Petrochemical wastewater, which is a complex solution of various chemicals, can cause severe environmental problems after being disposed to environment. Here, we investigate the removal of petrochemical contaminants at different periods and pH levels using three distinct processes: conventional ozonation, adsorption and nanostructured MgO-enhanced catalytic ozonation. The latter is found to be an economical and efficient new approach to treat petroleum contaminants, outperforming the other methods. For instance, after 30min of the treatment using the conventional ozonation, the removal percentages of benzene, toluene, ethylbenzene, and xylene are 35, 58, 81, and 92%, respectively; whereas the corresponding figures are 93.8, 83.5, 94.6, and 99.2% using the catalytic ozonation.
Las aguas residuales petroquímicas, que es una solución compleja de diversos productos químicos, pueden causar graves problemas ambientales después de ser eliminadas en el medio ambiente. Aquí, investigamos la eliminación de contaminantes petroquímicos en diferentes periodos y niveles de pH utilizando tres procesos diferentes: zona convencional, adsorción y ozono catalítico mejorado por nanoestructuras MgO. Este último es visto como un nuevo enfoque económico y eficiente para el tratamiento de los contaminantes del petróleo, superando a otros métodos. Por ejemplo, después de 30 minutos de tratamiento utilizando ozono convencional, las tasas de eliminación de benceno, tolueno, etilbenceno y xileno son 35, 58, 81 y 92%, respectivamente; mientras que las cifras correspondientes son 93, 8, 83,5, 94,6 y 99,2% utilizando ozonación catalítica.
Hazardous chemicals such as benzene, toluene, ethylbenzene, and xylene, which naturally exist in crude oil and gasoline, can easily be transferred into surface and groundwater due to either the leakage from industrial petrochemical equipment or inappropriate waste disposal [1,2]. Considering that the petroleum industry consumes large volumes of water, especially for cooling purposes, a large amount of petrochemical wastewater is produced [3,4]. Therefore, the appropriate treatment of polluted water sources produced in such industries is a challenge. Not to mention that the disposal of untreated petrochemical wastewater can cause grave environmental issues [5], expanding the presence of contaminants such as phenols and toxic hydrocarbons in the environment [6].
In general, wastewater from petroleum refineries contains large quantities of environmentally problematic materials; somewhere around 150-250mg/L BOD (Biological Oxygen Demand), 150-250mg/L COD (Chemical Oxygen Demand) and 20-200mg/L phenols, as well as 100-300mg/L other petroleum products and 5000mg/L salts [7], disrupting habitats of human and also wildlife communities [8,9]. The disposal of such wastewaters might destroy a considerable number of the biotic elements, leading to the gradual elimination of aquatic plants and animal species. It also simplifies the food chain through reducing the number and diversity of species [10]. Therefore, organic and inorganic contaminants present in petrochemical wastewaters must be carefully treated and removed, before the wastewater can be discharged into the environment [11]. A variety of methods have been suggested, and used, for the removal of petroleum contaminants from water resources, including chemical coagulation and sedimentation, filtering, photocatalytic oxidation, and adsorption [12]. Unfortunately, the appropriate implementation of such technologies is often very expensive, complicated and time-consuming, providing various challenges particularly in developing countries [13].
Advanced oxidation processes (AOPs) are among the most effective methods for the removal of organic contaminants. These methods are based on the production of highly oxidizing free radicals such as hydroxyl radicals, capable of mineralizing various toxic organic compounds [14]. AOPs are operationally easy, low cost, and efficient while being able to stabilize organic compounds through converting them into water and carbon dioxide [15–18] often using ozone [19]. APOs are suitable for treating contaminants that are resistant to biological decomposition [20,21].
Catalytic ozonation processes (COPs) are APOs in which solid catalysts play a role in overall ozonation process, facilitating the decomposition of ozone into free radicals. In fact, the use of catalysts may eliminate the most important limitations of simple ozonation, including the low solubility and stability of ozone in water, and its slow reaction with organic compounds [22,23]. The high efficiency, as well as low operation costs influenced by the short duration of COPs [24,25] also facilitates the implementation of these technologies, particularly, in developing countries.
Metal oxides, such as magnesium oxide (MgO) nanoparticles, have a high potential to be used in wastewater treatments, due to their possible large surface areas and the low cost of recovery and production. MgO also provides advantages of having antibacterial properties and high adsorption and/or photocatalysis performance for toxic compounds [26–28].
Various studies have been conducted on using MgO in COPs for the removal of toluene [29], textile dyes [30] and humic acid [31]. Moosavi et al. [32] studied the effect of MgO-enhanced COP on the removal of Reactive Red198, indicating that the removal efficiency improves with the increase of the pH value, due to the increased rate of ozone decomposition into free radicals at higher pH values. The decomposition of the dye molecules was attributed to an indirect oxidation process brought about by the active radicals. The present research intends to study the feasibility of using MgO nanoparticles in COP for the effective treating of petrochemical wastewater.
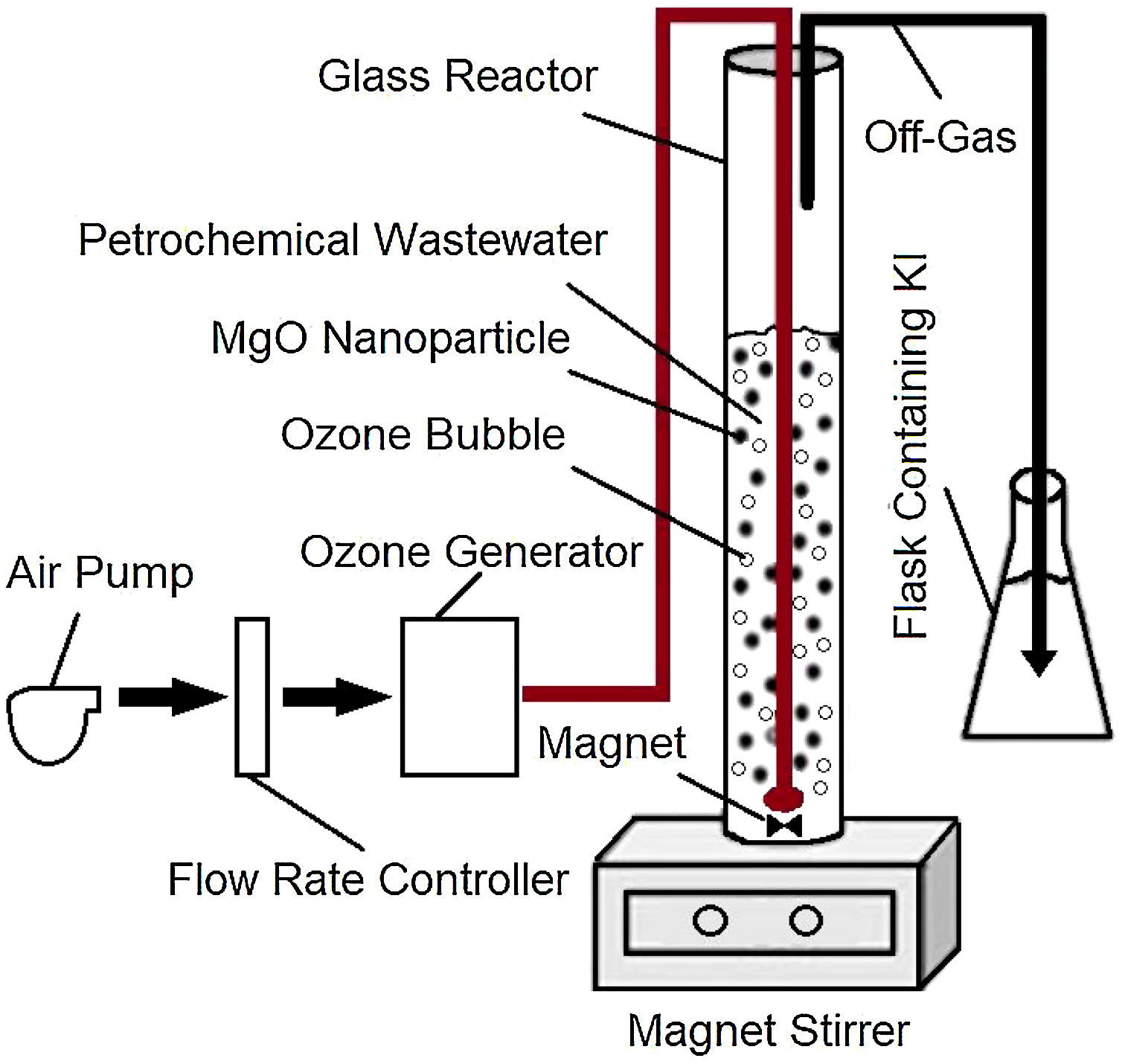
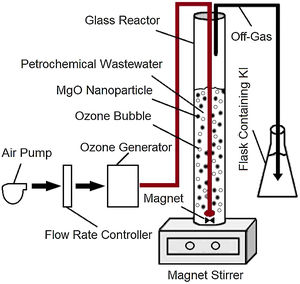
Materials and methodsReal wastewater samples were provided by a large petrochemical unit located in southern Iran. All other chemicals used in the research were of high purity and manufactured by the German Company Merck. The wastewater experiments were conducted using a laboratory scale semi-continuous ozonation reactor, shown in Fig. 1.
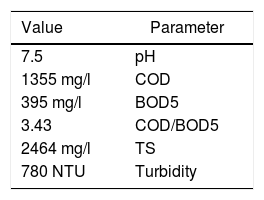
Before performing water treatment processes, the wastewater sample was tested to determine the values of COD, BOD, TS (Total Solid), and turbidity, as well as the amounts of benzene, toluene, ethylbenzene, and xylene. For this, 1μL of the wastewater was injected into an Agilent's Gas Chromatography (GC) with the GC column HP-5 30m, and 0.50μm film thickness using a flame-ionization detector. The incubator temperature was maintained at 40°C for 10min before the injection. The extraction was performed at 120˚C and 500rpm. The initial furnace temperature was kept at 40˚C for 2min, gradually raised to 80˚C at the rate of 20˚C/min, and kept at this temperature for 1min. The temperatures of the injector and detector were set at 250 and 300˚C, respectively, and nitrogen gas flow rate was maintained constant at 3mL/min. The amount of each component was calculated through comparing the diagram provided by the GC machine with that obtained by injecting standard samples into the GC equipment. Table 1 represents the characteristics of the petrochemical wastewater used in this study.
MgO nanoparticles, used as nanocatalysts in the wastewater treatment, were prepared by the calcination approach. For this, Mg (NO3)26H2O was first dried at 100°C for 8h. Then, the dried powder was calcined at 500˚C for 2h in an electrical furnace to form MgO nanoparticles. The morphology of nanoparticles was studied using scanning (SEM), and transmission (TEM) electron Microscopy. A Philips X-ray diffractometer (XRD) with Cu-Kα radiation (1.54Å) was employed for the structural study.
Catalytic ozonation process (COP)A semi-continuous cylindrical reactor with the internal volume of 1500mL was assembled for the catalytic ozonation of the wastewater (Fig. 1). Using this reactor, ozone could continuously be bubbled into the wastewater. The off-gas containing the excess ozone could then be directed from the reactor to gas scrubbers containing 20% potassium iodide where the excess ozone could be decomposed.
The catalytic ozonation experiments were conducted at room temperature using 50mL of the wastewater samples mixed with 450mL double distilled water and 0.3mg MgO nanoparticles. The pH was adjusted at various values of 3, 5, 7 and 9 employing 0.1M solutions of HCl or NaOH. For a typical experiment, the solution was transported into the glass reactor shown in Fig. 1, connected to a DONALIO3 ozone generator with an ozone production capacity of 0.2g/h, and treated for 10, 20, and 30min. After specific periods, the quantity of remaining benzene, toluene, ethylbenzene, and xylene present in the wastewater samples were measured by injecting 1μL of the sample into the GC equipment.
Simple ozonation process (SOP) and adsorption process (AP)In addition to the COP, SOP and AP were also evaluated using petrochemical wastewater samples in order to distinguish the effect of various phenomena which could be involved in the removal, or the reduction, of contaminants present in the wastewater. To perform the SOP, 50mL of the wastewater was mixed with 450mL of double distilled water at different pH values of 3, 5, 7 and 9. For this, the pH of wastewater was adjusted with solutions containing 0.1N of HCl or NaOH. Then, the ozonation process was carried out at reaction durations of 10, 20, and 30min to evaluate the influence of time on the process.
The AP was performed under the same conditions as mentioned above for SOP, with the difference that no ozone was involved, and instead, around of 0.3mg MgO nanoparticles was added to the wastewater. Moreover, the solution was aerated to create turbulence in order to achieve an appropriate contact between the solution and MgO nanoparticles.
At the end of both processes, the samples were rested for 5min, and 5mL aliquots were collected from the middle level of the reactor, from which 1μL samples were injected into the GC instrument for determining the quantities of contaminants remaining in the solution.
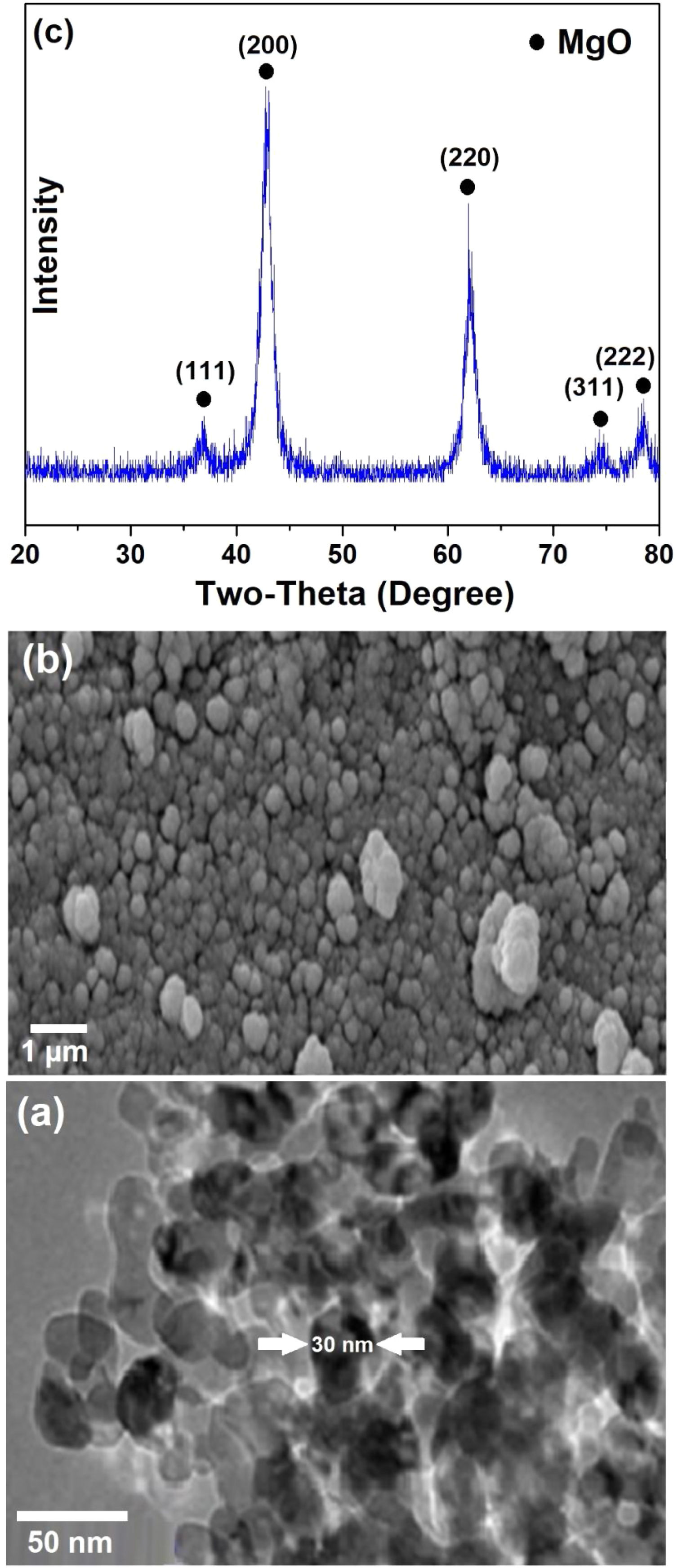
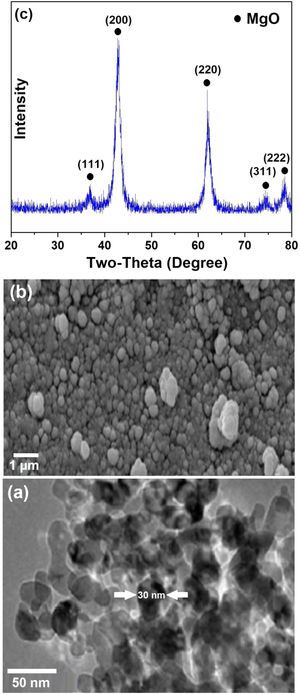
Results and DiscussionCharacterization of MgO nanocatalystsThe SEM surface morphology of MgO nanoparticles is shown in Fig. 2a, from which the presence of spherical shaped and agglomerated particles is evident. TEM image of the material (Fig. 2b) clearly indicates that individual MgO nanoparticles have an average dimension of around 30nm. X-ray diffraction pattern of MgO nanoparticles, presented in Fig. 2c, indicate the reflections at 2θ = 37°, 43°, 62°, 74°, and 78° corresponding to (111), (200), (220), (311), and (222) characteristic XRD peaks of MgO with a cubic structure (JCPDS 01-075-1525), respectively. Scherrer equation can be employed to evaluate the size of crystals in ceramic materials [33–35]. The mean crystallite size of MgO nanoparticles could be calculated using this approach, based on the most intense (002) XRD peak observed in Fig. 2c, and found to be around 21nm. This is in agreement with the nanostructured nature of the material.
It should be mentioned that there are several techniques to prepare ceramic nanoparticles including hydrothermal methods [36,37], sol-gel [38,39], spark discharge [40], electrochemical discharge machining [41], laser ablation [42,43], ball milling [44], aqueous wet chemical method [45], combustion synthesis [46–49] and molten salt-based techniques [50–53]. Comparing to these techniques, the simple calcination method used in this study for the preparation of MgO nanoparticles (reaction (1)) may benefit from relative simplicity, low cost and minimal requirement for complex equipment.
Mg(NO3)2 = MgO + 2NO2(g) + 1/2O2(g) ΔG°500°C = -80kJ (1)
It should be mentioned that NO2 produced as the by-product of the reaction (1) can be used in the manufacturing of nitric acid by treating with water and air [54–56], improving the economic performance of the process. It is worth noticing that the demand for nitric acid is considerably increasing due to its wide range of applications, including in the processing of fertiliser, mining explosives and gold extraction [57]. The surface area of the MgO nanoparticles produced in our study was measured by N2 adsorption/desorption measurements (TriStar II 3020 Micromeritics) and found to be 221 m2/g, with BJH Adsorption cumulative pores volume of 0.85cm3/g, and BJH adsorption average pore diameter of 27.4nm.
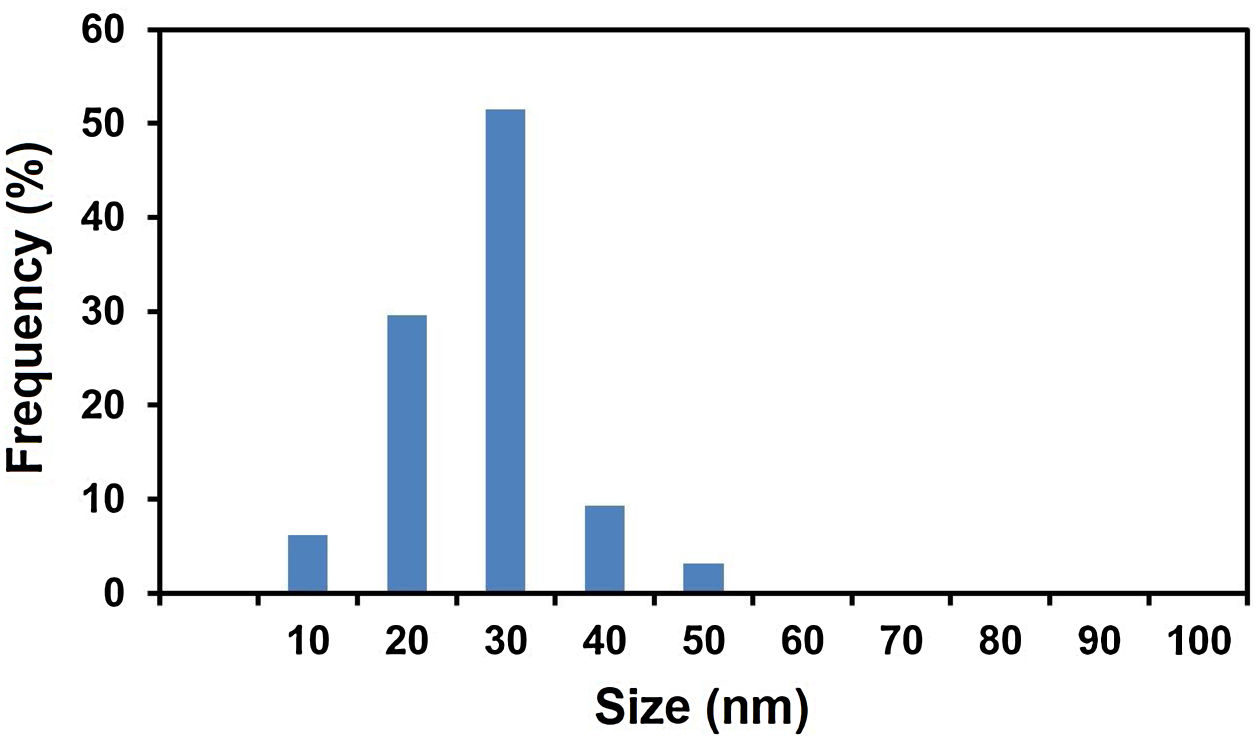
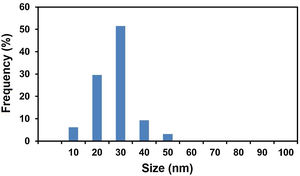
Fig. 3 shows the particle size distribution histogram of MgO nanoparticles calculated from TEM analysis, which was built up by counting more than 100 particles. From this, an average diameter of 22.6nm can be obtained. We can compare this value with those presented in the literature. Wong et al. [58] prepared MgO nanoparticles employing an ultra-sonication incorporated Pechini method [59] using magnesium acetate tetrahydrate and citric acid as the raw materials. The value of average size of MgO nanoparticles produced under different conditions were measured to be around 21 -122nm. In another work, Hajengia et al. [60] produced MgO nanoparticles with an average particle size of 16nm by the microwave heating of a mixture comprising Mg(CH3COO)2·4H2O and benzylamine to form Mg(OH)2 which was separated, washed and calcined at 550°C for 5h. The particle size distribution histogram of the sample demonstrated that the most of the particles were in the range of 12–22nm. Furthermore, Kumar et al. [61] produced MgO nanostructures with particle size of 20-25nm by a combustion route using Mg(NO3)2 .6H2O as the magnesium source and vetiver as the fuel. Overall, the characteristics of MgO nanoparticles produced in this study by a simple calcination technique are compatible with those produced by alternative, generally more complicated/expensive techniques.
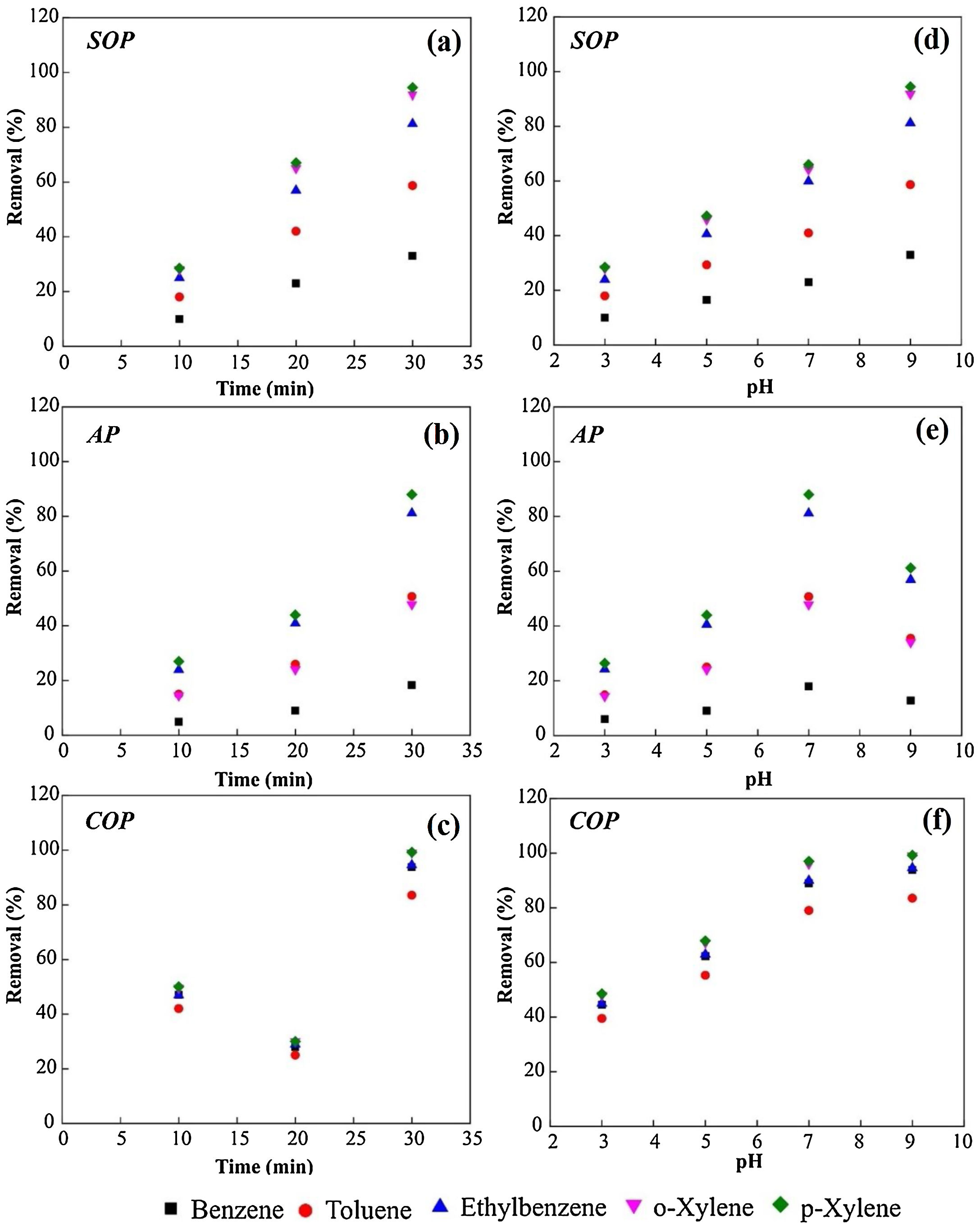
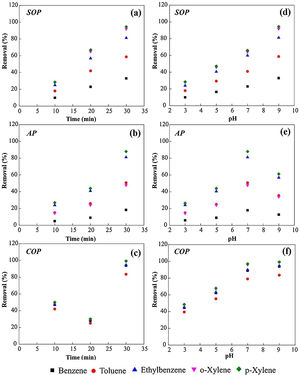
The effect of variation time of wastewater treatmentThe effects of time variation on the efficiency of COP, SOP and AP to remove chemicals from the petrochemical wastewater are exhibited in Fig. 4a-c. As can be depicted from Fig. 4a, the benzene removal efficiency in the SOP improved from 9% to 33% when the reaction time increased from 10 to 30min. At this time slot, the improvement in the removal efficiency of toluene, ethylbenzene, and xylene could be recorded to be 18 to 58%, 25 to 81%, and 28 to 92%, respectively. Likewise, in the AP, the increase in reaction time from 10 to 30min improved the benzene removal efficiency from 5 to 18%. For toluene, ethylbenzene, and xylene, the improvement in the removal efficiency was measured to be 15 to 50%, 24 to 81%, and 27 to 88%, respectively (Fig. 4b). As shown in Fig. 4c, the removal efficiency in the COP for benzene rose from 47 to 93% when the reaction time increased from 10 to 30min. The improvement recorded for the removal efficiencies of toluene, ethylbenzene, and xylene was from 42 to 83%, 47 to 94%, and 50 to more than 99%, respectively. The COP procedure presents the highest efficient of removal for all contaminants, denoting the positive influence of the catalytic process promoted by MgO nanoparticles.
Influence of the variation of time and pH on the performance of SOP, AP and COP for the removal of contaminates from the petrochemical wastewater. The variation of reaction durations at the pH value of 7.5 (Table 1) for (a) SOP, (b) AP, and (c) COP. The variation of the pH solution at the contact time of 30min for (d) SOP, (e) AP, and (f) COP.
Since pH is an important factor in ozonation process, its effect on the efficiency of SOP, AP and CPO for the removal of various contaminants from the petrochemical wastewater was evaluated, and the results can be observed in Figs. 4d-f. As shown in Fig. 4d, the removal efficiency increases in the SOP by increasing the pH value. For p-Xylene, the removal efficiency increased from about 28% at pH 3 to 94.5% at pH 9. As shown in Fig. 4e, in the AP, the adsorption efficiencies of MgO nanoparticles for all the chemicals increase when the pH increases to about the neutral value, but considerably decline by further increasing the pH value to 9. As presented in Fig. 4f, in the COP, the efficiencies for the removal of chemicals increase by increasing the pH values. For instance, the removal efficiency of ethylene, benzene and xylene increased from 45-50% at pH 3 to more than 90% at pH 9. As presented in Fig. 4f, by increasing the pH level of the solutions from 3-9, the efficiency of COP process to remove various contaminants from the petrochemical wastewater increases. It is noteworthy that more than 90 percent of all the petroleum compounds present in the wastewater could be decomposed at pH = 9 after 30min of treatment. It should be noticed that the positive relation of pH with SOP efficiency to degraded organic compounds was also previously observed [23]. Such a relation can also be indicated for the case of COP as evident in Fig. 4f.
Results obtained indicate that the duration of ozonation and contact time enhance the overall performance of SOP, AP and COP. The highest removal efficiencies achieved can be summarized as 94.5, 88, and 99.2% for the SOP, the AP, and COP, respectively, obtained after 30min of treatments. Therefore, the order of removal efficiency of organic pollutants can be regulated as AP < SOP < COP.
The adsorption proprieties and catalytic performance of MgO for the degradation of n-alkanes have been evaluated in the literature [62,63], highlighting the electron-acceptor and electron-donor abilities of the MgO surface. Those studies identify that the dye adsorption performance of MgO nanoparticles is maximized at the pH value of 6, which is in an approximate agreement with our results obtained for AP presented in Fig. 4e.
For the case of COP, the removal efficiencies are approximately same at the pH values of 7 and 9, providing the maximum pollutants degradation performances. This results is in agreement with those obtained by Moussavi et al. [64].
These authors suggested a mechanism for the degradation of phenol by COP, based on which, in the presence of ozone, the Lewis acid sites on the surfaces of MgO particles (MgO-s) react with ozone, and eventually with phenol, leading to the degradation event. This mechanism may also shed light on the degradation of pollutants in the current study, as explained by the occurrence of reactions (1)-(4).
O3 + MgO-s → MgO-sO3 (1)
MgO-sO3 → MgO-sO• + O2 (2)
MgO-sO• + pollutant→ CO2 + H2O + intermediates (3)
MgO+pollutant + O3 → CO2 + H2O + intermediates (4)
Reaction (4) can be regarded as the direct oxidation of pollutant with O3 molecules.
On the other hand, regarding the degradation of organic molecules in the presence of O3 as an oxidizer (E0 = + 2.07eV), it is known that under alkaline conditions, the decomposition of O3 molecules occurs with the production of hydroxyl radicals (•OH) [65]. These radicals have an even greater oxidative potential (E0 = + 2.8eV) for decomposition of organic molecules [66,67].
In the presence of a catalyst, the production of •OH can be stimulated. Also, the increase in the pH value of the reaction medium can accelerate the mass transfer of O3 and its decomposition rate, promoting the formation of highly reactive free radicals, such as •OH, •O2H and •O3H, leading to the increase of pollutants degradation efficiency [32,68,69].
The formation of ●OH and MgO-hydroxyl (MgO-sOH) radicals have been explained to occur based on reactions (5) and (6) [32].
MgO-sO• + 2H2O + O3 → MgO-sOH + 3 •OH + O2 (5)
•OH + O2 + pollutant → CO2 + O2− + H2O (6)
The ●OH radicals could also be produced in COP, providing a more effective environment for the oxidation of organic pollutants present in the petrochemical wastewater, in comparison with radicals produced by SOP. The reactions (3), (4) and (6) can occurs simultaneously, improving the overall degradation efficiency.
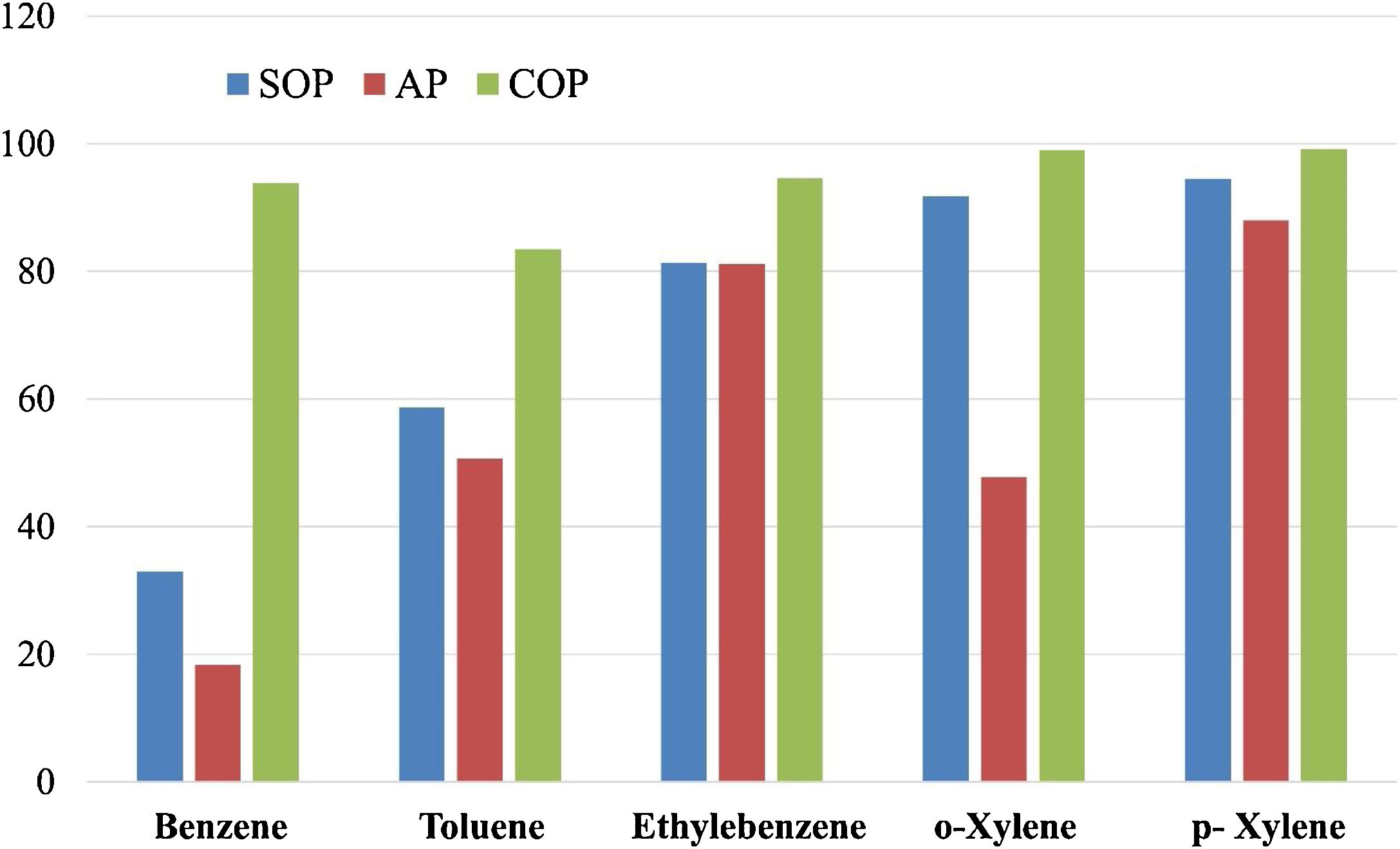
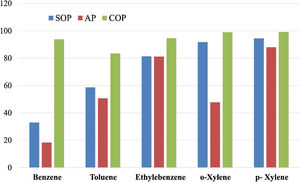
Treatment of the petrochemical wastewater at the pH value of 12Inspired by the experimental results, the petrochemical wastewater was further treated using the three distinct approaches; SOP, AP and COP, to reduce its contamination level under deviated conditions from those exhibited in Fig. 4, i.e. the enhanced pH value of 12, and extended contact duration of 50min. The results obtained are presented in Fig. 5. As indicated, in the SOP, the xylene removal percentage is higher compared to that of ethylene benzene, toluene, and benzene. Moreover, the removal percentage of xylene and ethylbenzene in the AP are very close to each other and both higher than those of benzene and toluene. The lowest removal efficiency is related to that of benzene (33%). These observations can be explained by the combination of effects, comprising the lower solubility of benzene and toluene compared to ethylbenzene and xylene, and the higher molecular weights and melting points of xylene and ethylbenzene compared to benzene and toluene [70–72].
As shown in Fig. 5, in the AP, the highest and lowest (18%) removal efficiency could be achieved for p-Xylene and benzene, respectively. Moreover, the removal percentage of o-Xylene is lower than those of ethylbenzene and paraxylene.
As indicated by Lu et al. [72], at the initial benzene and toluene concentrations of 60 and 200mg/L, respectively, toluene adsorption per unit of the adsorbent mass was greater than that of benzene. Moreover, Aivalioti et al. [70] showed that the adsorption capacity of diatomic adsorbents was higher for toluene than benzene. Yakout and Daifullah [73] found that a higher amount of toluene could be adsorbed per unit mass of the activated carbon than benzene. Furthermore, Su et al. [74] showed that the capacity of carbon nanotubes modified by sodium hypochlorite for the adsorption of benzene and toluene are 212 and 225mg/g of the adsorbent, respectively, at their initial concentration of 200mg/L, the contact time of 240min, and adsorbent concentration of 600mg/L. Other studies show that activated carbon adsorbent could reduce petroleum contaminants from water by up to 80% [75], although it cannot completely remove organic matter from water. Garoma et al. [76] found that the removal efficiencies of petroleum compounds by SOP could be 27%. Furthermore, they noticed that SOP was more efficient than the AP using MgO nanoparticles in removing contaminants, except for ethylbenzene, for which SOP and the AP exhibited almost the same efficiency.
As mentioned earlier, it should be noticed that molecular ozone has a high oxidation-reduction potential of 2.07V vs. SHE, which is greater than those of most oxidants such as chlorine (1.48V), but lower than that of hydroxyl radicals (2.8V) [77]. As shown in Fig. 5, the COP is much more efficient than the SOP and the AP. The reason for this might lie in the fact that the simultaneous use of ozone and adsorbent accelerates the transformation of ozone from its molecular state into hydroxyl radicals. This is in agreement with the work of Dehouli et al. [78], who found that the utilization of ozonation together with the activated carbon could enhance the wastewater treatment performance, and this was attributed to the greater quantities of hydroxyl radicals present in the COP.
The performance of COP for the removal of Reactive Red 198 in the presence MgO nanocrystals was found to be related to the decomposition of dye molecules due to indirect oxidation by the active radicals [32]. Valdez et al. [79,80] examined the effects of COP together with volcanic rocks in the benzothiazole removal, and related the high efficiency of the process to the intense inclination of ozone to react with Lewis acids of the metal oxides in the volcanic rocks. Moreover, results indicated that the COP performed better than the SOP at contact time of 30min. Since both direct and indirect oxidations are involved in contaminant removal in the COP, the process exhibits a higher performance at shorter contact periods compared to the common SOP. It should also be noticed the ozone decomposition can be enhanced on the adsorbent surfaces results in higher concentrations of the free radicals in the system, promoting the decomposition of organic pollutants [80].
It has been found that ozone decomposition can be promoted on the surfaces of crystals of natural zeolite, and particularly on volcanic rocks [79–81]. It was because such materials are capable of adsorbing ozone, followed by its conversion into free radicals.
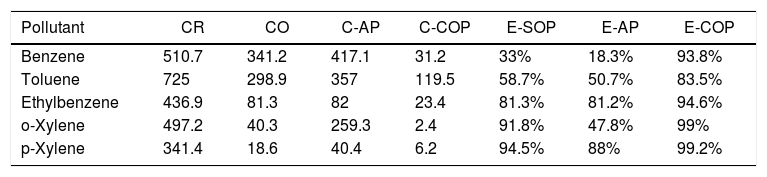
From Figs. 4 and 5, the enhanced performance of the heterogeneous COP process, comparing to SOP and AP is evident. The catalyst surface properties, therefore, play an important role in the ozonation process, as also indicated by Zhang et al. [82]. Based on the results obtained by Mohammadi et al. [29], COP is an efficient and rapid method for removing of toluene from aqueous solutions. It is known that metal oxides are able to adsorb water molecules, and this causes the formation of surface hydroxyl groups. Such hydroxyl groups exhibit various electric charge patterns at different pH values [83], affecting the decomposition of ozone on the metal oxide's surface. For instance, Qi et al. [84] studied the degradation of 2, 4, 6-trichloroanisole by the ozonation process in the presence of Al2O3 particles, and found that the decomposition of ozone on the alumina particles is enhanced when pH level approaches around 8. Furthemore, Garoma et al. [76] concluded that in ozonation experiments, the presence of sufficient amount of iron in groundwater samples would improve the removal of BTEX, MTBE, TBA, and TPHg. These observations are in agreement with our experimental results. Table 2 summarizes the performance of the SOP, AP and COP processes for the removal of the pollutants from the petrochemical wastewater.
A summary on the performance of various techniques used for the removal of contaminants from the petrochemical wastewater: Concentrations of poullants in the raw wastewater (CR), and those after the ozonation (CO), the adsorption (C-AP), and the catalytic ozonation (C-COP). The removal efficiency in the ozonation process (E-SOP), adsorption (E-AP), and the catalytic ozonation (C-COP).
| Pollutant | CR | CO | C-AP | C-COP | E-SOP | E-AP | E-COP |
|---|---|---|---|---|---|---|---|
| Benzene | 510.7 | 341.2 | 417.1 | 31.2 | 33% | 18.3% | 93.8% |
| Toluene | 725 | 298.9 | 357 | 119.5 | 58.7% | 50.7% | 83.5% |
| Ethylbenzene | 436.9 | 81.3 | 82 | 23.4 | 81.3% | 81.2% | 94.6% |
| o-Xylene | 497.2 | 40.3 | 259.3 | 2.4 | 91.8% | 47.8% | 99% |
| p-Xylene | 341.4 | 18.6 | 40.4 | 6.2 | 94.5% | 88% | 99.2% |
It is known that the physical and chemical properties of contaminants significantly influence their removal performance from wastewaters [85].
For instance, in advanced oxidation treatments, xylene can be eliminated more than chemicals such as ethylbenzene, toluene, and benzene. Furthermore, the amount of adsorbed ethylbenzene and xylene is very close, whilst their adsorption values are considerably higher than those of benzene and toluene. These characteristics can be explained based on the properties of these chemicals. Based on this, the relatively high dissolution rate of benzene in water (1780ppm) in comparison to other compounds such as toluene (512ppm), ethyl benzene (152ppm), and xylene (175ppm) [86,87], makes it more difficult to be removed from wastewasters. The considerably higher molecular weight and boiling points of xylene and ethylbenzene than toluene and benzene around (around 144, 136, 111, and 80°C, respectively) can also influence their removal efficiency [70,88].
Remarks and conclusionsPetroleum-derived chemicals can be hazardous and toxic threatening the human health and environment security. These compounds may enter the food chain via various routes, one of which is the water resources. Therefore, the removal of petrochemical contaminants from water resources is of significant importance. This can be carried out by various methods, including adsorption and ozonation; considering that each technique has its own advantages and disadvantages. Catalytic oxidation processes are among promising water treatment methods which have the advantages of both adsorption and oxidation approaches. Here, we investigated the performance of AP, SOP, and COP on the removal of various contaminants (benzene, toluene, ethylbenzene, and xylene) from petrochemical wastewaters, collected from an Iranian petrochemical industry. MgO nanoparticles produced by the calcination of Mg (NO3)26H2O with a mean particle size of around 23nm was used as the catalyst. The effects of contact time and pH were evaluated for each approach. Results showed the considerably higher performance of COP in comparison with those of SOP and AP. The increase in the contact time and the pH value of the solutions led to the improvement of removal efficiencies. During 30min of the COP, the removal efficiencies of benzene, toluene, ethylbenzene, and xylene were recorded to be 93, 83, 94, and 99.2%; which are considerably greater than those obtained in SOP; 33, 58, 81, and 92%, respectively. MgO-enhanced COP can be considered a promising and economical approach for the removal of petroleum-based pollutants from aqueous environments.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: