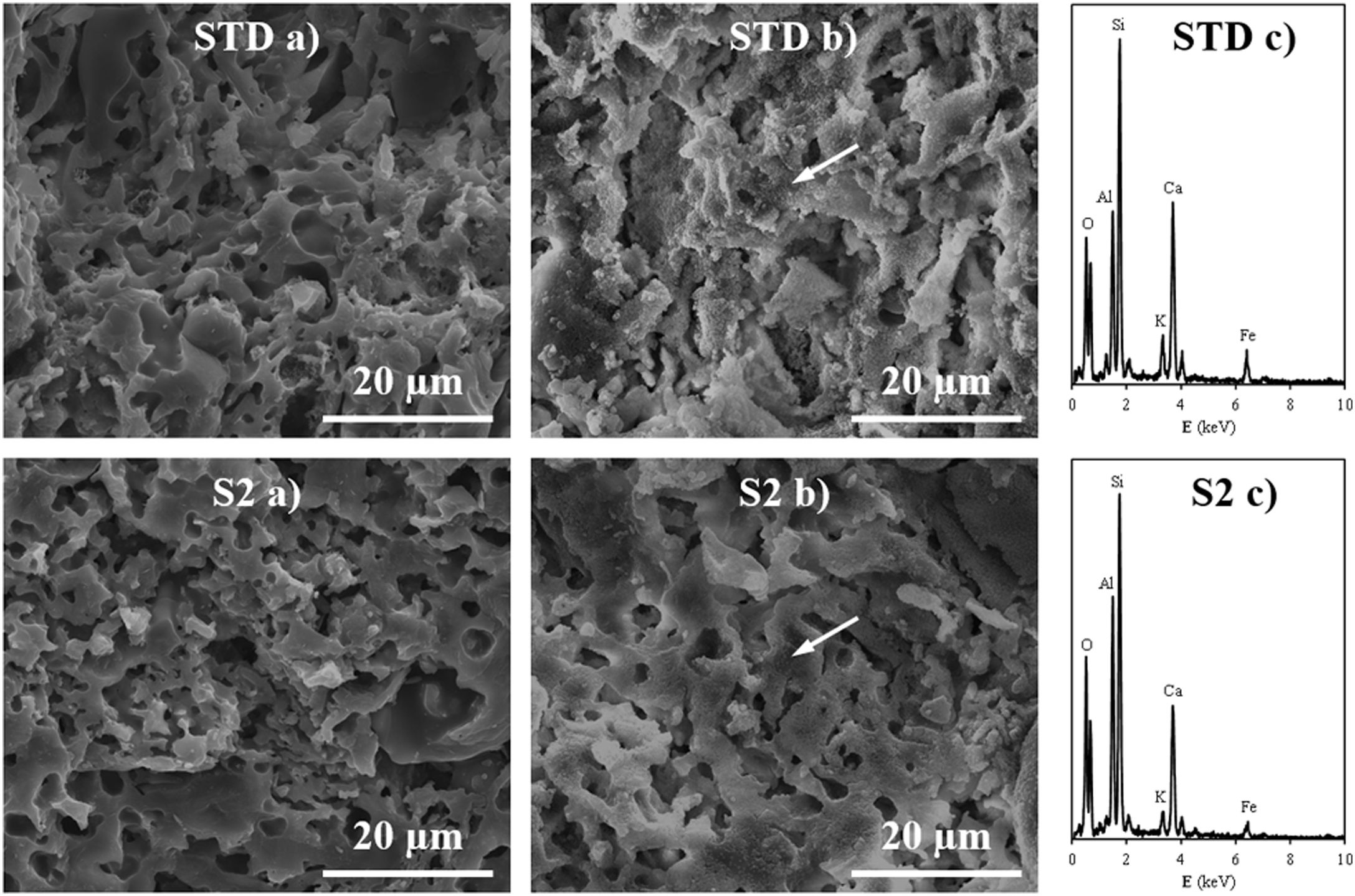
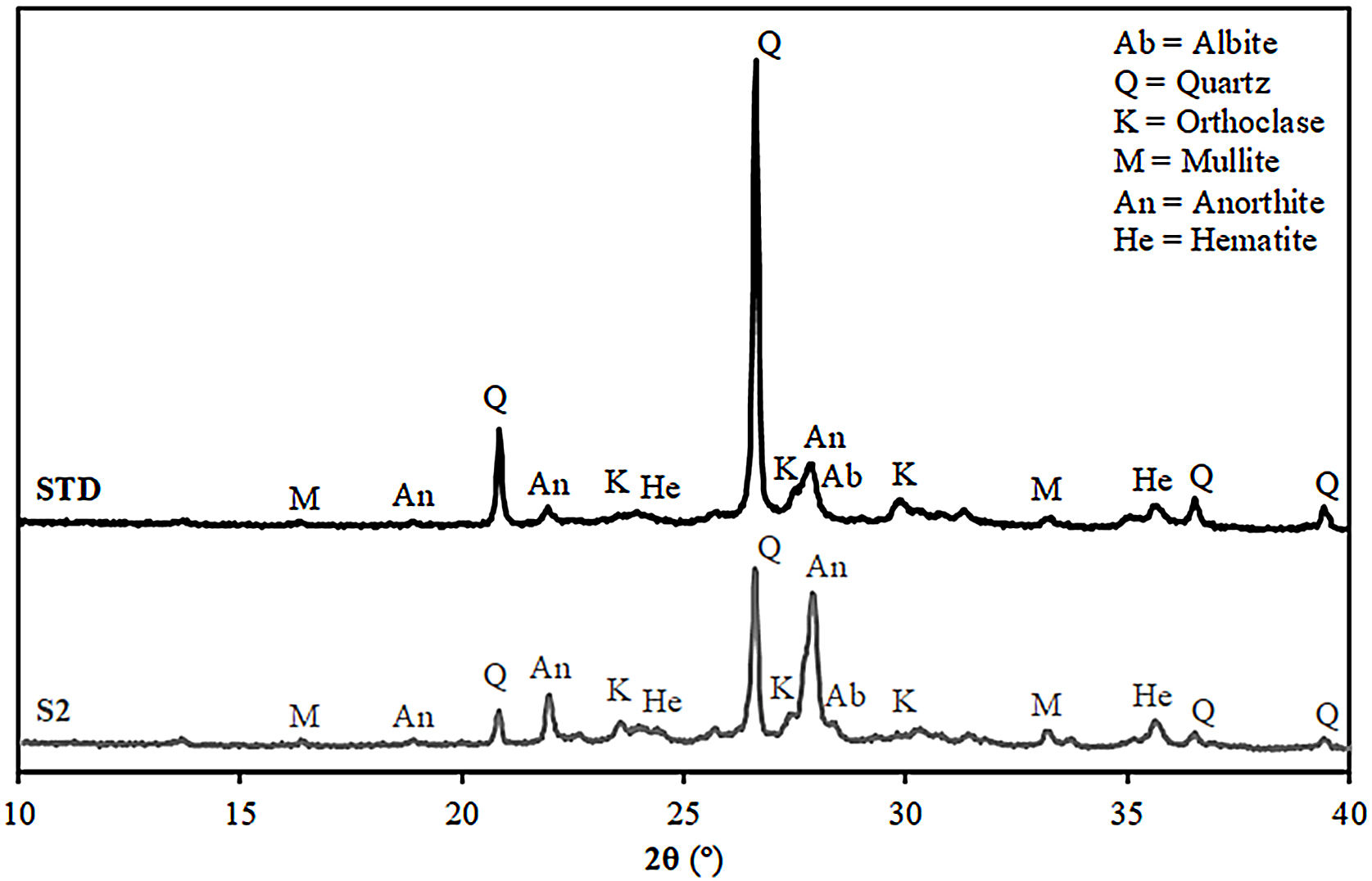
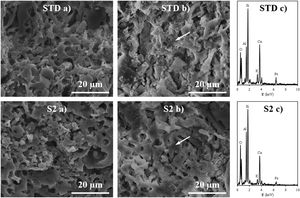
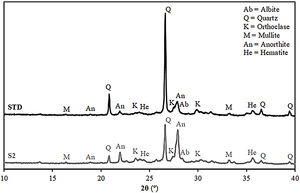
Fired bodies characterisationFig. 5 shows the electron microscope micrographs of specimens fired at 1120°C of the compositions STD and S2. S2 is the intermediate composition of those studied and it is the most interesting based on the characterised firing behaviour and the amount of waste used in its formulation. As can be seen, both specimens show very similar microstructures, characteristic of porous wall tiles. Thus, the abundant presence of vitreous phase, somewhat more plentiful in the composition S2, and porosity impede a clear observation of the presence of crystalline phase, which is produced by chemical reaction during the sintering process or due to unreacted starting particles. To reveal the presence of these crystalline phases, it is necessary to subject the specimens to an attack with hydrofluoric acid of the vitreous phase present, as is usually done in this type of sample treatment in electron microscopy. With this, a series of microcrystals appear on the surface which were analysed by EDX in order to know the composition at the points marked with an arrow. The analyses reveal a significant amount of silicon, aluminium and calcium (major components) with low amount of potassium, iron and other metals (minor components). XRD analyses of the compositions STD and S2 in Fig. 6 confirm the presence of quartz and anorthite as main phases. Both samples show very similar diffractograms with the same crystalline phases, although the proportion of unreacted quartz is higher in the STD composition and there is more anorthite formed during firing in composition S2.
Apart from their aesthetic characteristics, fundamentally marked by their decorated surface, wall tiles must comply with a series of technical requirements specified in the different national and international standards (UNE and EN standards). Among others, mechanical strength and water vapor adsorption represent two main quality requirements for long service use of wall tiles. To check if both requirements were met, the diametral compressive strength of pieces fired at 1100°C of the STD composition (optimum firing temperature based on the firing diagram in Fig. 3) and pieces fired at two temperatures of the intermediate composition S2, 1120°C and 1140°C (temperatures belonging to the optimum firing range as also set out above) as well as moisture adsorption in autoclave of these same pieces, were determined. Table 4 shows the results obtained. This table also includes the porosity values of the specimens obtained from the bulk density (Archimedes method) and water absorption values of fired specimens. As can be seen, the composition proposed in this study clearly improves the technical characteristics assessed when compared with STD composition. This finding relates to the higher degree of sintering promoted by the presence of the glassy phase coming from the fly ash, as set out previously in firing and sintering diagrams (Figs. 3 and 4) as well as microstructure micrographs (Fig. 5). Indeed, the presence of a higher proportion of glassy phase during firing results in a decrease in the porosity of the fired specimen of the composition S2, which leads to an increase in its mechanical strength, as described extensively in the literature on the effect of firing porosity on mechanical strength [34,35]. The decrease in porosity and the higher formation of crystalline phases with calcium oxide (higher peak intensity for anorthite in XRD analyses) are the reasons for the lower moisture adsorption value. The effect of porosity due to the higher degree of sintering progress can be verified by observing the values of mechanical strength and moisture adsorption of the fired specimens at 1140°C. However, it should be noted that, although the increase in temperature would lead to an improvement in the properties of the fired product with the composition S2, the linear shrinkage of the piece and, above all, the variation of the linear shrinkage with temperature as shown in the firing diagram in Fig. 4 would lead to greater difficulty in the processing of industrial tiles and, in particular, the manufacture of large-format tiles with uniform sizes.
Mechanical strength by diametral compression of pieces fired at 1100°C of the STD composition and of pieces fired at 1120°C and 1140°C of the intermediate composition S2, as well as the moisture adsorption and the apparent porosity value of these same pieces.
| Composition | Mechanical strength (MPa) | Moisture adsorption (‰) | Porosity (%) |
|---|---|---|---|
| STD (1100°C) | 8.1 | 3.7 | 33.1 |
| S2 (1120°C) | 14.0 | 2.4 | 30.9 |
| S2 (1140°C) | 14.5 | 2.3 | 29.9 |
Compositions have been designed for the manufacture of ceramic wall tiles, using three widely available industrial wastes in a geographic area close to the ceramic tile industry in Castellón (Spain): marble dust, fly ash and fired tile scraps, all of which play different roles in the designed composition. In all cases, it is necessary to incorporate a certain proportion of plastic clay in order to facilitate the press-forming of the pieces.
It has been shown that it is possible to develop ceramic tiles whose behaviour during the stages of the manufacturing process (pressing and firing), as well as the properties of the fired product (microstructure, porosity, mechanical strength and moisture adsorption) resemble (and in some cases exceed) an industrial spray-dried powder used as a reference. Based on the results obtained, it can be concluded that it is technically feasible to manufacture wall ceramic tiles incorporating up to 55wt% of the 3 addressed wastes, while maintaining 15wt% of marble powder to ensure the development of the desired crystalline phases in the fired product.
Acknowledgements to the Ministry of Science and Innovation of the Government of Spain and the European Regional Development Fund (ERDF) of the European Union for the funding received within the project “CONFORTMA RTC-2017-5904-5 New materials for a more sustainable and comfortable construction”.