In this study, the effect of perlite from NW Turkey on the physical properties of ceramic tile bodies was investigated. The phases present in the raw materials were measured by X-ray diffraction while chemical analysis of the raw materials were determined by X-ray flouresence. Colour, water absorption and firing shrinkage of the raw materials were investigated. The data obtained from this study showed that the addition of NW Turkey perlites improved sintering behaviour of ceramic tile bodies, Therefore, alternative tile compositions were formulated using perlite as a substitute for common alkali bearing raw materials. The sintering behaviour of the standard bodies and that of the perlite containing bodies were characterized using a double-beam optical non-contact dilatometer. Moreover, the effect of perlite on firing shrinkage (%), water absorption (%), apparent porosity (%), apparent density (g/cm3), bending strength and colour values (L, a, b) were studied and resultant microstructures were characterized by X-ray diffraction (XRD) and scanning electron microscope studies (SEM). It was clearly observed that perlite improves sinterability of the tile bodies but increases the firing shrinkage due to the presence of water in its structure. The low thermal expansion values of the sintered bodies containing perlite makes it a visible raw material for porcelain stoneware tiles.
Se ha investigado el efecto de una perlita de la región de Biga (noroeste de Turquía) sobre las propiedades físicas de composiciones estándar de gres porcelánico. Las fases presentes en las materias primas y su composición química se han determinado por difracción de Rx y fluorescencia de Rx, respectivamente. También se determinó algunas propiedades físicas de las materias primas cocidas como el color, la contracción y la absorción de agua. Se han planteado composiciones alternativas empleando perlita como sustituto de materias primas aportadoras de óxidos alcalinos. El comportamiento en la sinterización de las composiciones propuestas ha sido evaluado por dilatometría. Además, también se ha estudiado el efecto de la perlita sobre las propiedades físicas de las piezas sinterizadas, así como sobre las fases y la microestructura de las mismas mediante difracción de Rx y microscopía electrónica de barrido. Se observó que la perlita mejora la sinterabilidad de las piezas, pero aumenta la contracción de cocción debido, en parte, al contenido de agua estructural de este mineral. Se observó claramente que la perlita mejora la sinterización de las de los azulejos, pero aumenta la contracción de cocción debido a la presencia de agua en su estructura. Los bajos valores de expansión térmica de piezas sinterizadas que contienen perlita lo convierten en una materia prima visible para las baldosas de gres porcelánico.
Porcelain stoneware tile is a very compact product obtained by fast firing, in the 1200–1250°C temperature range. It has low porosity, which is essential feature that provides the tiles with relevant physical-mechanical properties, such as bending strength, surface hardness and wear resistance [1–3]. The starting composition of the porcelain tile is made from a mixture of quartz, feldspar, kaolin and clay in specific proportions. The quartz serves as a filler material and forms the skeleton of the body. Clay and kaolin provide plasticity to the body for shaping [4–6]. Feldspars are used as fluxing agents to lower the sintering temperature during firing by forming a glassy phase [7–9]. Tile bodies are manufactured using large amount (40–50wt%) of fluxes, such as sodic and potassic feldspars [10], Nepheline syenite [11,12], talc [13], glasses [14–16], intrusive and extrusive rocks [17–24] and glass ceramics [25]. Therefore, the chemical and mineralogical properties and the proportions of the raw materials affect the shrinkage, water absorption, breaking strength and colour properties of the body depending on firing temperature and time. From the economic point of view, utilization of cheap raw materials able to replace the traditional fluxes without altering the process and product characteristics has to be highly efficient because of the resulting reduction in product cost.
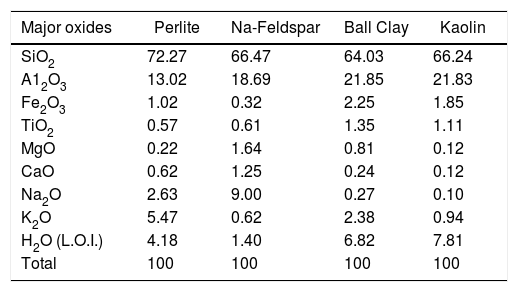
Perlite, which is an amorphous alkali alumina silicate is an acidic volcanic glass that contains 3–6% water in its structure and can be separated as pearl grains when broken due to its small circular fractures. Formation of perlite is thought to be related to volcanic activities. The eruption time and the cooling rate of the volcanic lava determines the colour and grain size of the perlite. Cooling of lava controls the formation of amorphous and crystalline forms. The appearance of perlitic rocks is very diverse being compact, fine-grained, porous (pumice-bearing perlite), easily disintegrating, hand-friable in sand and sandstone. Aluminium silicate in perlite is chemically bonded with the water in composition. Water in the composition causes expansion of perlite 10–20 times in volume during firing, producing flaky shaped particles [26,27]. Chemical composition of the perlite sample is given in Table 1 where small amounts of CaO, MgO, Fe2O2, TiO2, MnO2, SO3 may associate. About 90–97% of the volume is composed of glassy and crystallizing minerals such as K-feldspar and biotite. Quartz, apatite and magnetite may be the accessory phases. Montmorillonitic clay and very fine-grained zeolite crystallization formed in macro and micro concentric cracks resembling the onion skin structure, may also be among the secondary minerals of the perlites. The presence of substantial amount of alkali is in its composition as well as its glassy nature makes it a potential fluxing material which could replace Na-Feldspar therefore used widely in tile composition. Na-feldspar formations are mainly concentrated in Western Turkey, away from many ceramic plants, requiring transportation from these regions. Therefore, alternative fluxes could be available solution to avoid the transportation.
Chemical analysis of the raw materials (L.O.I.: loss on ignition).
| Major oxides | Perlite | Na-Feldspar | Ball Clay | Kaolin |
|---|---|---|---|---|
| SiO2 | 72.27 | 66.47 | 64.03 | 66.24 |
| A12O3 | 13.02 | 18.69 | 21.85 | 21.83 |
| Fe2O3 | 1.02 | 0.32 | 2.25 | 1.85 |
| TiO2 | 0.57 | 0.61 | 1.35 | 1.11 |
| MgO | 0.22 | 1.64 | 0.81 | 0.12 |
| CaO | 0.62 | 1.25 | 0.24 | 0.12 |
| Na2O | 2.63 | 9.00 | 0.27 | 0.10 |
| K2O | 5.47 | 0.62 | 2.38 | 0.94 |
| H2O (L.O.I.) | 4.18 | 1.40 | 6.82 | 7.81 |
| Total | 100 | 100 | 100 | 100 |
In this study, the use of perlite could help to achieve the complete bodies densification at low firing temperature. In fact, during firing both feldspar and montmorillonite present in perlite could support the formation of a liquid phase that could contribute to obtain the final product at lower temperature. The objective of this study is replacing Na-Feldspar used in the porcelain body compositions by perlite from NW Turkey as alkali source. Using such an alternative raw material would considerable reduce the production and transportation costs.
Materials and methodsThe major raw materials used to prepare various formulations in this study include sodium feldspars from Çine region of Aydın/Turkey, ball clay from Sile region of Istanbul/Turkey, kaolin sample from Düvertepe region of Balıkesir/Turkey, perlite sample from Biga Region of NW Turkey. It is well known that characteristics of raw materials used to prepare ceramic bodies considerably affect the ultimate product quality. In this respect, the chemical analyses of the starting raw materials were carried out by an Rigaku ZSX Primus type X-ray fluorescence device and the results are reported in Table 1. Melting behaviour of fluxing materials such as Na-feldspar and perlite was studied using a hot stage optical microscope (MISURA, Expert System Solutions, Italy) by using fast heating rate 50°C/min. resembling industrial firing conditions.
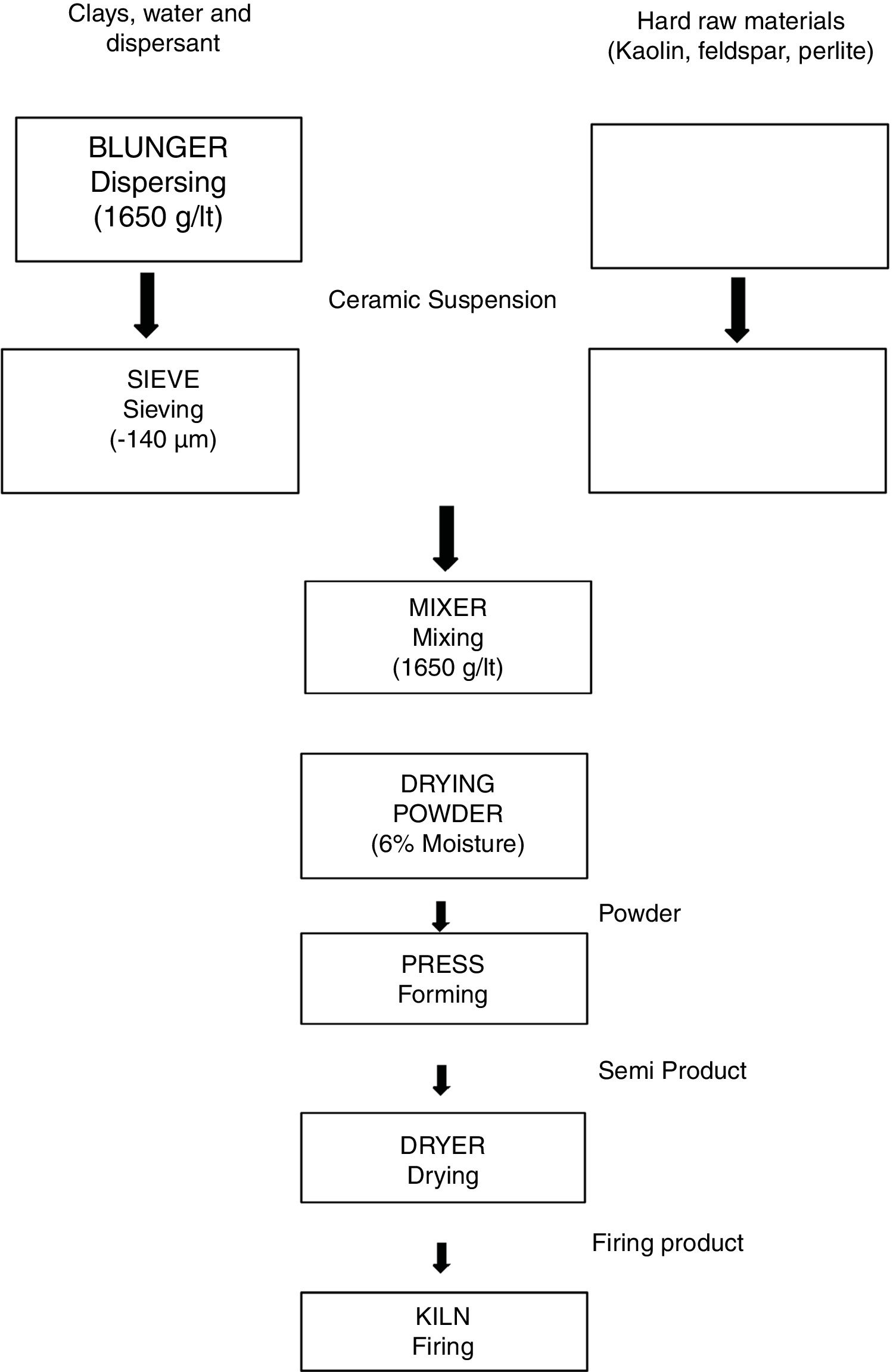
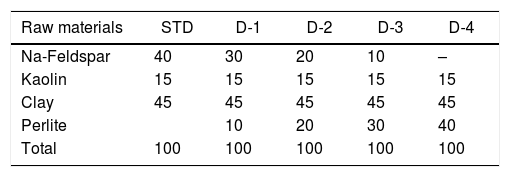
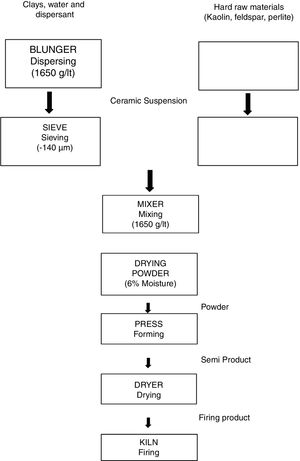
In the first part of the study, the representative experimental tile formulations were prepared by direct and progressive incorporation of perlite into a standard porcelain tile formulation (Table 2) in different amounts ranging from 10 to 40wt%. The amounts mentioned were chosen in order to highlight the effect of perlite addition. These formulations were further designated as STD (Standard), D-1, D-2, D-3 and D-4 for porcelain tile. The compositions were prepared according to Laboratory process as shown in Fig. 1. The process consists of slurry preparation (grinding, clay blunging, mixing, sieving), drying powder, pressing, drying and firing. The raw material prepared according to the percentage of compositions was milled below 45μm in which the weight of oversize material was about 3.5–4%. The prepared slurry was then dried at 105°C, in 4h and powdered by grinding. The moisture content of the powder was adjusted to around 6.5–7% and rendered ready for pressing. Dried powder was shaped at a pressure of 380kg/cm2. Finally, samples were single fast fired in a roller furnace under industrial conditions (at a peak temperature of 1200°C for 50min cold to cold).
Qualitative determination of major crystalline phases of the perlite and fired bodies was achieved by X-ray diffraction (Rigaku, Rint 2000, Japan) with Cu K-α radiation.
In the second part of the study, the densification behaviour was described in terms of linear firing shrinkage, water absorption and breaking strength in accordance with UNI EN ISO 10545-3 standard procedures. Apparent density and apparent porosity measurements were made according to the ASTM C20-74. The fired bodies were also subjected to colour measurements using a UV–Vis spectrophotometer (Minolta 3600d) and the change in chromatic co-ordinates of L*, a*, b* values with peak firing temperature was compared. Linear thermal expansion coefficients of perlite, albite and studied bodies were determined using a fully computer controlled Netch thermal dilatometer (Model: 402EP) at a heating rate of 10°C/min to 650C. The vitrification behaviour of rectangular compacts of the bodies was studied using a double beam optical non-contact dilatometer (Misura, Expert System Solutions, Italy) according to the corresponding industrial firing profiles.
Microstructural observations were performed on fractured and etched (with HF solution) surfaces of some selected fired samples using Scanning Electron Microscope (SEM, Zeiss Supratam 50VP) as secondary electron imaging mode, after sputtering with a thin layer of gold-palladium alloy in order to prevent charging. Qualitative EDS (Oxford Ints.5108 Link) analyses were performed simultaneously with microstructural observations in order to distinguish the various phases.
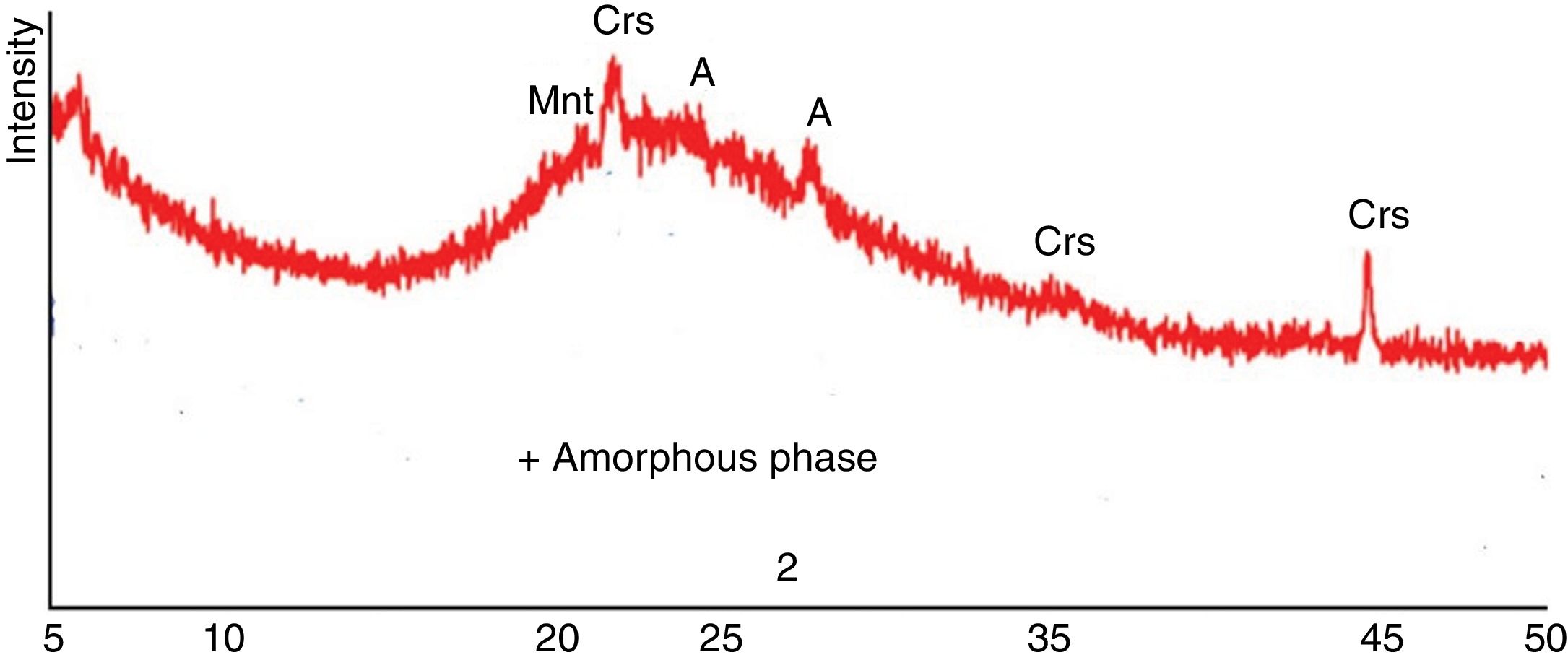
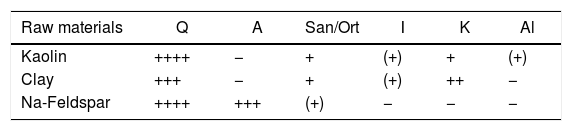
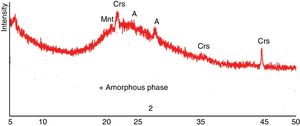
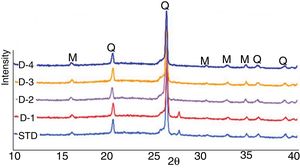
Results and discussionRaw material propertiesAs seen in Table 1, Perlite is mainly an alumina – silicate system, containing 8.90wt% alkali oxide (Na2O+K2O) and 1.02wt% Fe2O3. Fig. 2 is the XRD spectrum taken from perlite which predominantly contains an amorphous phase and minor amount of cristobalite (Crs), montmorillonite (Mnt) and albite (A). Furthermore, Table 3 gives the mineralogical abundances of albite, clay and kaolin used in the study. Na-Feldspar contains quartz (Q) and sanidine (San/Ort) besides albite which is the main phase. Quartz, kaolinite (K), illite (I) and sanidine are common minerals in clay and kaolin, whereas alunite (Al) is only in kaolin.
Mineralogical contents of the raw materials.
| Raw materials | Q | A | San/Ort | I | K | Al |
|---|---|---|---|---|---|---|
| Kaolin | ++++ | − | + | (+) | + | (+) |
| Clay | +++ | − | + | (+) | ++ | − |
| Na-Feldspar | ++++ | +++ | (+) | − | − | − |
Legend: Q: Quartz; A: Albite; San/Ort: K-feldspar; I: Illite; K: Kaolinite; Al: Alunite; ++++ (>20%); +++ (>15%); ++ (>10%); + (>5%); (+) (>5%); – not present.
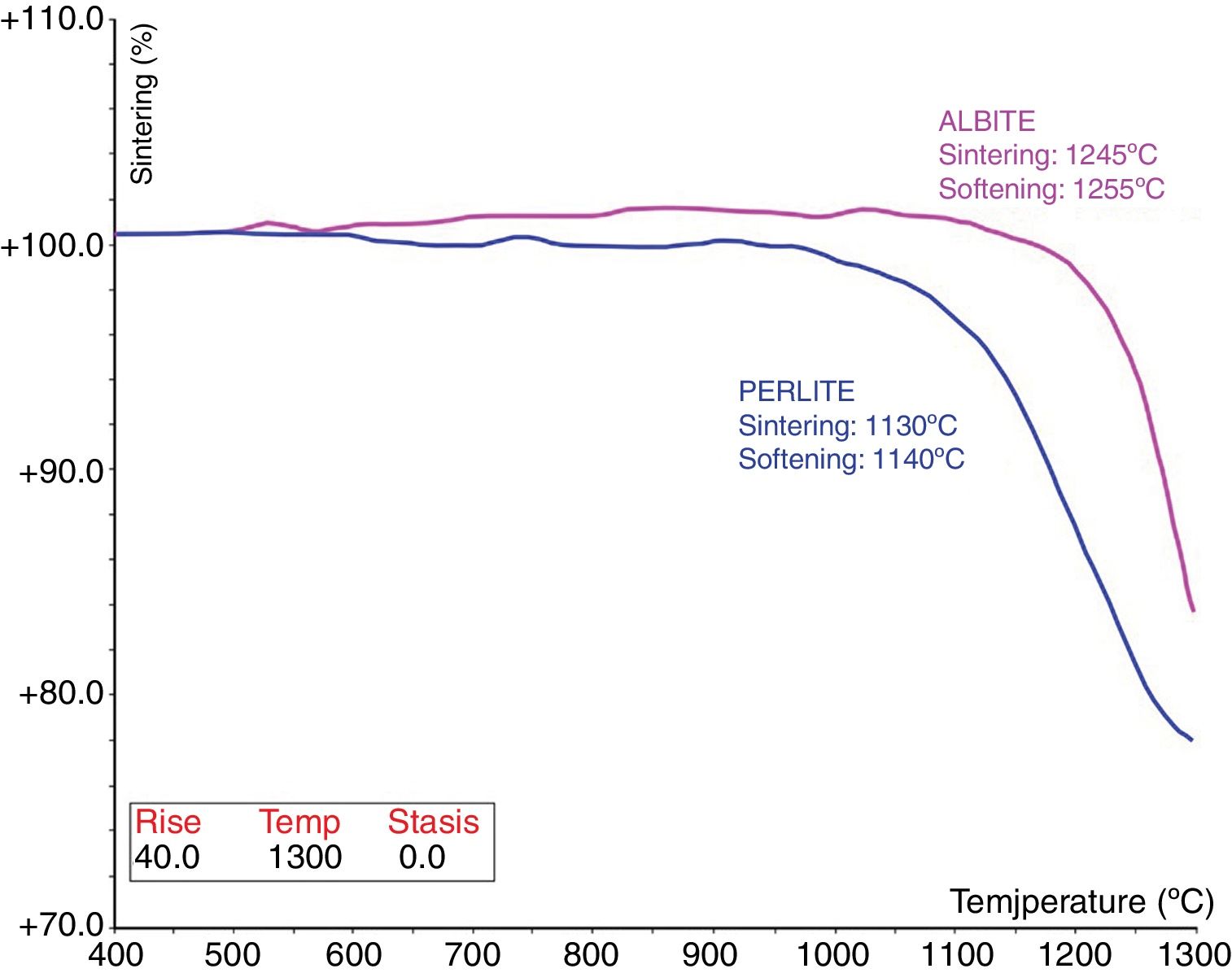
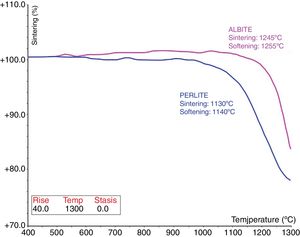
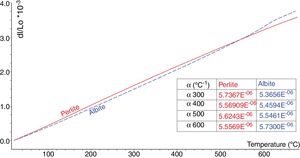
Fig. 3 shows the melting and sintering points of the fluxing materials, namely albite and perlite. Albite has both sintering and softening points at 1245°C and 1255°C. Perlite has the lowest sintering and softening temperatures at 1130°C and 1140°C. As known from the literature, the melting point of feldspar depends on the composition, especially on its alkali oxide (Na2O+K2O) content. Both the total amount of alkali oxides and the sodium-to-potassium ratio (Na2O/K2O) influence the melting behaviour, mixed alkaline melts at lower temperature than their pure forms [4].
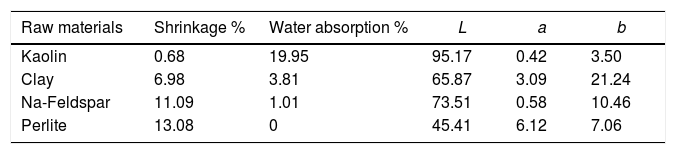
Table 4 indicates the shrinkage, water absorption and colour L, a, b values of all the raw materials individually used in porcelain tile bodies when fired in an industrial fast firing kiln at a peak temperature of 1200°C. Considering the technological properties of fluxing raw materials; perlite shrinkage values (13.08%) was higher compared to albite (11.09%) and water absorption value of perlite was 0% whereas albite had 1.01% water absorption. This use indicates more active flux nature of perlite compared to albite. The colour L value of perlite was very low (L: 45.41), possibly due to its high iron content (Fe2O3:1.02%).
Shrinkage, water absorption and firing colour values of the raw materials (Firing cycle: 1200° C and 34min).
| Raw materials | Shrinkage % | Water absorption % | L | a | b |
|---|---|---|---|---|---|
| Kaolin | 0.68 | 19.95 | 95.17 | 0.42 | 3.50 |
| Clay | 6.98 | 3.81 | 65.87 | 3.09 | 21.24 |
| Na-Feldspar | 11.09 | 1.01 | 73.51 | 0.58 | 10.46 |
| Perlite | 13.08 | 0 | 45.41 | 6.12 | 7.06 |
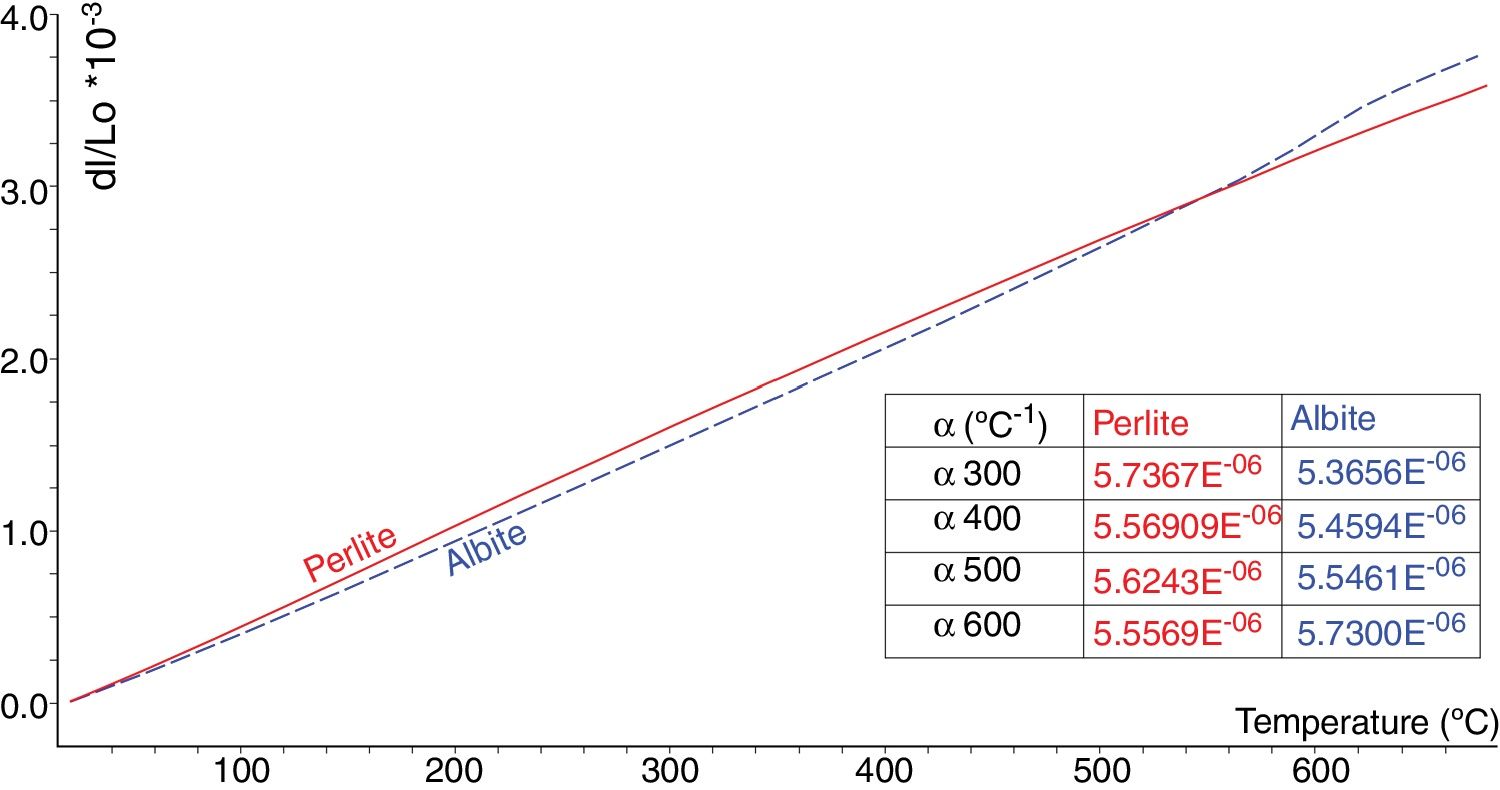
Fig. 4 shows thermal expansion curves of sintered albite and perlite blocks. Due to higher SiO2 content and lower alkali content perlite has lower thermal expansion than albite. As widely known, silica shows a volumetric expansion at 573°C during firing, corresponding to the transition from quartz to quartz, which is accompanied by a sharp increase in volume. The same situation occurs even more markedly just beyond 220°C, when cristobalite passes from the to form. This is a particularly critical moment in ceramic manufacturing as excessively fast cooling can cause certain types of damage to the tile [4]. As seen in the thermal expansion graphs in Fig. 4, no sudden volume expansion due to cristobalite and quartz is seen in perlite whereas a volume expansion is noticeable in feldspar due to the presence of some amount quartz. The presence of quartz in feldspar also contributes to its higher thermal expansion coefficient.
Technological propertiesTable 5 shows some of the rheological properties of slips prepared and physical properties of the recipes after sintering. Slip density reduces with the perlite addition due to lower theoretical density (2.2g/cm3) of perlite than feldspar (2.6g/cm3). On the other hand, slip viscosity increases probably due to the presence of small amount of montmorillonite in perlite. It is known that the presence of mixed layer clays such as montmorillonite increases viscosity of the silicate slurries [4].
Technological properties of standard and experimental compositions.
| Compositions | STD | D-1 | D-2 | D-3 | D-4 |
|---|---|---|---|---|---|
| Weight volume (g/L) | 1725 | 1719 | 1701 | 1677 | 1660 |
| Viscosity (cP) | 180 | 250 | 360 | 400 | 550 |
| Sieve residue on 45μm (%) | 1.97 | 1.91 | 1.86 | 2.05 | 2.08 |
| Apparent density (g/cm3) | 2.36 | 2.35 | 2.36 | 2.37 | 2.38 |
| Apparent porosity (%) | 1.16 | 1.05 | 0.88 | 0.56 | 0.23 |
| Firing shrinkage (%) | 6.87 | 6.93 | 7.59 | 8.03 | 8.59 |
| Water absorption (%) | 0.29 | 0.27 | 0.19 | 0.11 | 0.09 |
| Bending strength (MPa) | 44.20 | 44.35 | 47.80 | 49.10 | 50.67 |
| L | 55.26 | 53.88 | 53.64 | 53.12 | 53.02 |
| a | 2.6 | 3.07 | 3.08 | 3.52 | 3.62 |
| b | 16.10 | 16.39 | 16.90 | 16.97 | 17.52 |
| Linear thermal expansion (CTE. 10−7C−120–400°C) | 74.6 | 71.6 | 69.0 | 65.4 | 64.6 |
As also seen in Table 5, the water absorption and apparent porosity values significantly decrease with increasing perlite content in the body composition. Correspondingly, the apparent density and linear shrinkage values increase. It appears that higher sintering activity of the perlite alone as compared to the feldspar is reflected also in the body composition, as expected.
According to the UNI ISO EN standards and on the basis of water absorption percentage the obtained samples could be classified into BIa group having water absorption less than 0.5%, respectively, and belong to porcelain ceramic tiles [28].
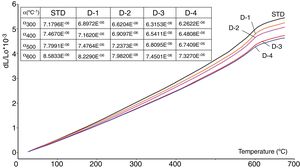
The values of the bending strength measured on the standard (STD) and new developed bodies (D-1, D-2, D-3 and D-4) show that it is affected by the porosity and perlite content (Table 5). Apart from porosity, strength development of silicate ceramics also depends on the amount and size of quartz and mullite crystals in the microstructure [29,30]. Although the quantification with respect to the amount of quartz has not been made in this study, it can be qualitatively judged from Fig. 5 by comparing expansion amount due to the quartz transformation at 573C in that the free quartz amount in all the samples are similar. It can also be elucidated that the quartz grain size in all the samples is similar as their grinding time was the same during sample preparation. Likewise, Fig. 6 shows that the amount of mullite in the sintered samples and their size (Fig. 8) was similar. Therefore, increasing bending strength of the sintered samples with increasing perlite content can simply be related to progressive reduction in porosity as it is known that porosity reduces strength of ceramics exponentially [31].
From the colorimetric analysis, it is evident the L value decreases with the increasing perlite content while the opposite trend characterized the a and b parameters (Table 5). This behaviour is owing to the chemical composition of used raw materials. In fact, as the perlite content is increased, Fe2O3 and TiO2, of the bodies will be increase and this led to the decreasing of L value and increasing a and b values.
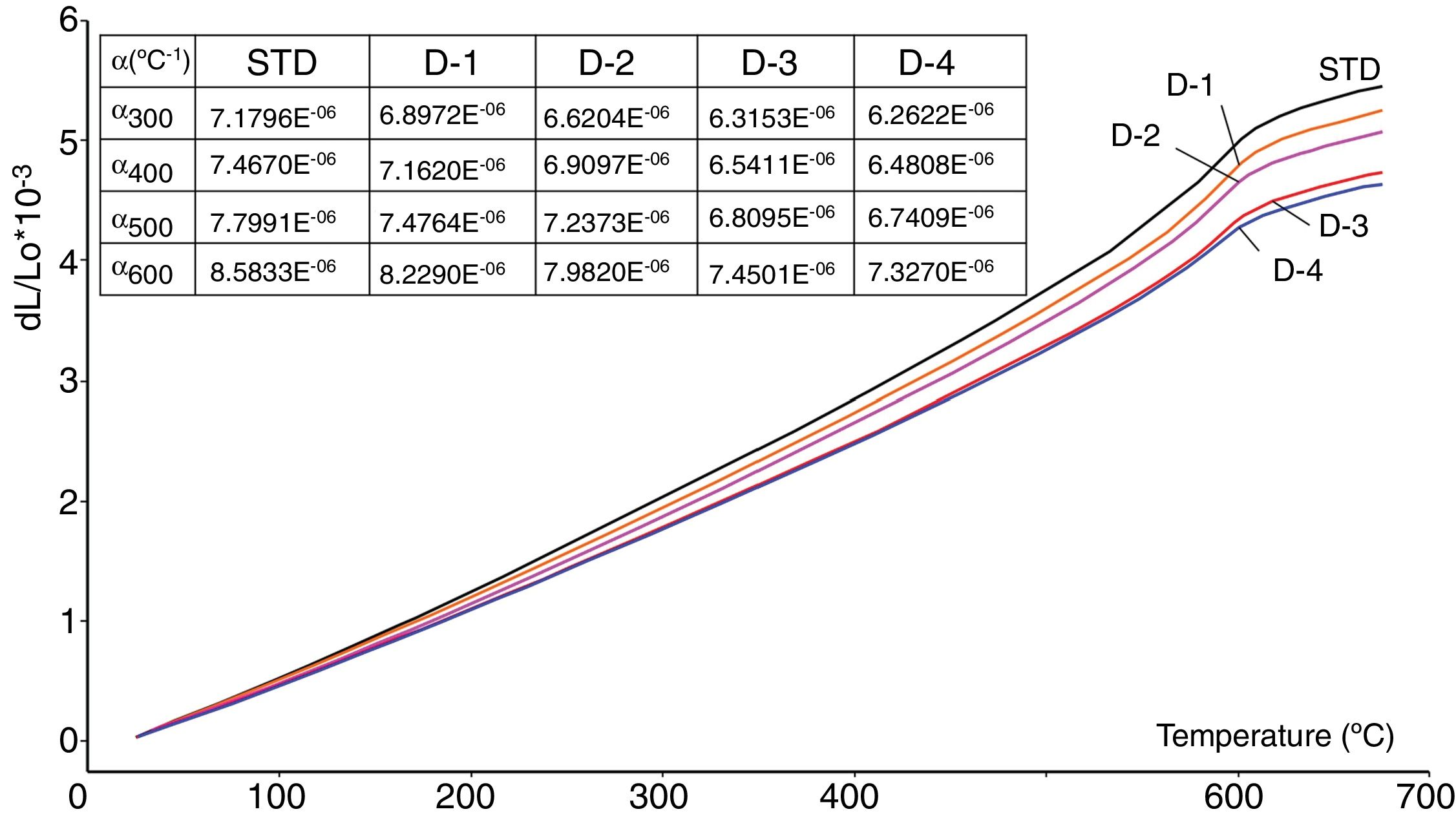
Thermal expansion coefficientsThe values of the thermal expansion coefficients of standard and new bodies containing perlite are showed in Fig. 5. Increasing perlite addition in the body compositions progressively reduces thermal expansion of the fired bodies. This is due to the high silica and low total alkali content of perlite compared to Na-feldspar and the absence of residual crystal phases of silica such as cristobalite and quartz. Unlike perlite, fired Na-feldspar contains residual quartz after firing (Figs. 2 and 4), causing an increase in its thermal expansion coefficient. As it is well known, silica in amorphous form has very low thermal expansion coefficient (0.52×10−6/K) [32] and likewise increasing silica content in an amorphous glass, such as the one in fired perlite containing bodies, causes reduction in thermal expansion coefficient. It is to be noted that there is no formation of cristobalite due to perlite in sintered bodies which indicates that the low amounts of cristobalite present in perlite is dissolved in the liquid phase during firing. This is particularly important as the risk of cracking due to cristobalite transformation during cooling, which occurs around 220°C, is eliminated. Substantial reduction in the thermal expansion coefficients of perlite containing bodies as the perlite content increases (from 7.16×10−6 to 6.48×10−6 between 20 and 600°C, see Fig. 5) indicates that perlite is an effective mineral for reducing thermal expansion values of the sintered bodies.
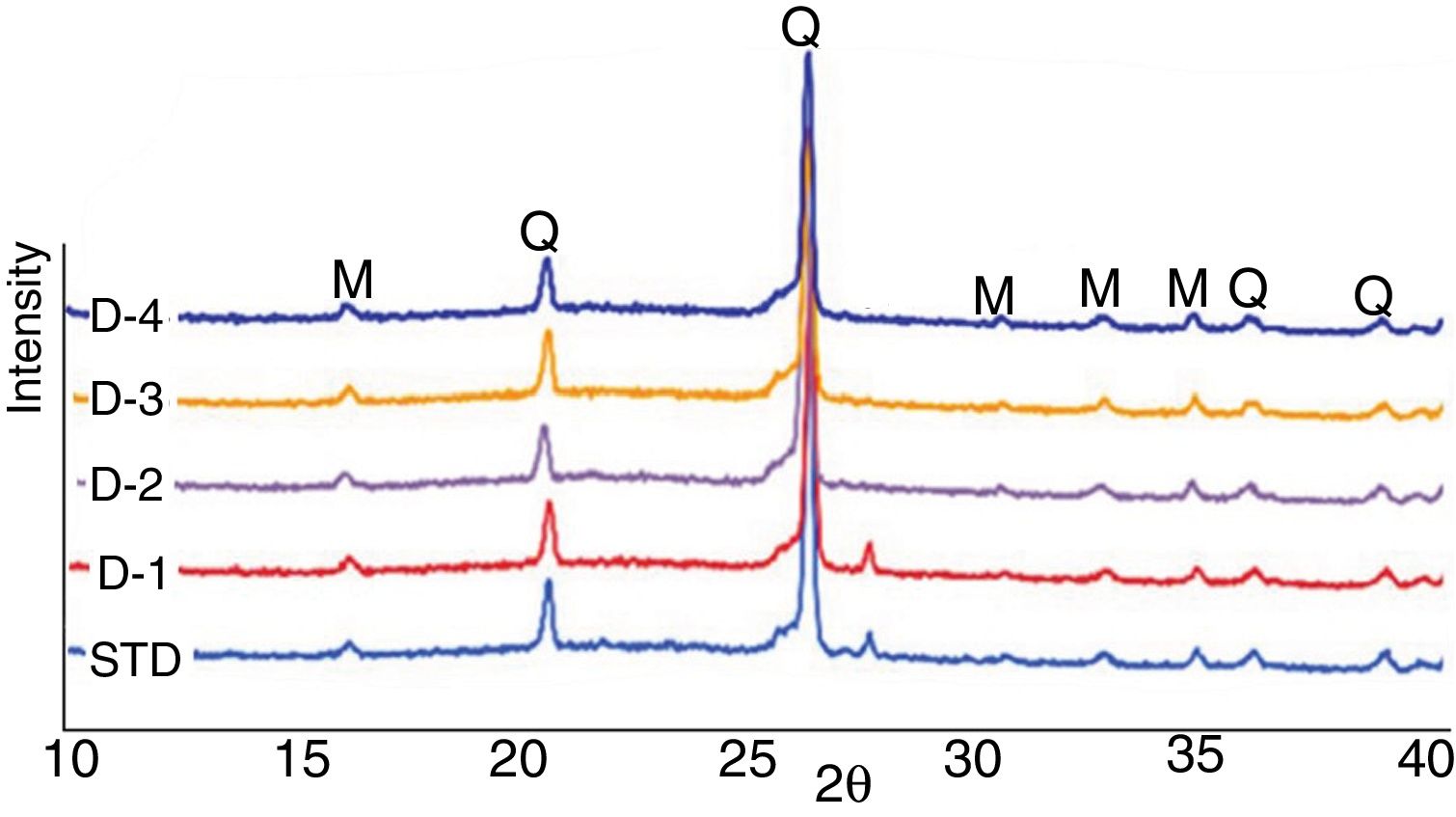
X-ray diffractionXRD patterns of the studied compositions fired at a peak temperature of 1200°C (Fig. 6) indicate the presence of mullite (M) and quartz (Q) as major phases. In addition, weak diffraction peaks of albite phase (A) (Na2O·Al2O3·6SiO2) were observed. Most of the reactions occurring during firing of traditional tile bodies are kinetically governed processes that do not reach thermodynamic equilibrium due to rather short (<60min.) industrial fast firing cycles. This explains the common presence of crystals of quartz and feldspars that have not been entirely transformed in the fired bodies. However, no residual albite is observed in D-2 recipe indicating that perlite helps albite melt completely. This further helps densification by increasing volume of the liquid during sintering. As can be explained from the same figures, the introduction of perlite into the standard formulation did not cause the development of new phases, particularly cristobalite.
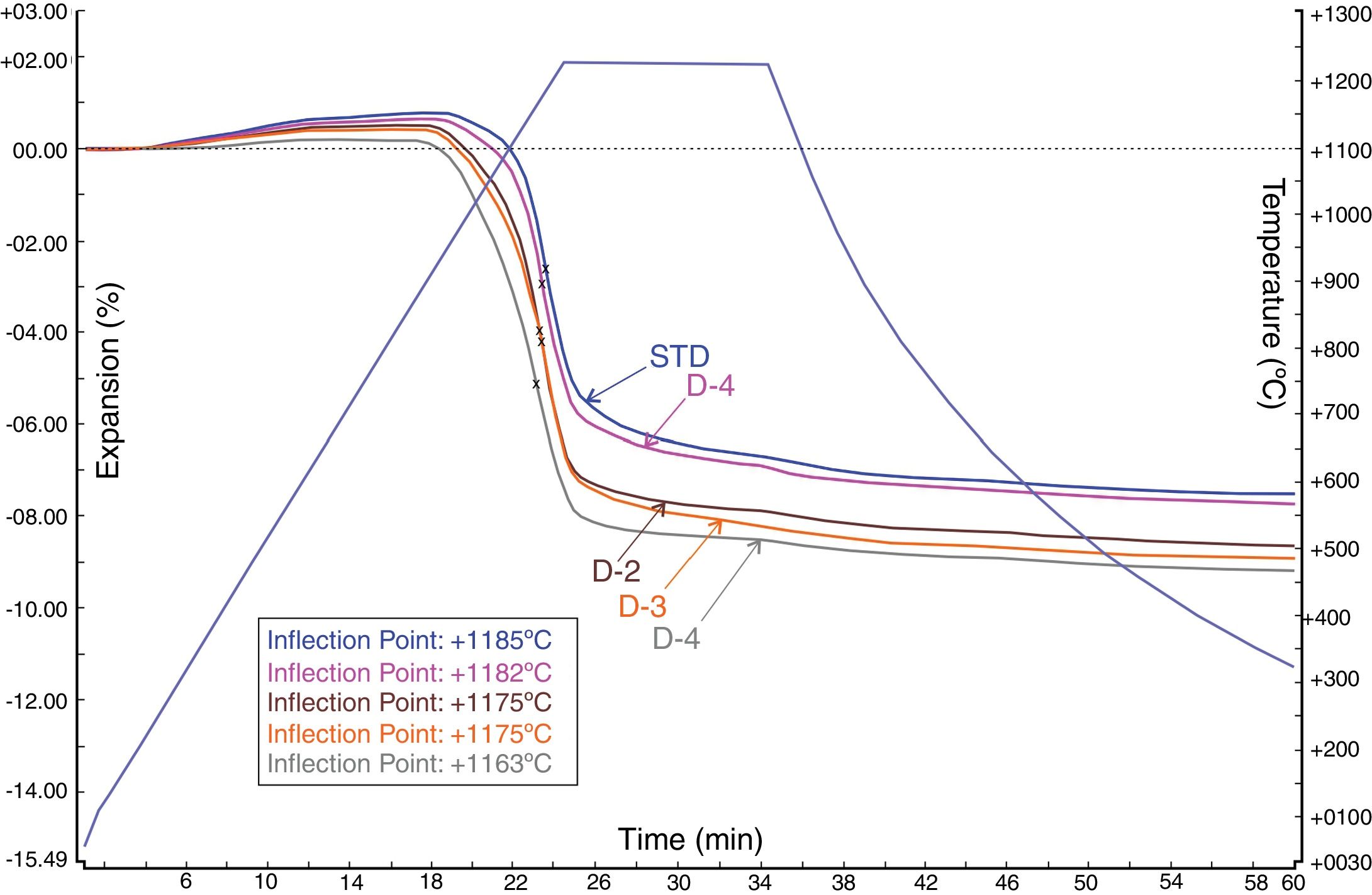
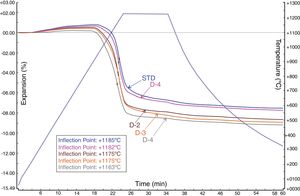
Sintering behaviour of the developed bodiesFig. 7 shows the sintering behaviour of the standard (STD) and investigated new body formulations with perlite. In the figure, the graph was plotted with time on the x-axis and both temperature and expansion percentage on the y-axis (negative expansion indicating shrinkage). According to the dilatometric curve of the STD and new developed bodies with perlites (D-1, D-2, D-3 and D-4) in Fig. 7, all bodies show expansion up to around 1000°C (first inflection point) before densification occurs. The maximum sintering rates (the temperature of maximum shrinkage rate, indicated as inflection point at the expansion curves) were observed for all bodies between 1163°C and 1182°C, the lowest being with the highest amount of perlite. On the other hand, in Fig. 7, D-4 body shows the maximum sintering activity with respect to the standard (STD) and alternative bodies (D-1, D-2, D-3), since it has the highest shrinkage amount that is around 5.09%. That finding is also supported by the technological properties of the product (Table 5), namely water absorption and linear firing shrinkage.
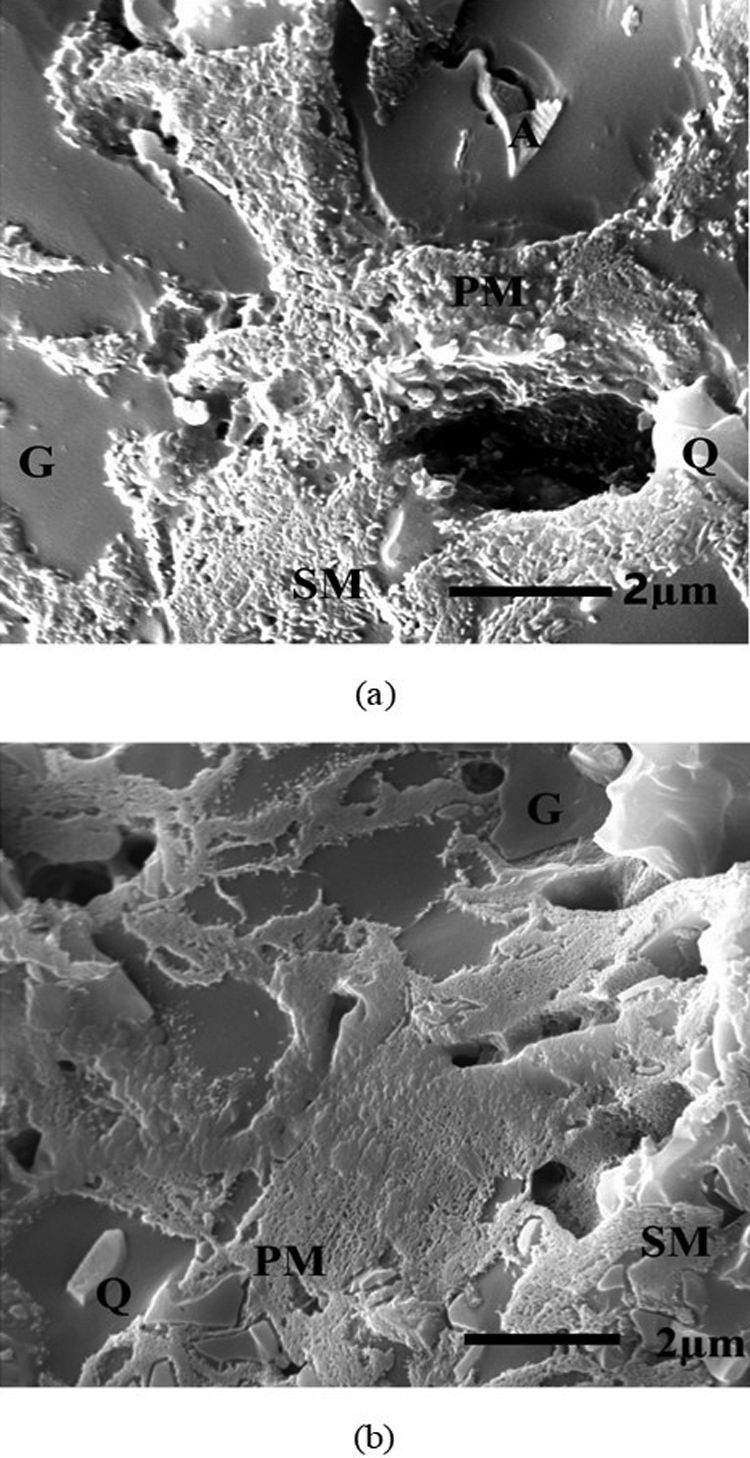
Microstructural analysisStandard body (STD) and the new developed body containing 40% perlite (D-4) was compared to each other in micrograph of SEM (Fig. 8). Both bodies show similar microstructural features comprising of mullite and partially dissolved quartz within glassy matrix, which is in agreement with the results reported by Iqbal and Lee [33]. SEM image of standard body (STD) has revealed the presence of primary mullite (PM) and secondary mullite (SM) particles, unreacted albite, and dissolved quartz crystals (Fig. 8a). 40wt% perlite containing body shows more developed and longer needle like mullite particles and lack of unreacted albite crystals (Fig. 8b).
ConclusionsNa-Feldspar usage can be reduced by using perlite in STD compositions. Perlite has more shrinkage properties than other alkali-containing raw materials during firing because of its water content and its mineralogically amorphous structure. Therefore, firing shrinkage of perlite containing bodies tend to increase. However, by the use of raw materials containing quartz at high mineralogical levels in such bodies or decreasing of firing temperature or cyle, firing shrinkage values can be reduced without changing the water absorption value too much. Thermal expansion of porcelain tile is also a significant factor in determining the surface quality and dimensional stability of the tiles. The major finding in this study was the decrease in the thermal expansion coefficients owing to the addition of perlite, showing that final products with high dimensional stability and suitable deformation properties can be obtained. Therefore, perlite can be suggested as an alternative fluxing agent to Na-Feldspar in porcelain tile compositions.
This research has been carried out in Kaleseramik Research and Development Center that is supported by Republic of Turkey, Ministry of Science, Industry and Technology and Turkish Ceramic Research Center (SAM).