The conflict in Ukraine has meant that the supply of ball clay from this country has been cut off, giving rise to a serious setback for the manufacture of porcelain tile, as this raw material was a basic component for the formulation of this type of ceramic tile. This work proposes the use of a clay from the Afyon area in Turkey as an alternative, which is formed by a complex mixture of clay and non-clay minerals.
In the first part of the study, a complete characterisation of the clay has been carried out, comparing it with a standard Ukrainian clay. In a second part, a composition in which Afyon clay is the main clay component has been formulated and characterised in comparison with a standard composition formulated with Ukrainian clays. Overall, the results show that, although some properties for Afyon clay differ from those for Ukrainian clays, the behaviour of the proposed composition in the different stages of the process, milling/deflocculation, pressing and firing, is similar to that of the standard composition, thus adapting it to the requirements of industrial practice. This provides a feasible alternative way of replacing Ukrainian ball clays.
El conflicto en Ucrania ha supuesto el corte de suministro de arcilla «ball clay» procedente de este país, causando un serio contratiempo para la fabricación de gres porcelánico, ya que esta materia prima era un componente básico para la formulación de este tipo de baldosa cerámica. Este trabajo propone como alternativa el uso de una arcilla procedente de la zona de Afyon en Turquía, la cual está formada por una mezcla compleja de minerales arcillosos y no arcillosos.
En la primera parte del estudio, se ha llevado a cabo una caracterización completa de la arcilla, comparándola con una arcilla ucraniana estándar. En una segunda parte, se ha formulado una composición en la cual la arcilla de Afyon es el principal componente arcilloso y se ha caracterizado comparándola con una composición estándar formulada con arcillas ucranianas. En conjunto, los resultados muestran que, aunque algunas propiedades para la arcilla de Afyon difieren de las de las arcillas ucranianas, el comportamiento de la composición propuesta en las diferentes etapas del proceso, molienda/desfloculación, prensado y cocción, es similar al de la composición estándar, adaptándose así a los requisitos de la práctica industrial. Ello constituye una alternativa viable para sustituir las arcillas plásticas ucranianas.
The consumption of white plastic clay (“ball clay”) from Ukraine has acquired enormous importance in the ceramic tile industry in Europe, basically due to the irruption of porcelain stoneware, back in the 1990s of the last century, which has increasingly demanded high quality raw materials [1]. Ukrainian clay deposits originate from the Donetsk basin (Donbas region) where sedimentary strata from the Miocene are exploited [2,3]. The reason for its rapid and successful implementation in porcelain tile compositions lies in the right combination of properties it possesses, in particular the trinomial whiteness-plasticity-flux, which is difficult to find in ball clays of other origins or provenances [4–6]. The excellence of this type of clay has been widely described in the literature, relating its technological properties to its mineralogical composition and particle size distribution. Thus, Zanelli et al. [7] indicate that Ukrainian clay is characterised by a very fine particle size, a clay mineral with a poor degree of crystallinity and a low proportion of quartz. Such has been its growth that, until 2022 year, consumption was estimated at over 3 million tonnes per year, destined for the main European porcelain tile producers such as Italy and Spain. Basically, two qualities are marketed, although with multiple variants, which are differentiated by their plasticity, in other words, by the clay mineral content. In terms of chemical composition, these two qualities have alumina contents of 23–24% and 27–28% [2].
The outbreak of the war in Ukraine in February 2022 led to an unexpected cut in the supply of this clay, and porcelain stoneware formulations containing this type of clay, in percentages of between 30% and 45%, quickly had to be reconsidered. This approach has not been easy because, in addition to the undoubted technical and processing challenges of replacing a major component of the formulation with others, the availability of possible alternatives is also an issue, given the enormous volume of supply of Ukrainian clay.
One of the most appreciated characteristics of Ukrainian clay is its plasticity, which is also provided by a set of clay minerals (mainly kaolinitic in nature) with a low proportion of iron oxide, the main chromophoric impurity of white clays, which gives it a high whiteness after firing. For this reason, the search for alternative clays to Ukrainian clay must focus, to a large extent, on a clay that provides a high degree of plasticity to the composition, without affecting the final degree of whiteness of the whole [7].
One clay that can provide this plasticity is from the Afyon region of Turkey. Commercially known as “Afyon clay”, this material grows out around the Alanyurt village of Afyonkarahisar City. The Afyon clay is an ignimbrite type pyroclastic rock which is variously altered [8]. There are basically 3 type clay-like zones occurring in the mine: 1 – white coloured zones: illitic parts, 2 – beige and creamy levels: kaolinite dominated parts, 3 – green-pale or green zones: contain smectitic clays [9]. However, all the different zones of the Afyon clay cannot be operated separately, due to the complex alteration events. Thus, supply companies usually operate all clay groups together, marketing them as a single product of Afyon clay.
Although different studies on the origin and geological structure of these deposits are known [10,11], there are practically no works that deal with the physico-chemical characterisation of this clay, nor its possible application in the manufacture of ceramic tiles. The lack of availability of Ukrainian plastic clay increases the interest in the characterisation and study of the ceramic potential of this clay. Thus, in a recent work in Turkish [12], Tarhan et al. proposed the reduction of Ukrainian clay by introducing 7–10% Afyon clay, however, all the proposed compositions preserved Ukrainian clay in a proportion of not less than 12%. No other work has been found that complements the above or goes further for the total elimination of Ukrainian clay.
As a consequence of the above, the present work has a twofold purpose. On the one hand, carrying out a physico-chemical and technological characterisation of Afyon clay, and as a second objective, testing a porcelain tile composition without Ukrainian clay by using Afyon clay as the main clayey raw material. This research will propose solutions, through rigorous analysis, to the drastic situation caused to the ceramic tile industry by the cut in the supply of Ukrainian clay.
ExperimentalMaterialsA commercial sample of Afyon clay supplied by the R&D department of the Çanakkale Seramik company was used. Together with this clay, other commercial raw materials typically used in porcelain stoneware compositions in the industry were also employed in this work. These raw materials, also supplied by Çanakkale Seramik were: Ukrainian clays of around 25% (U-25) and 30% (U-30) alumina content, Istanbul clay (I) and Turkish sodium feldspar (F). These clays contain kaolinite and illite/muscovite as main clayey phases together with different amounts of quartz, while the main phase of feldspar is albite. The properties and potential uses of Istanbul clay in ceramic tiles have been determined in previous works [13,14]. Table 1 shows the chemical composition of these raw materials.
Chemical composition of the standard raw materials used (in wt%).
| Ref. | SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | Na2O | K2O | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| U-25 | 60.6 | 25.5 | 1.3 | 0.8 | 0.2 | 0.5 | 0.2 | 2.0 | – | 8.9 |
| U-30 | 55.1 | 30.4 | 1.5 | 0.7 | 0.3 | 0.6 | 0.4 | 2.1 | 0.2 | 8.7 |
| I | 54.6 | 28.6 | 1.4 | 2.5 | 0.1 | 0.6 | – | 2.1 | 0.5 | 9.6 |
| F | 71.9 | 16.4 | 0.2 | 0.2 | 0.3 | 0.1 | 9.2 | 0.6 | 1.0 | 0.7 |
Afyon clay sample has been characterised from a physico-chemical point of view, following a characterisation protocol in accordance with its subsequent use in the manufacture of ceramic tiles.
Thus, in the first place, chemical analysis by X-ray fluorescence spectrometry (XRF; Axios, Panalytical) and mineralogical analysis by X-ray diffraction (XRD; D8 Advance Diffractometer, Bruker Theta-theta) were carried out. XRF was performed on beads of fused material, using a mixture of lithium tetraborate and lithium metaborate as fluxing agent, a Rh anode tube, 4kW working power and certified reference materials. XRD included an identification of the clay fraction by multiple analysis following typical treatments [15]. This analysis comprises an initial separation of the clay mineral by sedimentation and sieving below 5μm, a preparation as oriented aggregate by filtration to improve the diagnostic signal of the basal reflections, a glycolation with ethylene glycol and a heat treatment at two temperatures: 350°C and 550°C. XRD analyses were carried out using a Cu Kα radiation (λ=0.154nm) at 30kV and 40mA, with a range of 2θ from 5° until 90°, a step size of 0.02° and a scanning speed of 0.5s/step.
Additionally, the particle size distribution was determined by laser diffraction (Mastersizer 3000, Malvern). A simultaneous thermal analysis (DSC-TG) was also carried out in a thermal analysis equipment (STA 449 F5 Jupiter, Netzsch), obtaining the thermogravimetric (TG and DTG) curves and the differential scanning calorimetry (DSC) curve. The test conditions for this thermal analysis were maximum temperature 1205°C, heating rate 10°C/min, platinum crucible and dynamic air atmosphere.
Moreover, the rheological behaviour of the Afyon clay was assessed by determining the deflocculation curve at a given solids content using a twist wire viscometer (Gallenkamp Torsion Viscometer). The target solid content and percentage of deflocculant to solid is specified as that which allows to obtain a minimum viscosity value in the deflocculation curve lower than 1000cP [16]. A commercial liquid mixture based on sodium polyacrylate and sodium silicate (Fluidax 461, AMC) was used as dispersant. The viscosity variation with the addition of deflocculant was determined to construct the clay deflocculation curve. The degree of deflocculation was estimated as follows: after each addition of deflocculant, the mixture was stirred to homogenise it, the viscosity was measured after 1min of standing, and finally the viscosity curve was constructed as a function of the addition of deflocculant.
Together with these techniques, a technological characterisation has been done, consisting of the determination of the Atterberg plastic index by indentation method [17], as well as the assessment of clay behaviour in pressing and firing. For that purpose, a clay sample was subjected to a primary grinding in a hammer mill with an output sieve of 1mm and a subsequent secondary aqueous wet grinding in a ball mill for 10min at a rotation speed of 410rpm, determining the residue on a 63μm sieve which was observed using a stereo magnifying microscope (DSX-10, Olympus) with dark field observation and 105× magnification. For wet milling, 1000cm3 jars were used, filled with 180g of 1.4–2cm diameter alumina balls, 400g of solid and 400g of water. The resulting suspension was dried, crumbled and conditioned for subsequent pressing by spraying water until a powder moisture content of about 5.5% was obtained. The conditioned powder was pressed at a pressure of 300kg/cm2 to obtain cylindrical specimens of 4cm in diameter and around 5mm thick. Subsequently, the specimens were dried in an oven at 110°C and their bulk density was determined by the Archimedes method. To evaluate the firing behaviour, the dried specimens were then subjected to a heat treatment in a laboratory electric kiln, simulating industrial firing cycles. Thus, the cycle consisted of a rapid heating and cooling with a residence time at the maximum temperature of 6min, resulting in a total firing cycle of about 50min. The two maximum firing temperatures tested were 1120°C and 1220°C. Then, the linear shrinkage was determined from the difference between the diameter of the specimen before and after firing, defining this parameter on a dry basis, and the water absorption was obtained by the vacuum method following the ISO 10545-3:2018 standard.
Finally, the same determinations were carried out with the Ukrainian clay U-25 for comparison purposes, since this is the main type of Ukrainian clay quality used in the industry.
Porcelain tile composition design and characterisationUsing Afyon clay as main clayey material, a composition was designed in which the raw materials described above have been used. The composition formulation aimed at preserving the characteristics of porcelain stoneware as well as its ease of processing. This Afyon-based composition was designed from the findings obtained in the characterisation programme set out above. For comparison purposes, a standard composition was also formulated using Ukrainian clay, as it had been used for years until the supply was cut off.
The compositions were prepared in the form of aqueous suspension (50wt%) by wet grinding the raw materials in a ball mill to reduce the particle size, as prepared in the ceramic industry. The grinding time was 20min, which allows to reach a typical 40μm oversize below 2% for the two compositions. Then, the suspension can be dried and dry milled to obtain a ceramic powder. This was followed by conditioning of the press powder to a moisture content of 5.5% and subsequent pressing of cylindrical specimens with the same characteristics as in the case of Afyon clay, although in this case the pressing pressure used was 400kg/cm2, more in line with industrial porcelain tile manufacture. Then, the pressed bodies were dried at 110°C and characterised by determining the bulk density by Archimedes method and mechanical strength by 3-point bending test method (Universal Testing Machine, Hoytom) following the procedure set out elsewhere [18]. A minimum of 5 parallelepiped samples of dimensions 8cm×2cm×0.7cm were prepared for the determination of the mechanical strength, which were tested with a crosshead speed of 10mm/min. The firing of the dried specimens was carried out in the electric laboratory kiln as set out above at maximum temperatures between 1160°C and 1220°C with a dwell time of 6min. The linear shrinkage and water absorption of all the fired specimens were determined as well as the fired bulk density by Archimedes method.
As set out above for Afyon clay, the rheological behaviour of the compositions was assessed by determining the deflocculation curve at a solid content around 70% using the same twist wire viscometer as previously. This solid content is close to that used in the industrial practice.
Finally, the microstructure and the whiteness of the fired specimens obtained from the formulated compositions were analysed. On the one hand, the microstructural characterisation in fracture cross-section was carried out by means of field-emission gun environmental scanning electron microscope (FEG-ESEM; Quanta 200 FEG, FEI Company). A secondary electron detector signal was used under high vacuum conditions during the observation and the specimens were platinum coated by sputtering previously. Additionally, observations were also performed on samples attacked with a 2.5% hydrofluoric acid solution for an etching time of 2min to reveal the crystalline phases present. On the other hand, the whiteness was estimated from chromatic coordinates of the CieLab system (L*, a*, b*) by using a visible light spectrophotometer (Color-Eye 7000A, Gretag Macbeth). For each composition, two measurements were made with CIE standard illuminant D65, observation angle 10° and wavelength range from 360nm to 750nm.
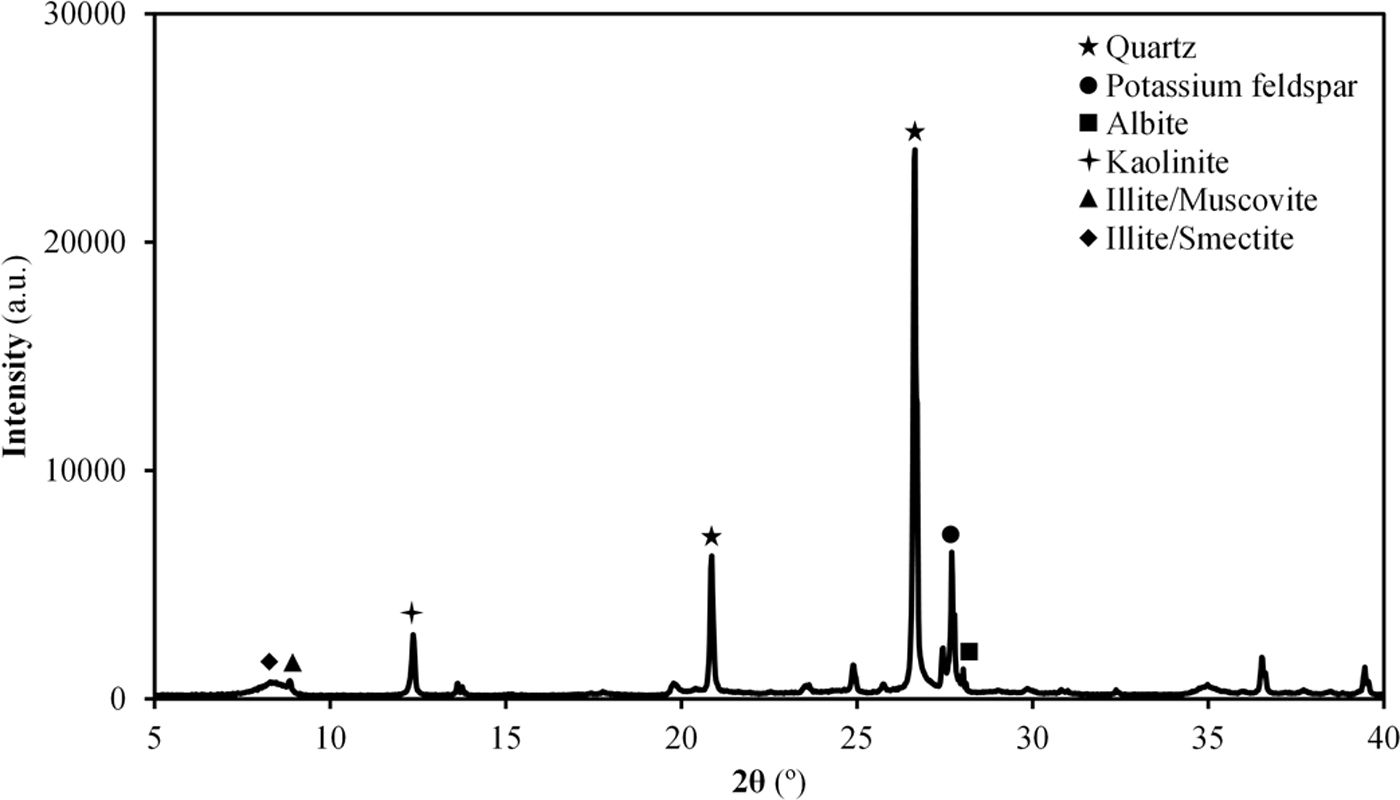
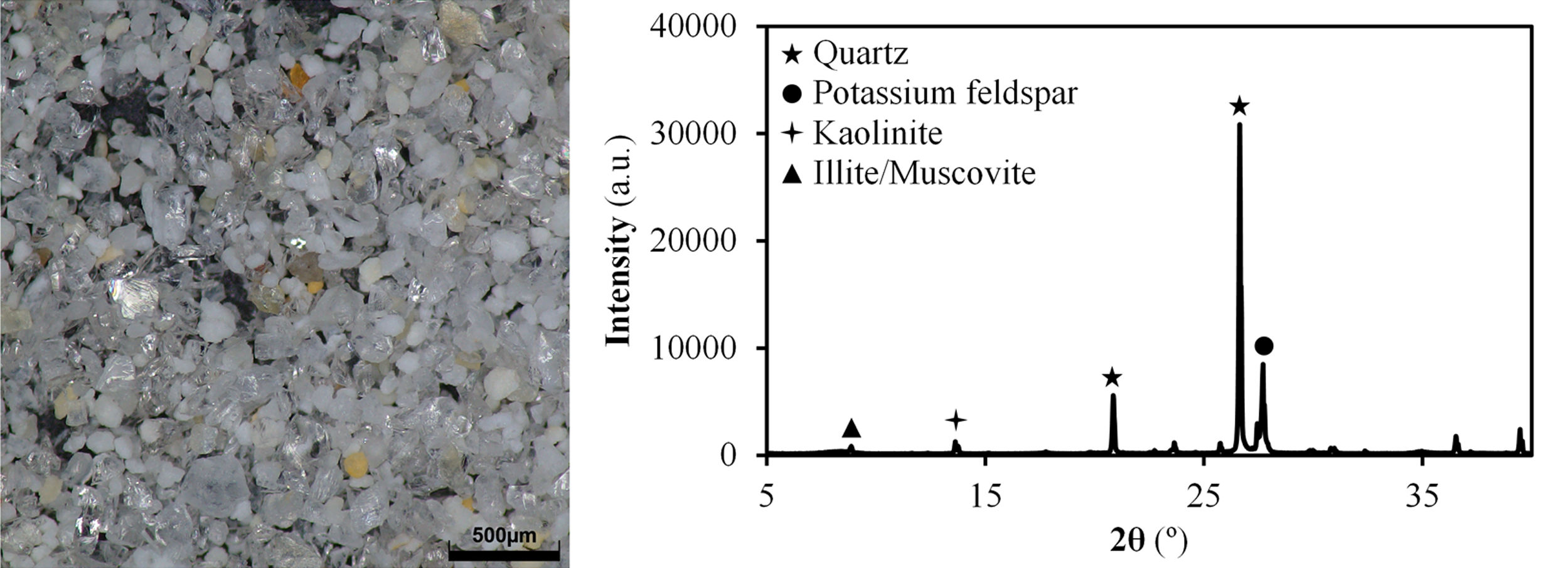
Results and discussionCharacterisation of Afyon clay for ceramic useTable 2 shows the chemical composition of Afyon clay sample and Fig. 1 presents its XRD pattern. As it can be seen, the clay is expected to present a very high quartz content, as deduced by the low loss on ignition of the sample, together with a mixture of other clayey and non-clayey minerals. From the silica content in the XRF analysis, an approximate estimate of 50% of quartz can be presumed. According to the XRD pattern in Fig. 1, the other 50% is made up of kaolinite, illite and potassium feldspar. This mineralogical description almost coincides with that previously reported [12]. Thus, in both cases, the clay mineral content is attributed to kaolinite and predominantly to the illite/sericite group, as a consequence of the presence of abundant potassium oxide in the composition. However, in contrast to the observations of these authors, a small peak has been identified which is associated with the presence of clay minerals of a smectitic nature. In fact, as the mineralogical studies of the deposit indicate, smectitic clay zone can be distinguished from the other illitic and kaolinitic zones [9]. Also noteworthy is the low presence of iron oxide in its composition, reaching values similar to those exhibited by Ukrainian clays (see Table 1).
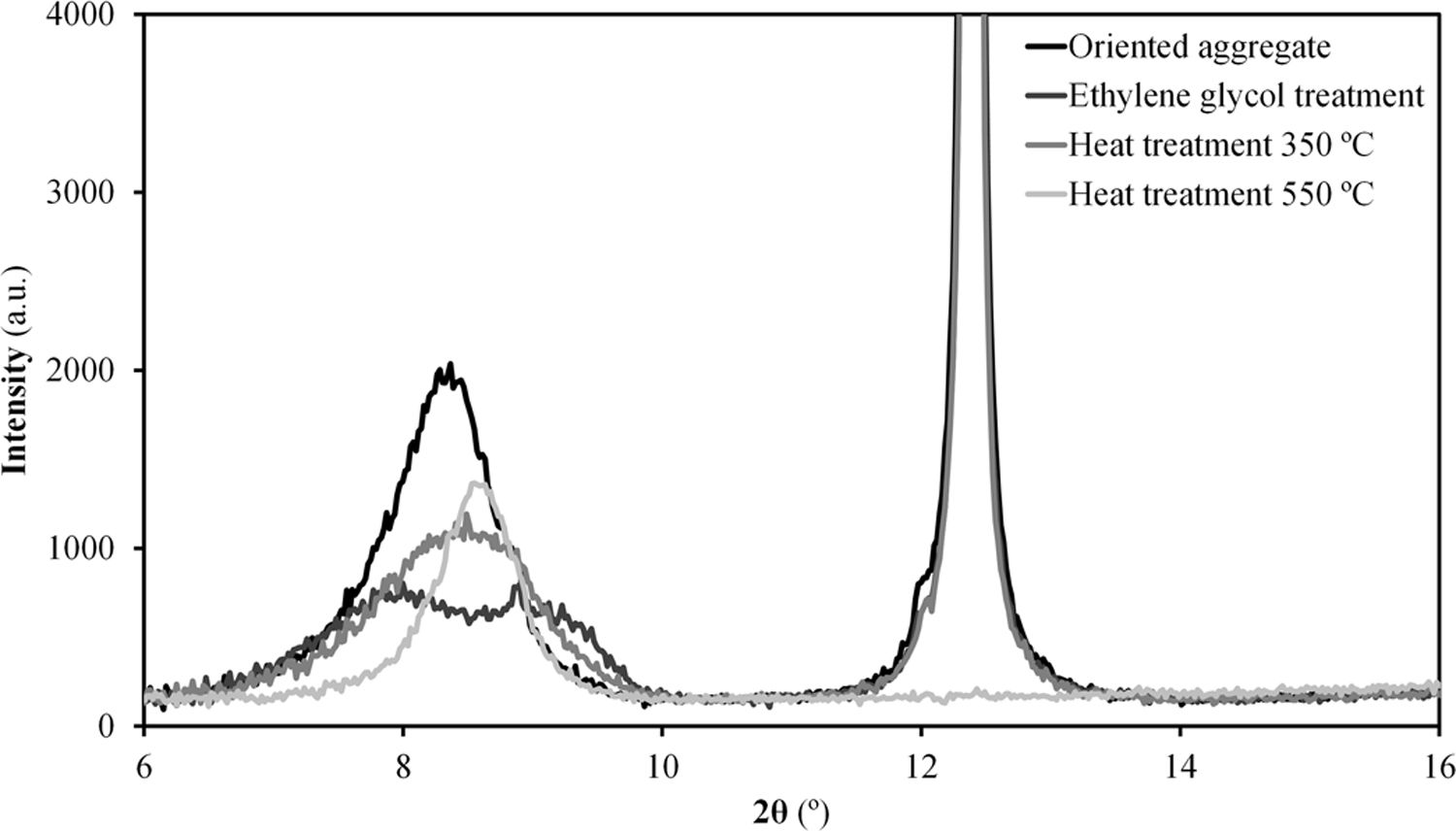
The results of the multiple analysis by means of XRD are detailed in Fig. 2. This analysis allows to establish more precisely the mineralogical composition of the mixed-layered clay minerals, which often pose difficult interpretation [19]. The results reveal the existence of an interstratified clay mineral of illitic–smectitic nature, with a peak of considerable intensity at angles below 10° after the treatment of oriented aggregates. The existence of illite is confirmed by a peak which remains after heat treatment at 550°C. Meanwhile, the presence of degraded smectite is evidenced by a peak further to the left of that corresponding to illite after glycolation, which disappears when the sample is heated. According to the literature [20,21], the smectite structure collapses with heat treatment and it is not detected in the diffractogram. The intense peak on the right (2θ≈12°) corresponds to kaolinite.
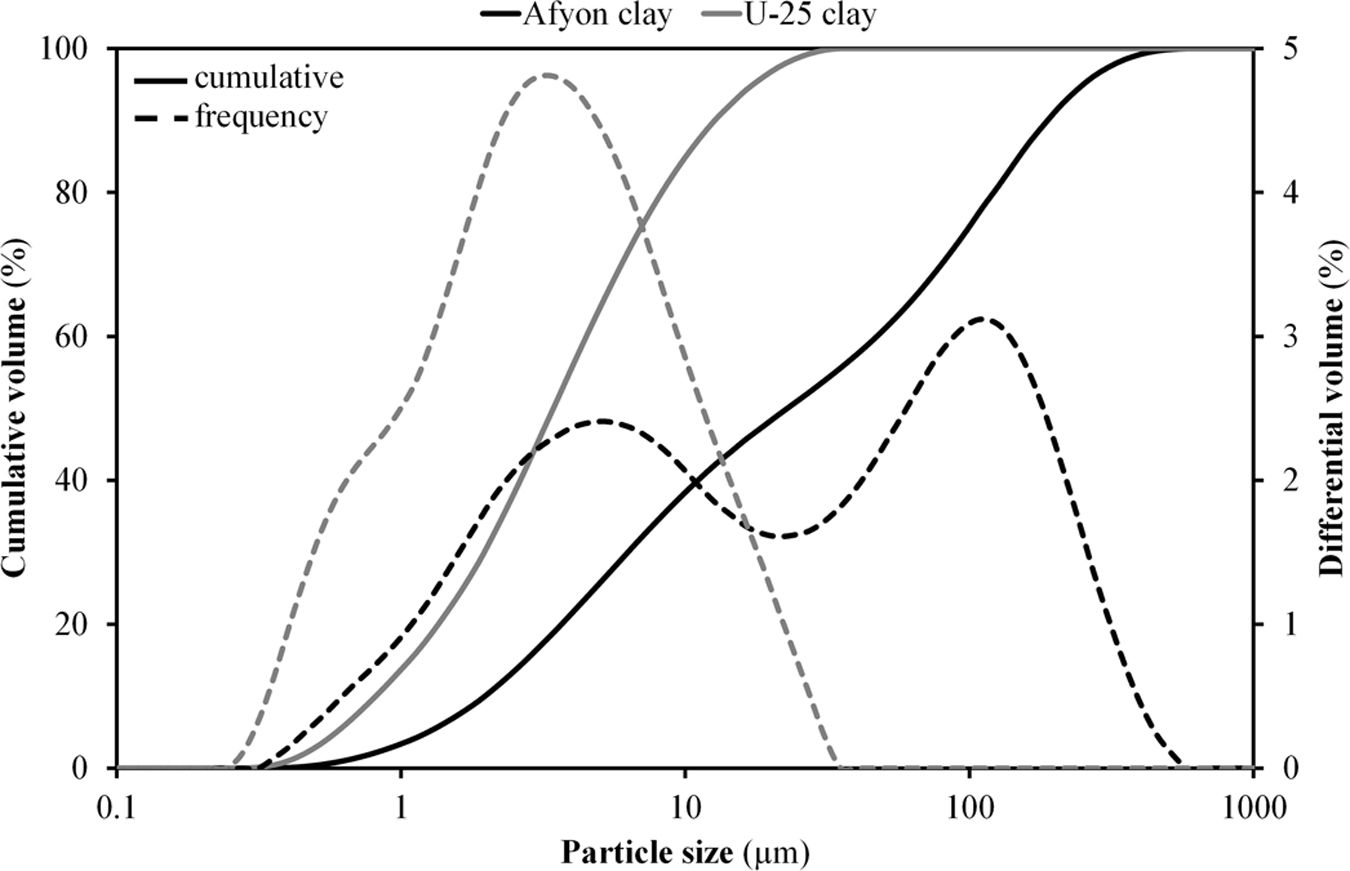
Fig. 3 shows the results of particle size distribution obtained by laser diffraction for Afyon clay in comparison with U-25 sample. As it can be observed the amount of colloidal fraction of U-25 clay is much higher than that of Afyon clay as a consequence of its lower amount of quartz [5,7]. Furthermore, the quartz contained in the Afyon clay displays a fairly large particle size giving rise to a clear bimodal particle size distribution as observed in Fig. 3.
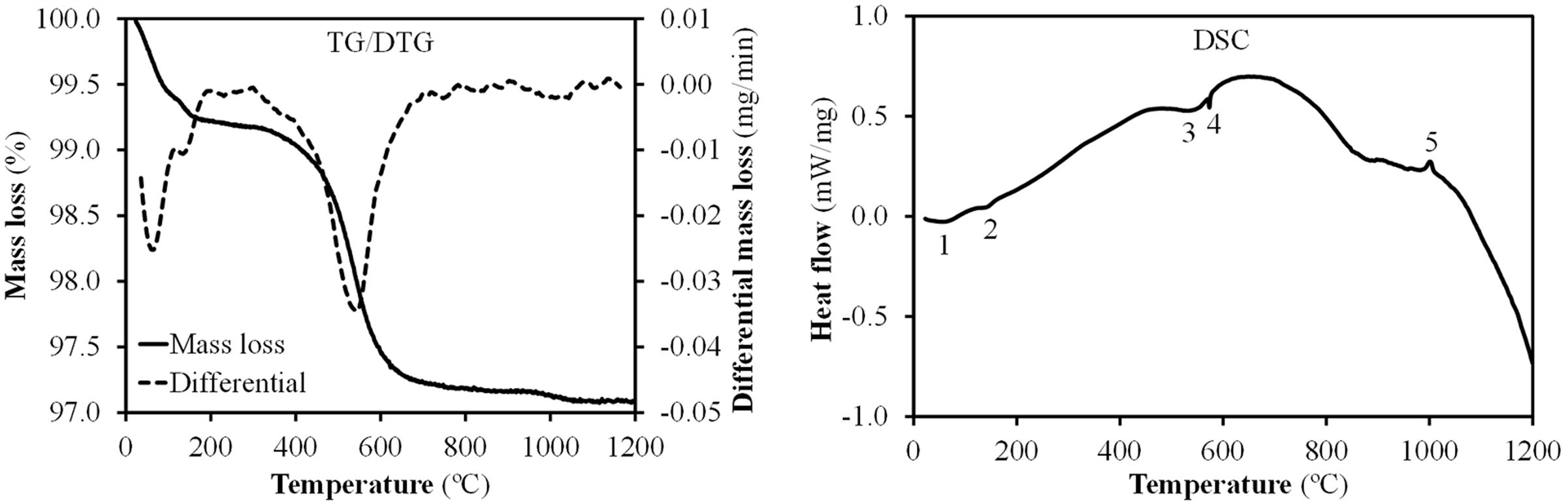
Fig. 4 shows the results of simultaneous thermal analysis (TG/DTG-DSC) for Afyon clay sample. Relevant peaks are marked by numbers. Peaks 1 and 2 relate to moisture loss of absorbed water and more specifically, peak 2, at slightly higher temperature, normally is assigned to adsorbed water loss from colloidal clay minerals [22]. Between 500 and 600°C dehydroxylation of clay minerals occurs as indicated by the weight loss as well as the large endothermic area in DSC curve (peak zone number 3) which corresponds to 540°C and 560°C assigned to illite group and kaolinite minerals breakdown respectively [2]. The high amount of quartz is responsible for peak number 4 in DSC curve which represents the allotropic alpha-beta transformation of this mineral. Finally, the small crystallisation peak number 5 could be attributed to the crystallisation of the silica-alumina spinel from kaolinite (temperature above 980°C), rather than to the spinel formed from the breakdown of the illitic or smectitic clay mineral (temperature above 950°C) [23].
Regarding the technological characterisation, i.e., the behaviour of the clay evaluated with a view to its possible application in the manufacture of porcelain tile, the results of the following determinations are detailed in Table 3, where:
- •
Grinding behaviour: residue on 63μm sieve after a standard grinding time (10min).
- •
Deflocculation behaviour: viscosity in torsion viscometer (η) at a specific solid content (SC) and percentage of deflocculant (Defloc.) for each clay.
- •
Pressing behaviour: plasticity index (PI) and dry bulk density (ρdry) after pressing of the wetted powder under standard pressing conditions.
- •
Firing behaviour: linear shrinkage (LS) and water absorption (WA) of fired specimens at two maximum firing temperatures (1120°C and 1220°C).
Technological characterisation summary of Afyon clay in comparison with standard Ukrainian clay (U-25).
| Clay | Residue (%) | SC (%) | Defloc. (%) | η (cP) | PI (%) | ρdry (g/cm3) | LS1120°C (%) | LS1220°C (%) | WA1120°C (%) | WA1220°C (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| U-25 | 3.4 | 58 | 0.90 | 570 | 38 | 1.80 | 6.8 | 10.4 | 9.0 | 0.7 |
| Afyon | 22.9 | 63 | 0.45 | 560 | 37 | 1.91 | 0.1 | 1.2 | 14.2 | 11.5 |
For all these determinations, set out in previous section, comparison with standard Ukrainian clay (U-25) has been done as included in this same table.
The first striking observation deals with the large 63μm sieve residue of Afyon clay when ground in a laboratory planetary ball mill, especially when compared to Ukrainian clay. Fig. 5 shows a micrograph of this residue, together with the XRD diffractogram. Both figures show that the high amount of residue is mainly a consequence of the large content of quartz (around 50%) in this clay, also highlighting that it displays a coarse particle size that must be ground when incorporated into a ceramic tile composition, in order to adapt the milling residue to the values dictated in the manufacture of porcelain stoneware (between 1% and 2% residue at 40μm). This high proportion of quartz would explain other characteristics shown in the technological characterisation of the clay as summarised in Table 3. Thus, on the one hand, it is observed that the solid content achieved by the Afyon clay in the deflocculation test is higher than that obtained with the Ukrainian clay sample with a lower amount of deflocculant, despite the fact that the plasticity value determined was almost the same for both clays. The effect of clayey mineral content and nature in ball clays used in porcelain tiles has extensively analysed in clays from different origins and countries [6,24]. While the plasticity of clays depends to a large extent on the specific surface, i.e., the content and nature of clayey mineral, a minor influence of the kaolinite and illite crystallinity is appreciable. In addition, the presence of a smectitic component, even in low proportion, can strongly impact on plasticity and technological properties as set out elsewhere [24,25]. Thus, for example, assuming a plasticity index value of zero for quartz, the plasticity of all the clay minerals comprising the Afyon sample should exceed a value of 75%, which in all probability is again evidence of the presence of smectitic clay minerals which characterise by extremely high plasticity values [26].
Image of 63μm sieve residue of Afyon clay with a stereo magnifying microscope and the XRD diffractogram (phases identified with the same identification cards as in Fig. 1).
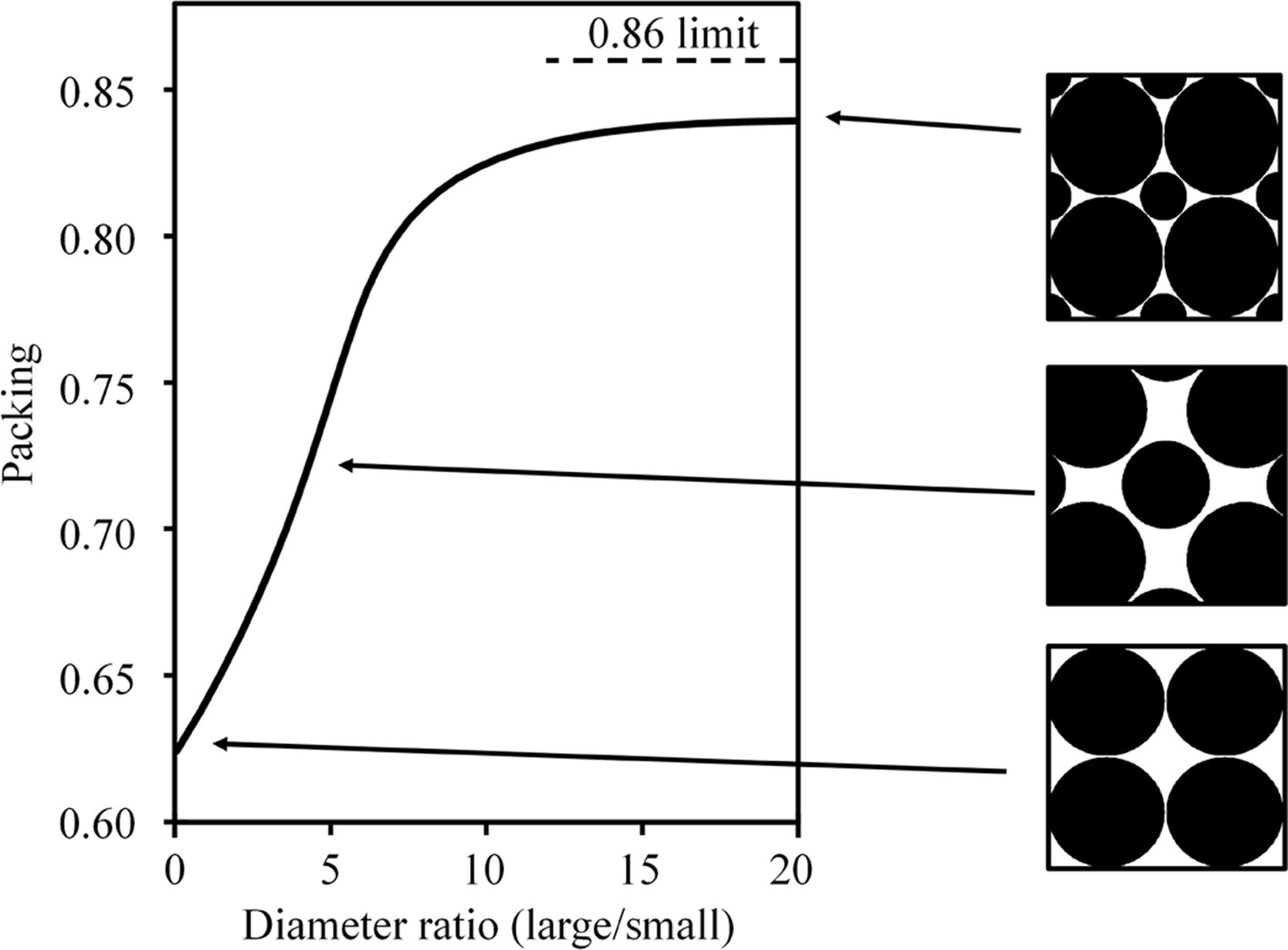
For the same reason, the high amount of coarse quartz particles in Afyon clay gives rise to good compactness as deduced by the high dry bulk density value which is larger than the corresponding value for U-25 sample. Indeed, as widely described in the literature, the greater or lesser presence of quartz in ball clays largely defines their pressing behaviour and, consequently, the final compactness value (bulk density) achieved [27]. Thus, an adequate balance of fine particles, mostly provided by colloidal clay minerals and coarse particles, usually coming from quartz mineral gives rise to optimum compactness as predicted by ideal particle packing models for spherical particles [28]. This behaviour observed with the Afyon clay can be interpreted in Fig. 6. In this figure, ideal particle packing model that would explain the high compactness observed with Afyon clay is shown. The ideal particle packing refers to a cubic arrangement of bimodal spherical particles. Large particles would be assigned to quartz and small particles to clayey minerals. Indeed, the good balance between large-sized quartz particles and a high proportion of colloidal-sized clay particles optimises the occupation of the voids that are generated during particle packing, since the size difference between the two types of particles is large enough to maximise this occupation (see Fig. 3). This observation coincides with the findings reported by Sánchez et al. [29]. In this research mixtures of different percentages of a plastic ball-clay containing a very high amount of clay minerals (around 90%) and quartz were tested. The authors found a maximum bulk density of 1.92g/cm3 (very similar to the value obtained with Afyon clay) for a mixture made up of 37% of ball-clay and 63% of quartz particles.
Ideal particle packing model that would explain the high compactness observed with Afyon clay (elaborated by the own authors). The ideal particle packing refers to a cubic arrangement of bimodal spherical particles. Large particles would be assigned to quartz and small particles to clayey minerals.
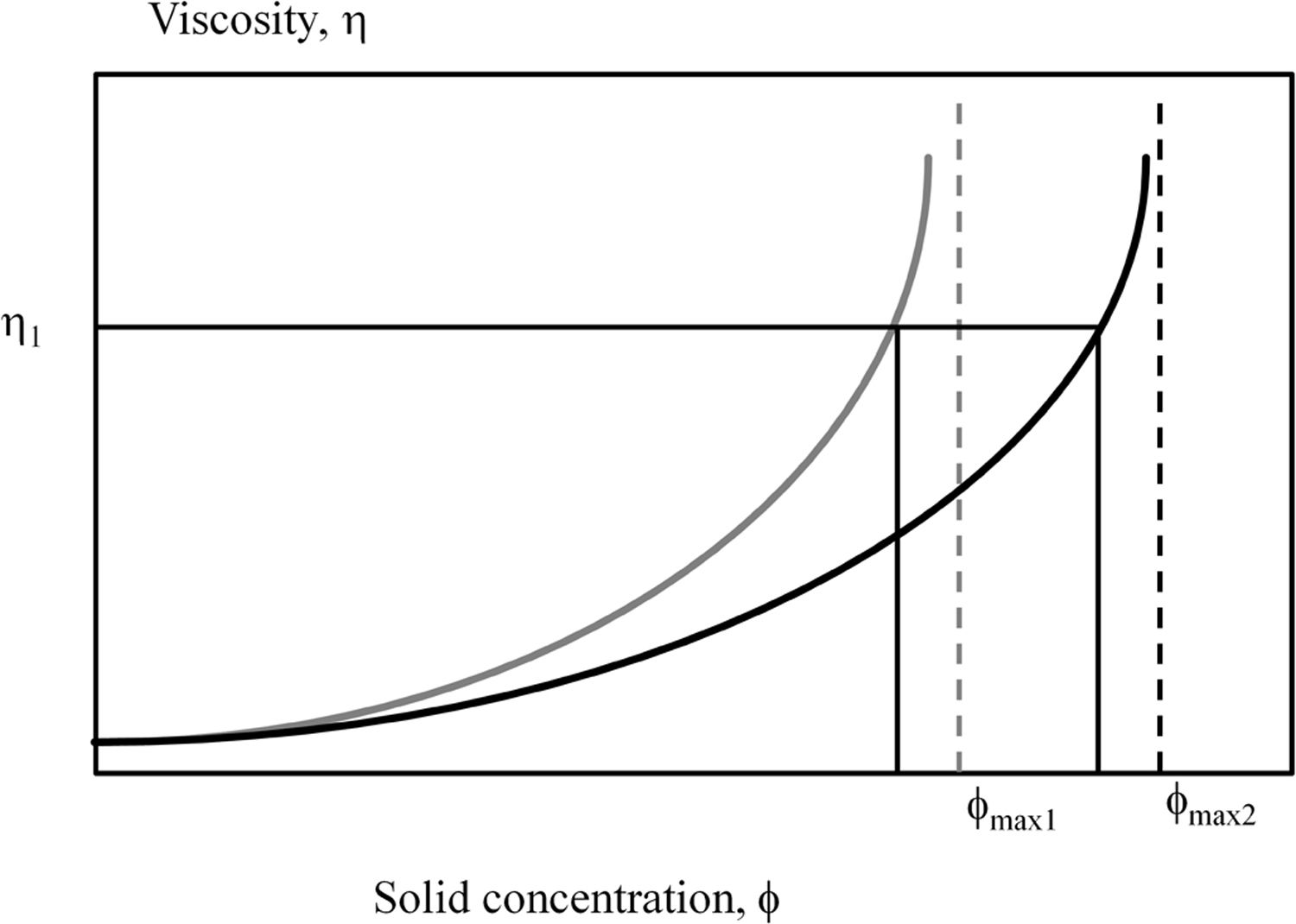
This particle packing efficiency would also explain the higher solids content obtained with Afyon clay in the deflocculation test. Thus, as Krieger–Dougherty model predicts the higher the particle packing efficiency the lower the viscosity for a given solids content is, in other words, a higher solids content suspension can be reached for a specific (industrial) viscosity constrain [30]. Fig. 7 shows this behaviour for two examples of particle size distributions in which the packing efficiency is different. As observed in both cases, the viscosity shows an exponential profile with an asymptotic value given by the particle packing efficiency. However, a higher packing efficiency shifts the curve of estimated relative viscosity to higher solids content as occurring when comparing U-25 (assigned a lower packing efficiency ϕmax1) with Afyon clay (assigned a higher packing efficiency ϕmax2).
Observation of theoretical variation of relative viscosity (η) versus volumetric fraction of solids in two suspensions containing particles with two different packing efficiencies (ϕ), elaborated by the own authors. Afyon clay could be represented by the higher packing efficiency (ϕmax2) in comparison with U-25 clay (ϕmax1).
Finally, firing behaviour is assessed in terms of linear shrinkage and water absorption. As observed in Table 3, the amount of quartz contained in Afyon clay leads to a quite refractory behaviour as evidenced by the very high water absorption value even at the highest firing temperature of 1220°C which is above the typical firing temperatures employed in the industry for porcelain tile. As a consequence of this refractoriness, linear shrinkage hardly varies along the temperature range tested. This higher refractoriness should be compensated in the final composition by modifying the nature and/or amount of fluxing materials as is usually the case in industrial compositions.
Use of Afyon clay as main clayey material in porcelain tile formulationTable 4 shows the composition of porcelain tile formulated without Ukrainian clay by using Afyon clay as the main clayey material (A). For comparison, this table also contains the standard recipe of a Ukrainian clay composition (STD). The excessive amount of quartz in the clay is the main reason to limit its content in the final composition. Thus, 30% of Afyon clay was considered a reasonable compromise between plasticity contribution to the composition and ease of processing of pressed tiles. Together with Afyon clay, 20% of Istanbul clay was also used to complete the required amount of clay in the composition without further introduction of quartz. Istanbul clay is a Turkish ball-clay commonly used in porcelain tile recipes in Turkey as well as in other tile manufacturers countries [14,31].
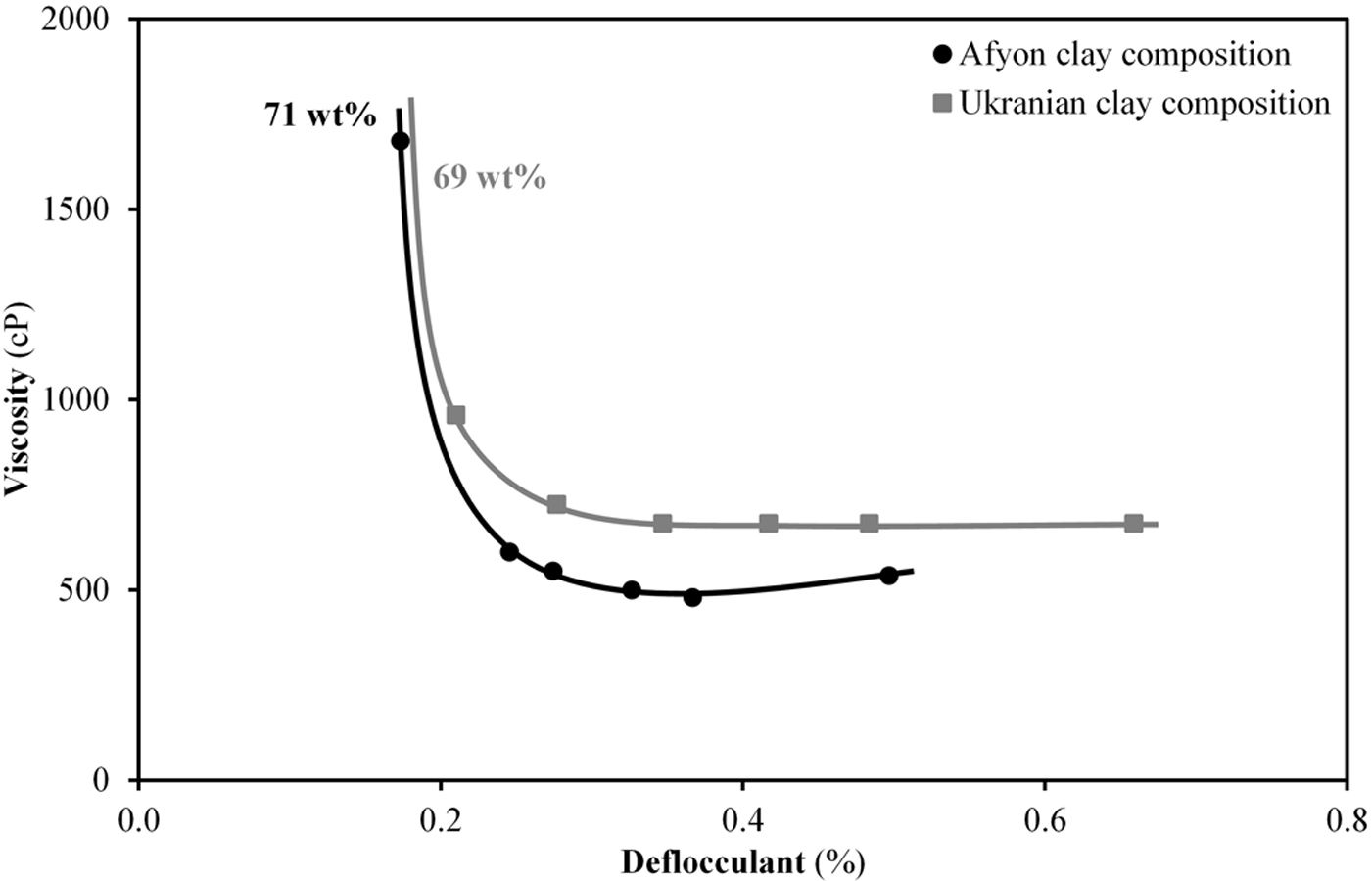
Fig. 8 shows the deflocculation curves obtained from the two compositions at a solid content around 70%, close to that used in the industrial practice. As it can be seen, the evolution of viscosity with deflocculant addition is quite similar for both compositions. The minimum of viscosity in both curves, below 1000cP, in both cases agrees with the requirement of the industrial milling and spray-drying operations since an excessive value of viscosity could impair these operations. As can also be seen, the composition with Afyon clay results in a suspension with a higher solids content which can contribute to significant energy saving benefits in the process.
Table 5 collects some technological parameters of pressed bodies, i.e., dry bulk density and mechanical strength. As observed, not very different values of dry bulk density have been obtained which indicate the good balance between fine and coarse particles in Afyon composition. The dry mechanical strength is lower for Afyon clay composition than that for Ukrainian clay composition, although the values are in the usual range for unfired porcelain stoneware bodies (2–3MPa) and higher than those needed to extract the tiles from the die and transport them along the manufacturing line [32]. Nevertheless, these values could be improved by slight changes in the composition plasticity or by means of some additives usually employed in tile manufacturing.
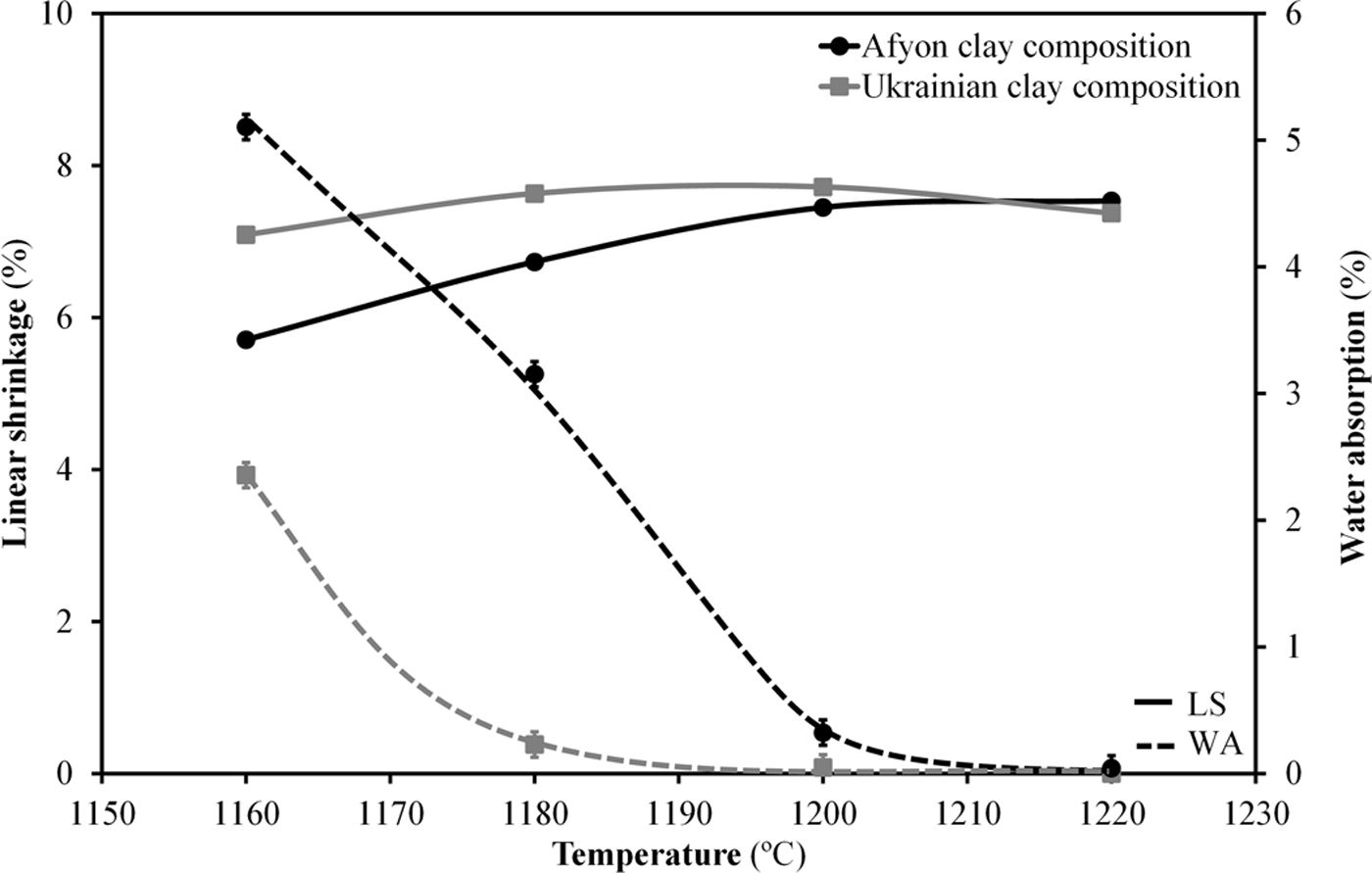
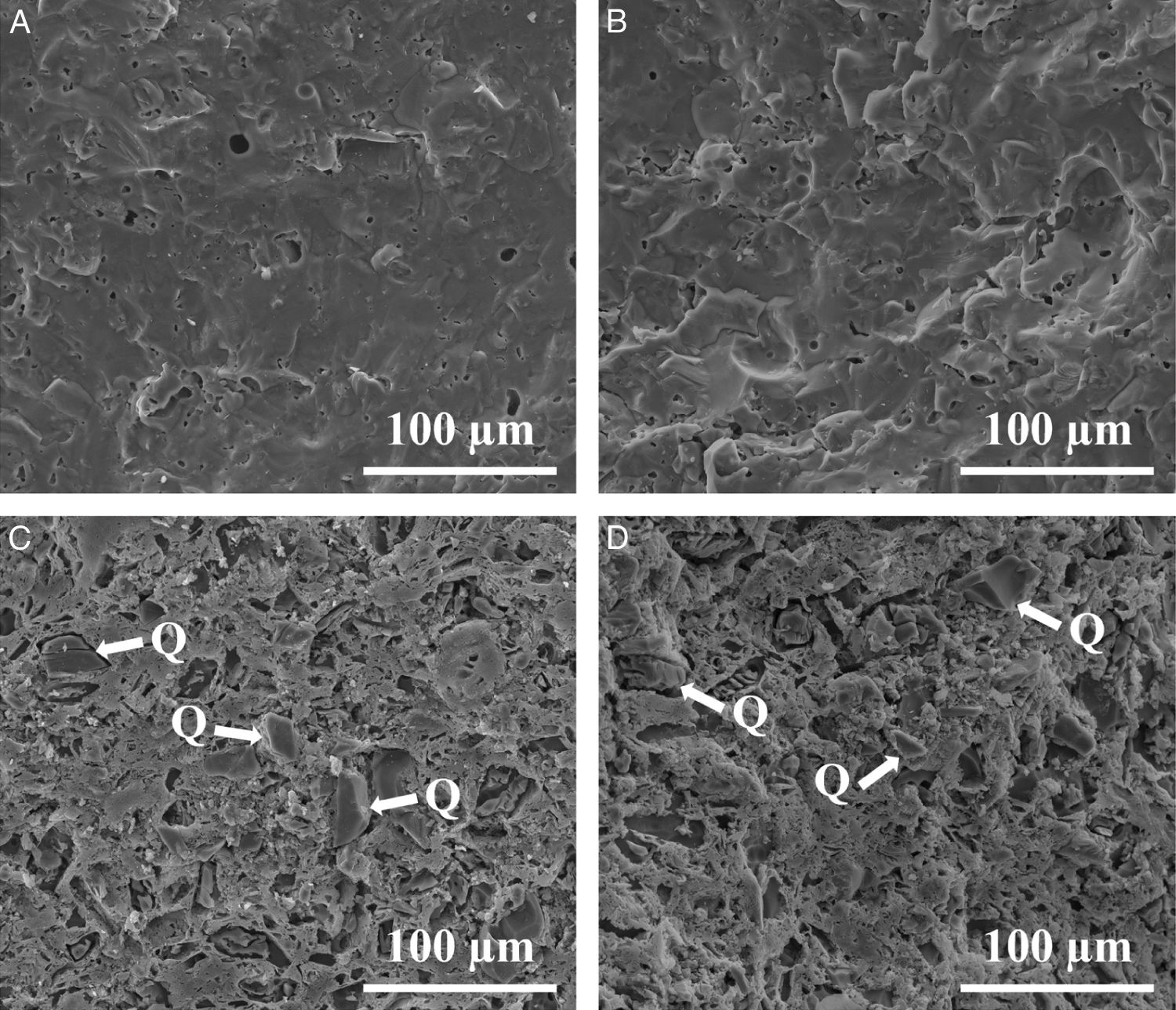
Fig. 9 shows the firing diagrams of both compositions. In these diagrams linear shrinkage and water absorption are plotted versus firing temperature. As it can be observed, in both cases the evolution of these two parameters follow similar profiles since the sintering mechanism operating during firing is exactly the same. Thus, for these compositions densifying occurs by a sintering process with the presence of liquid phase mainly provided by the sodium feldspar melt [33,34]. Nevertheless, the high amount of quartz in Afyon clay results in a slight rise in the temperature at which the maximum densification (minimum water absorption) occurs. Apart from this, firing behaviour of Afyon clay composition reproduces quite faithfully that of the one shown by a standard, Ukrainian clay composition. With regard to the microstructure of the specimens fired at temperature close to zero water absorption value for any composition, it can be observed in the SEM micrographs shown in Fig. 10. The figure set out the micrographs of the bodies of the two compositions (STD and A) fired at 1200°C for composition STD and 1210°C for composition A. There are two types of micrographs: as-fractured cross sections and the same cross sections after being attacked with hydrofluoric acid to better observe the crystalline phases. As it can be seen, in both cases the microstructure corresponds to that of a porcelain tile body as described elsewhere [35]. Thus, an abundant glassy phase provided by sodium feldspar melting is observed. The presence of some rounded pores contained in the body denotes the development of the sintering process by means of a liquid-phase mechanism which commonly operates with porcelain tile compositions [36]. Additionally, the removal of this glassy phase in the attacked microstructures reveal the expected presence of undissolved quartz particles (marked Q). No significant differences were observed between the microstructure of the two compositions.
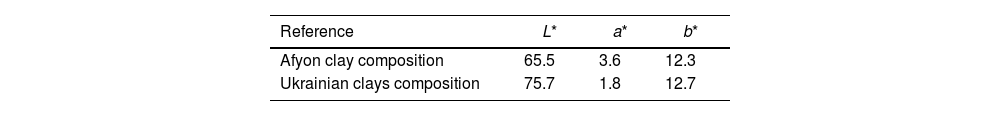
Finally, whiteness of the specimens fired at 1200°C and 1210°C for compositions STD and A respectively has been determined. Table 6 shows the chromatic coordinates (L*, a*, b*) of the CieLab system at these maximum densification temperatures. The results show a lower whiteness for the Afyon clay composition probably due to the presence of chromophores (mainly iron oxide) in the Istanbul clay used. These differences would make it imperative to use the formulated compositions for the manufacture of so-called “ultra-white” porcelain stoneware but not for the manufacture of majority porcelain stoneware with a moderate degree of whiteness. If very high whiteness is desired, another type of clay with a lower iron oxide content should be chosen and/or whitening additives should be used, as is currently the practice in the industry.
ConclusionsIn this work, a commercial sample of clay from the Afyon area in Turkey has been characterised as a possible alternative to ball clay from Ukraine in porcelain stoneware tile formulations. An exhaustive technological characterisation, compared with a Ukrainian clay sample, has revealed the following conclusions:
- 1.
The Afyon clay sample consists of a complex mixture of clay and non-clay minerals. Its high plasticity is attributed to the mixture of illitic–kaolinitic clay mineral together with the existence of an interstratified clay mineral of illitic–smectitic nature. The quartz content is abundant, exhibiting a coarse particle size.
- 2.
Its mineralogical composition largely determines its technological behaviour. Thus, despite its very high plasticity, the large quartz content gives rise to a clay with good deflocculation behaviour, ease of pressing and a very high sintering temperature.
A porcelain stoneware composition has been designed, also proceeding to its comparative technological characterisation with a standard composition formulated with Ukrainian clay. When making this comparison, it is observed that the composition that contains 30% Afyon clay presents a behaviour in the different stages of the manufacturing process that is very similar to that observed with the composition with Ukrainian clays. Although a decrease of mechanical strength of the unfired body was observed when compared with Ukrainian composition, the values obtained are in the usual range of industrial practice. In addition, a slight increase in the sintering temperature has been also observed, associated with the high quartz content, but this increase could easily be offset. Finally, a decrease of fired body whiteness occurred, nonetheless it was attributed to the presence of the Istanbul clay which was used together with the Afyon clay. Consequently, it can be concluded that a porcelain tile composition can be designed using Afyon clay as the major clayey component, adapting adequately to the requirements demanded in industrial practice.