Ca3Co4O9+xwt.% TiC (x=0, 0.25, 0.5, 0.75, and 1.0) polycrystalline thermoelectric ceramics have been prepared through the classical ceramic route. XRD characterization has demonstrated that all samples are mainly composed by the Ca3Co4O9 phase, while microstructural observation has shown that no reaction between both components has been produced. Moreover, TiC particles are well distributed for small additions, and start to agglomerate from 0.75wt.% content. Density measurements showed that nearly no changes have been produced by TiC addition and nearly the whole porosity appears as open one. Furthermore, electrical resistivity decreases up to 0.75wt.% addition, increasing for higher content. On the other hand, Seebeck coefficient has been maintained unchanged in all samples. In spite of an irregular behaviour of thermal conductivity with temperature, it tends to decrease when the temperature is raised, displaying the lowest values for the 0.25wt.% samples. These data led to an increase of about 40% in ZT values at 800°C for samples with 0.25wt.% addition, when compared with the pure ones. Finally, linear thermal expansion coefficient is decreased when TiC content is increased, which can be exploited to fit the thermal expansion coefficients of all the components used to build a power generation thermoelectric module.
Se han preparado cerámicas de composición Ca3Co4O9 + x% en peso de TiC (x=0, 0,25; 0,5; 0,75, y 1,0) utilizando el método clásico de estado sólido. La caracterización por XRD mostró que la fase termoeléctrica Ca3Co4O9 aparecía como mayoritaria. Las observaciones microestructurales determinaron que no se produce reacción entre esta fase y las partículas de TiC. Estas partículas aparecen bien distribuidas cuando hay pequeñas adiciones y comienzan a aglomerarse a partir del 0,75% en peso. Las medidas de densidad indicaron que este parámetro no se modifica con la adición de TiC y, además, que se produce una porosidad de tipo abierto. La presencia de TiC modifica la resistividad eléctrica de forma que disminuye hasta el 0,75% en peso de TiC, aumentando para contenidos mayores, sin afectar el coeficiente de Seebeck. La conductividad térmica muestra un comportamiento irregular, pero tiende a disminuir al aumentar la temperatura, alcanzando los menores valores en muestras con 0,25% en peso de TiC. Estos resultados llevaron a un aumento del 40% en los valores de ZT a 800°C en muestras con 0,25% TiC en peso, comparados con los medidos en muestras sin TiC. Finalmente, el coeficiente de dilatación lineal disminuye al aumentar el contenido de TiC, lo que puede utilizarse para ajustar los coeficientes de dilatación de todos los componentes al construir un módulo termoeléctrico de generación de potencia.
Thermoelectric (TE) materials are characterized by their ability to transform a temperature difference into useful electrical power, provided by the Seebeck effect. The efficiency of these materials when considered for this energy conversion can be described through the ZT (dimensionless figure of merit). Physically it can be described by the expression ZT=TS2/ρκ, where T is the absolute temperature, S the Seebeck coefficient, ρ the electrical resistivity, and κ the thermal conductivity [1]. Due to their characteristics, these materials can harvest heat in many applications [2], or from renewable sources [3], to produce electrical power from a temperature gradient. Consequently, they can be used to exploit waste heat produced in non-renewable energy transformation systems, increasing, in this way, their efficiency [4,5]. Usually, for integration in commercial thermoelectric generators, these TE materials should display ZT values≥1.
Today, there are TE commercially available devices, which are typically built using intermetallic legs. These intermetallic materials are characterized by high ZT values at relatively low temperatures [6–11]. Consequently, they are not useful at high temperatures due to the decrease of their performances, together with their high reactivity in these conditions under air, which leads to degradation processes or releasing heavy metals [12]. High temperature applications become feasible through the discovery of high TE performances in Na2Co2O4[13], which has led to intense research on this kind of materials and, to the discovery of new ones, as Ca3Co4O9[14,15] or CuCrO2[16], as p-type, and TiO- [17,18] or MnO-based materials [19] as n-type.
These Co-based materials can be crystallographically described using a monoclinic structure, which is formed by alternate stacking of two different layers. They are a conducting CoO2 layer with CdI2-type structure, and a rock-salt-type separating layer, acting as charge reservoir. Both layers have the same lattice parameters, only differing in their b-axis length, which leads to a misfit along this direction [20,21]. This high crystallographic anisotropy is reflected in the anisotropic grain growth, forming plate-like grains with favourable electrical conductivity along the ab-planes. These characteristics explain the large number of studies, which have been performed in the last years to obtain bulk materials with good grain orientation [22–27] to drastically decrease electrical resistivity with very slight modifications in the Seebeck coefficient values. On the other hand, many other works have also been studying the mechanisms to decrease thermal conductivity through different processes, as SiC nanoparticles addition, which can help to enhance phonon scattering [28,29]. The use of SiC nanoparticles has been justified as it is a semiconducting thermoelectric material with low electrical resistivity, as reported in [30], and it is able to decrease electrical resistivity and thermal conductivity without drastic modifications of Seebeck coefficient [28].
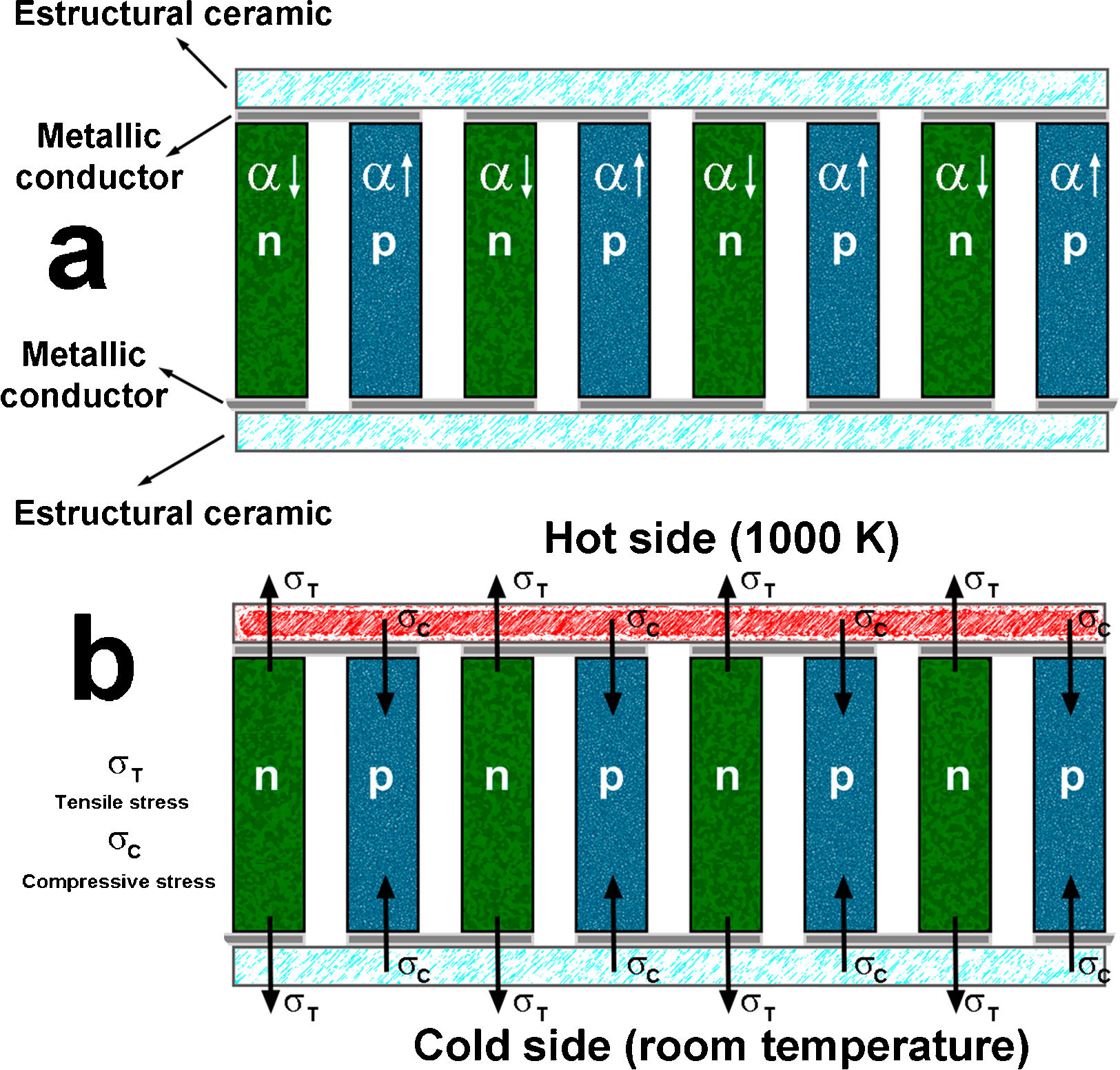
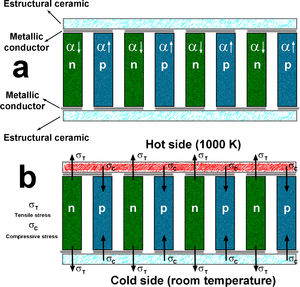
In spite of the large number of works published in the last years, it is very difficult to find studies reporting the materials characteristics from the point of view of their possible integration in practical thermoelectric modules, mostly related with their thermal expansion. This parameter is of the main importance, as it can be observed in the schematic presentation of a thermoelectric module of Fig. 1a, where the different types of materials are shown. The module consists in alternate n and p type thermoelectric materials, which are electrically connected in series and thermally in parallel. The connections between thermoelements are conducting materials (typically metals) to form the electric circuit. Moreover, the upper and lower parts of the module are electrically insulating materials (typically ceramics), which provide the mechanical robustness. Finally, all these materials are welded together to form the thermoelectric module. When heating this module, the situation is modified, as it can be seen in Fig. 1b, where the effect of different thermal expansion coefficients of thermoelements, is shown. The material with larger α will be subjected to compressive stress inside the module, while the one with lower α, to tensile stress. Consequently, these different stresses will affect the welding between different materials, which can degrade electrical connections and drastically affect the module performances and limiting its useful life.
Schema showing the different types of materials present in a thermoelectric module (a); stresses appearing on the thermoelements when the module is operating (b). α↓ and α↑ are the low and high linear thermal expansion coefficients; σT, and σC are the tensile and compressive stresses.
Consequently, this work will report the results obtained in Ca3Co4O9 ceramic materials with different additions of TiC nanoparticles. TiC has been chosen due to the relatively low mechanical properties of SiC, and especially very low fracture toughness [31]. Moreover, TiC addition to SiC has drastically increased mechanical properties in around 50% flexural strength, and two times for fracture toughness [32]. Furthermore, TiC is characterized by relatively good TE properties except by a very low Seebeck coefficient [33]. The effect of these additions on the structural, and microstructural properties will be studied and related to the thermal, and electrical properties of the final sintered materials.
ExperimentalCa3Co4O9+x wt.% TiC nanoparticles, with x=0.00, 0.25, 0.50, 0.75, and 1.0, have been prepared using the classical solid state route. The amount of TiC added to the samples has been fixed taking into account the optimal content of SiC nanoparticles in Bi2Sr2Co2Oy, which is much lower than 1wt.% [28]. For this purpose, CaCO3 (Panreac, 98 + %), and Co2O3 (Aldrich, 98 + %), were weighed in stoichiometric proportions, mixed and ball milled 30min at 300rpm in water media. The powder suspension was dried under infrared radiation, followed by manual milling, and two-step calcination procedure at 750 and 800°C for 12h under air, with an intermediate manual milling, to decompose the Ca carbonate and produce prereacted materials, as previously reported [34]. After manually milling these calcined powders, TiC (Aldrich, <200nm) was added in the adequate proportions and ball milled again for 30min at 300rpm to obtain a homogeneous mixture. The different mixtures were then cold uniaxially pressed into pellets under 400MPa, followed by sintering at 900°C for 24h under air atmosphere, and a final furnace cooling at room temperature.
Powder X-ray diffraction (XRD) patterns were obtained at room temperature in a theta-theta PANalyticalX’Pert Pro diffractometer (CuKα radiation, λ=1.54059Å) between 10° and 40°, where the main peaks of Ca3Co4O9 phase appear. Moreover, Rietveld refinement has been used to determine lattice parameters. Density measurements were performed using both Archimedes’ method and in a He pycnometer (AccuPyc 1340 from micromeritics) on several sintered samples for each composition. The relative density values were calculated with respect to the theoretical one of Ca3Co4O9 (4.677g/cm3[35]) and TiC (4.93g/cm3[36]).
Microstructural observations have been performed on several longitudinal fractured sections of samples, using a Field Emission Scanning Electron Microscope (FESEM, Carl Zeiss Merlin) fitted with an energy-dispersive spectrometry (EDS) system. Several micrographs obtained in each specimen have been used to determine the phases formed in each sample. Mechanical properties were measured in four samples of each composition by the three points bending test, in an Instron 5565 testing machine with 10mm span and a punch displacement of 30μm/s. Electrical resistivity and Seebeck coefficient were simultaneously measured using the standard four point contact in a LSR-3 measurement device (Linseis GmbH) between 50 and 800°C under He atmosphere. Thermal diffusivity (α) has been obtained using a laser-flash system (Linseis LFA 1000). The thermal conductivity (κ) has been determined using κ=αCpρ, where Cp is the specific heat and ρ is the sample density previously determined. Specific heat was calculated using the well-known Dulong–Petit law. Using electrical resistivity, Seebeck coefficient, and thermal conductivity data, ZT evolution with temperature has been calculated to determine the samples performances. The samples characteristics have been compared with the results obtained in pure ones and with the best values reported in the literature. Finally, dilatometric characterization has been performed between room temperature and 800°C in a L79 HCS dilatometer (Linseis GmbH) in order to evaluate the linear expansion coefficient variation as a function of the amount of TiC addition.
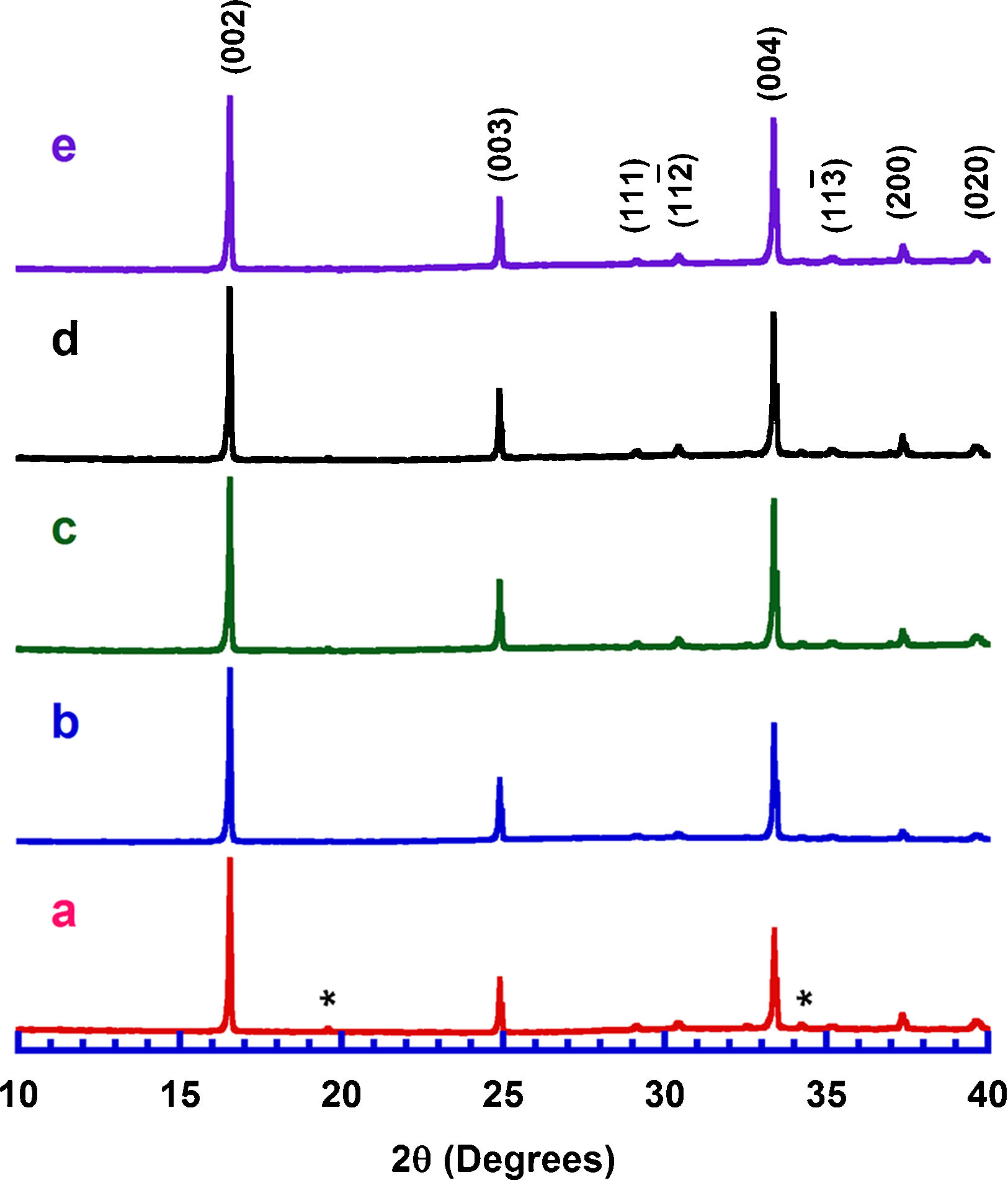
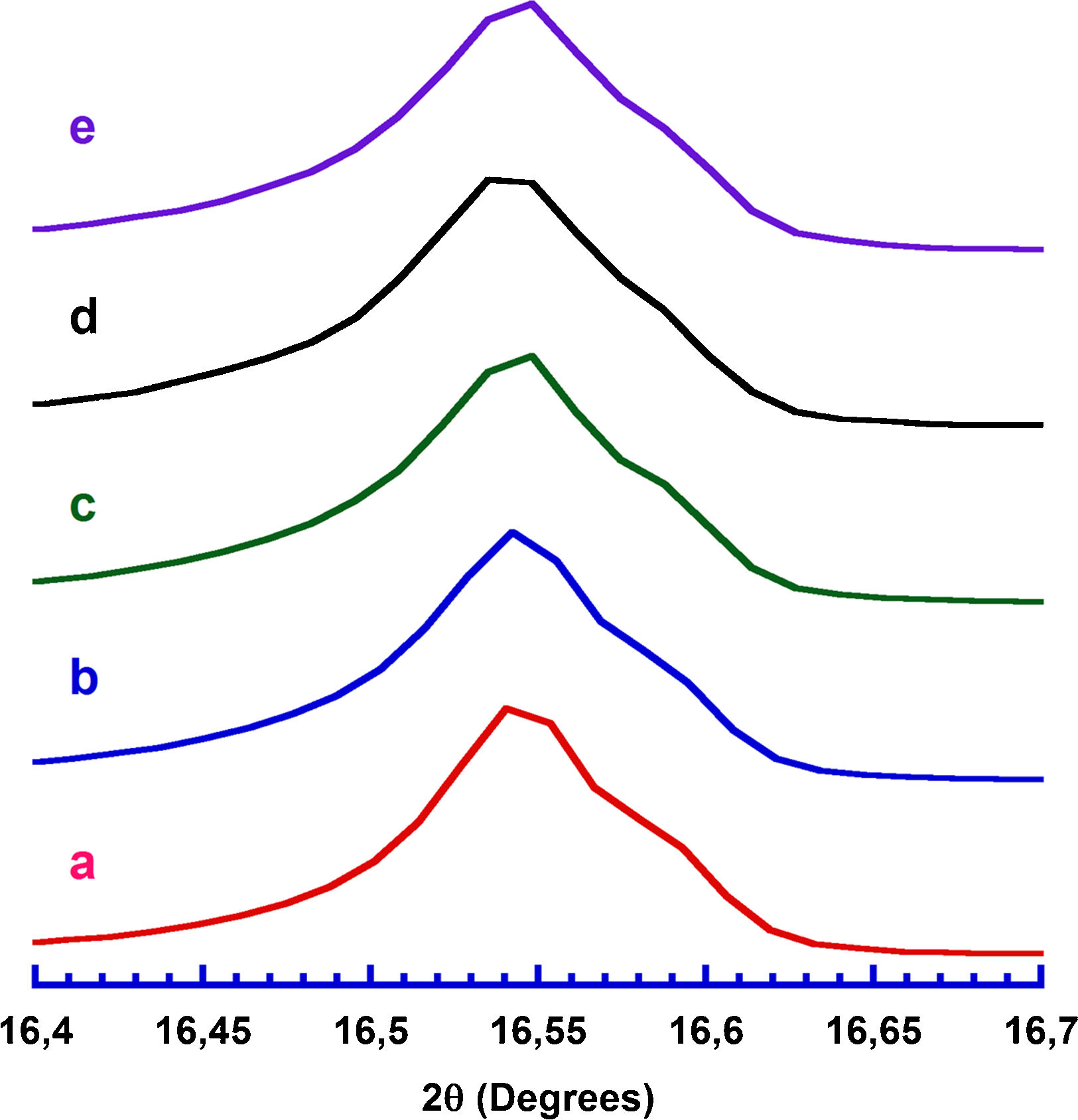
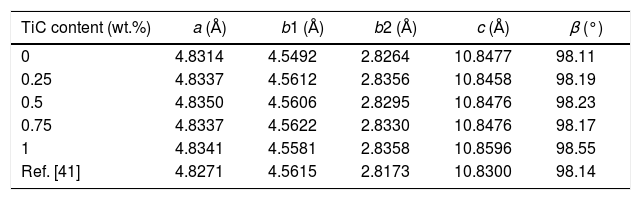
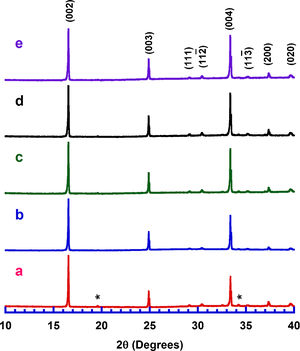
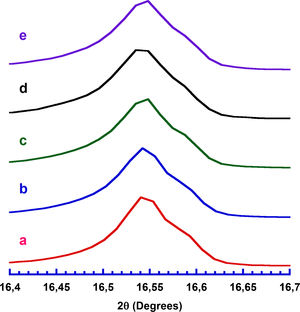
Results and discussionPowder XRD patterns determined in all Ca3Co4O9+x wt.% TiC samples (with x=0, 0.25, 0.5, 0.75, and 1) are displayed in Fig. 2. In the graph, it can be observed that all samples present the same patterns, independently of the TiC addition, and most of the peaks can be associated to the Ca3Co4O9 phase (with monoclinic symmetry) diffraction planes [37,38]. Moreover, the highest peaks have been indexed as corresponding to the ab-planes, indicating some degree of texture in the powders, which is induced by the samples preparation [39]. On the other hand, only small amounts of Ca3Co2O6 phase (indicated by * [40]), besides the thermoelectric one, have been identified with this technique, due to the small amount and sizes of TiC added to the samples. Furthermore, no shift has been detected in the peaks position, clearly showing that no cationic substitution has been produced in the Ca3Co4O9 phase. This fact has been confirmed through the close view of one of the main peaks of Ca3Co4O9 phase, (002) and presented in Fig. 3. As it can be easily observed in the plot, no significant shift of the peak has been produced, independently of the TiC addition, reflecting that Ti has not diffused into the crystal structure of the thermoelectric phase. Moreover, lattice parameters have been calculated from the Rietveld refinement of each pattern and they are presented in Table 1, together with the reported in literature [41]. As it can be observed in these data, there are differences between the parameters determined in these samples and the previously reported in the literature, but they could be associated to the typical texture-induced problems due to the plate-like nature of the thermoelectric grains. Consequently, it could be deduced that no significant element substitution has been produced.
Lattice parameters obtained after Rietveld refinement of XRD patterns of all samples.
| TiC content (wt.%) | a (Å) | b1 (Å) | b2 (Å) | c (Å) | β (°) |
|---|---|---|---|---|---|
| 0 | 4.8314 | 4.5492 | 2.8264 | 10.8477 | 98.11 |
| 0.25 | 4.8337 | 4.5612 | 2.8356 | 10.8458 | 98.19 |
| 0.5 | 4.8350 | 4.5606 | 2.8295 | 10.8476 | 98.23 |
| 0.75 | 4.8337 | 4.5622 | 2.8330 | 10.8476 | 98.17 |
| 1 | 4.8341 | 4.5581 | 2.8358 | 10.8596 | 98.55 |
| Ref. [41] | 4.8271 | 4.5615 | 2.8173 | 10.8300 | 98.14 |
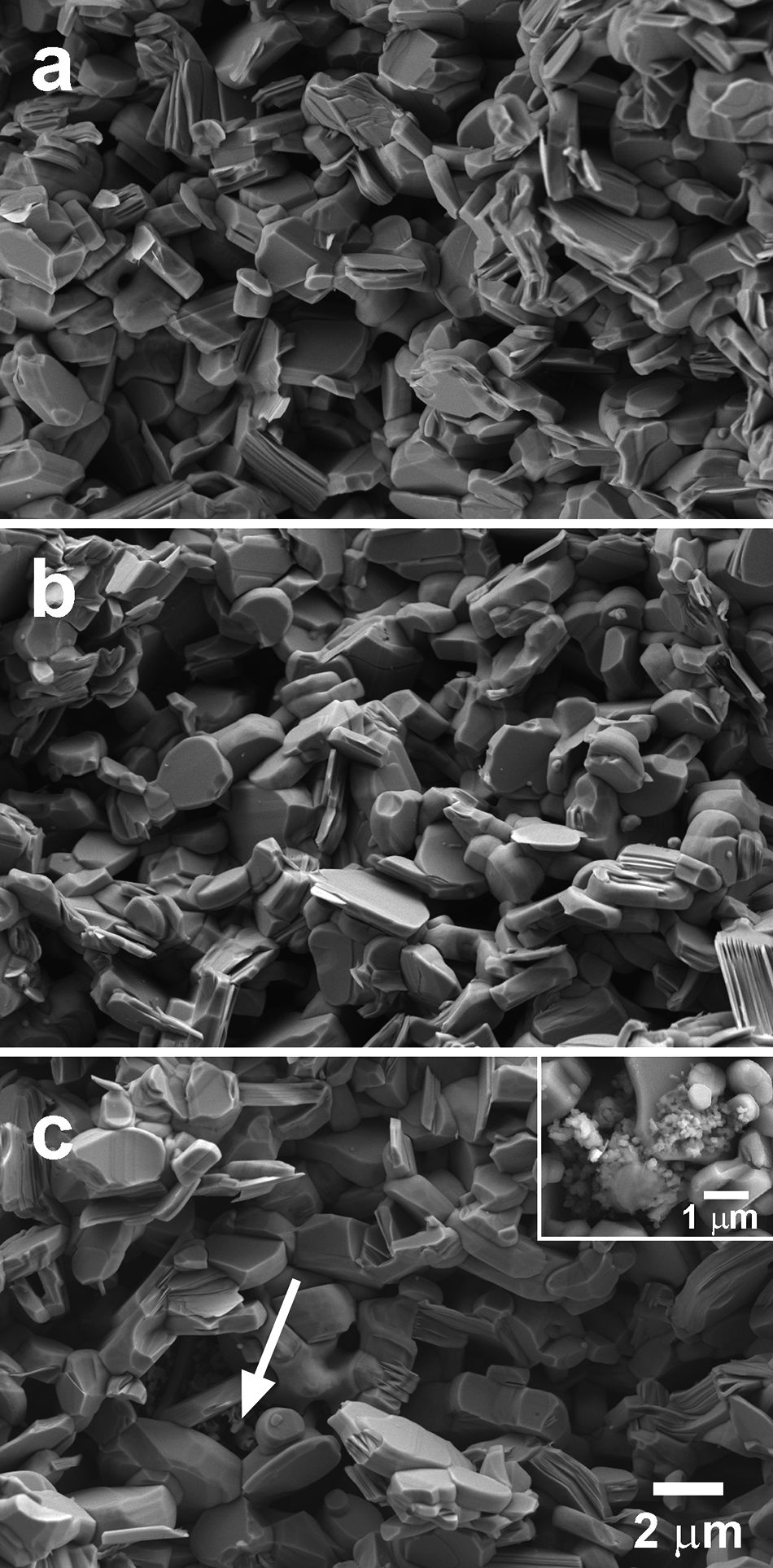
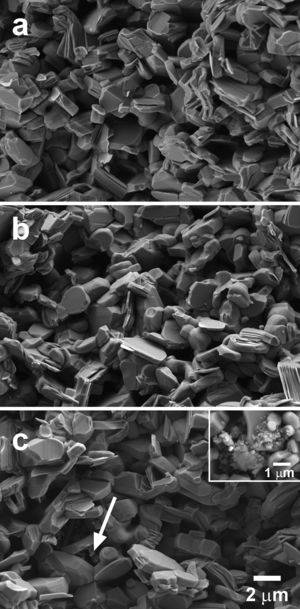
In Fig. 4 some representative SEM micrographs, performed on the fractured surfaces of samples, are presented. In these images, it is easy to see that all samples are composed of randomly oriented plate-like grains, which is the typical microstructure obtained through the classical solid-state method. Moreover, the images show a slight increase of grain sizes when the amount of TiC nanoparticles is raised. In order to confirm this observation, grain sizes have been evaluated on several micrographs for each composition using the line intercept method. The mean grain sizes have been 5.24, 5.59, 5.74, 5.85, and 5.92μm, for the 0, 0.25, 0.50, 0.75, and 1.0wt.% TiC addition, respectively. However, porosity seems to be nearly unchanged in all samples, which can be easily explained taking into account the relatively low sintering temperature used in this work, which is limited by the maximum stability temperature of Ca3Co4O9 phase (926°C). This temperature is far from the necessary to form a liquid phase in this system (∼1350°C) [42], hindering the samples densification during sintering. Additionally, it is also very far from the melting point of TiC (3067°C) [36], which cannot help in the sintering process. On the other hand, TiC has been found to be well distributed all around the samples for≤0.5wt%, while for higher contents, TiC starts to form agglomerates (indicated by an arrow in Fig. 4c) which are placed between the thermoelectric grains. A closer view of these agglomerates is shown in the insert in Fig. 4c, for clarity. On the other hand, EDS analysis of these agglomerates cannot give a clear indication of the possible oxidation of TiC during sintering process, even if it has been reported for the pure compound, or the TiC/SiC composite, in previous works [43,44]. However, TiC oxidation should not negatively affect transport properties, as TiO2 doping has been shown to decrease electrical resistivity of Ca3Co4O9 material [45].
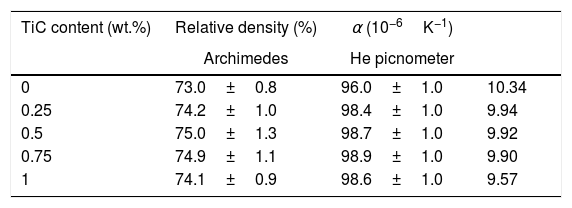
In order to confirm the microstructural evolution observed in the SEM micrographs, which showed that no modification in the porosity content has been produced by TiC addition, the samples density, evaluated through Archimedes’ method, has been determined on 4 samples for each composition. In order to minimize measurement errors, they were measured and weighed for three times, and the obtained results are summarized in Table 2. As it can be deduced from these data, all samples display very similar values, being slightly higher when TiC is added, in agreement with the SEM observations. The data clearly point out that TiC has no influence on the densification of samples during sintering procedure. Moreover, these values are of the order or higher than the reported for classically sintered Ca3Co4O9 materials [26,46–48]. On the other hand, density measurements have been also performed in an He pycnometer in order to distinguish between open and close porosity. The obtained data clearly showed that nearly the whole porosity found in the samples is open one, as the density values are nearly 100% of the theoretical one (see Table 2).
Relative density of sintered samples determined through Archimedes’ method and He pycnometer; and mean thermal expansion coefficient.
| TiC content (wt.%) | Relative density (%) | α (10−6K−1) | |
|---|---|---|---|
| Archimedes | He picnometer | ||
| 0 | 73.0±0.8 | 96.0±1.0 | 10.34 |
| 0.25 | 74.2±1.0 | 98.4±1.0 | 9.94 |
| 0.5 | 75.0±1.3 | 98.7±1.0 | 9.92 |
| 0.75 | 74.9±1.1 | 98.9±1.0 | 9.90 |
| 1 | 74.1±0.9 | 98.6±1.0 | 9.57 |
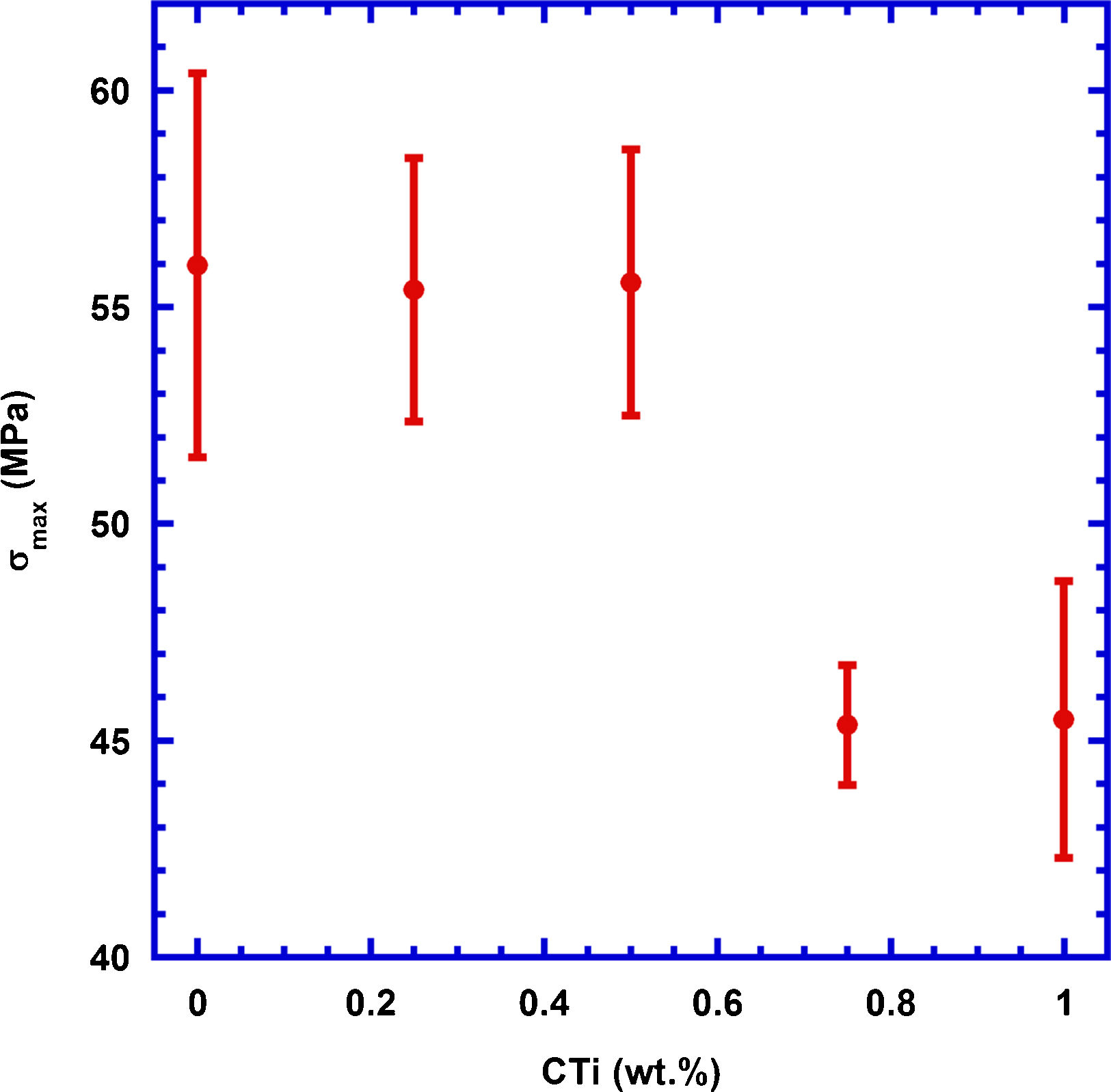
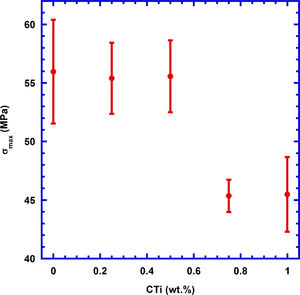
These results are reflected on the mechanical properties of these samples, determined through three points bending tests, and the results are displayed in Fig. 5, together with the corresponding measurement errors. As it can be deduced from the graph, the TiC addition in small amounts (≤0.5wt.%) does not significantly modify mechanical properties, when compared with pure Ca3Co4O9 samples, while higher content decreases them in a relatively important manner due to the agglomeration of TiC particles between the thermoelectric grains (see Fig. 4c). The fact that the mechanical properties are maintained for low TiC additions can be explained by the regular distribution of TiC grains between the thermoelectric ones, as observed in the SEM micrographs, which is counteracted by the thermoelectric grains growth. In the case of high TiC content, the decrease is associated to the agglomeration of TiC particles already observed and mentioned in the SEM discussion, which leads to the formation of weak zones in the samples. On the other hand, the large amount of porosity in these sintered samples lead to much lower mechanical properties than the reported in nearly fully dense materials (287MPa) [49].
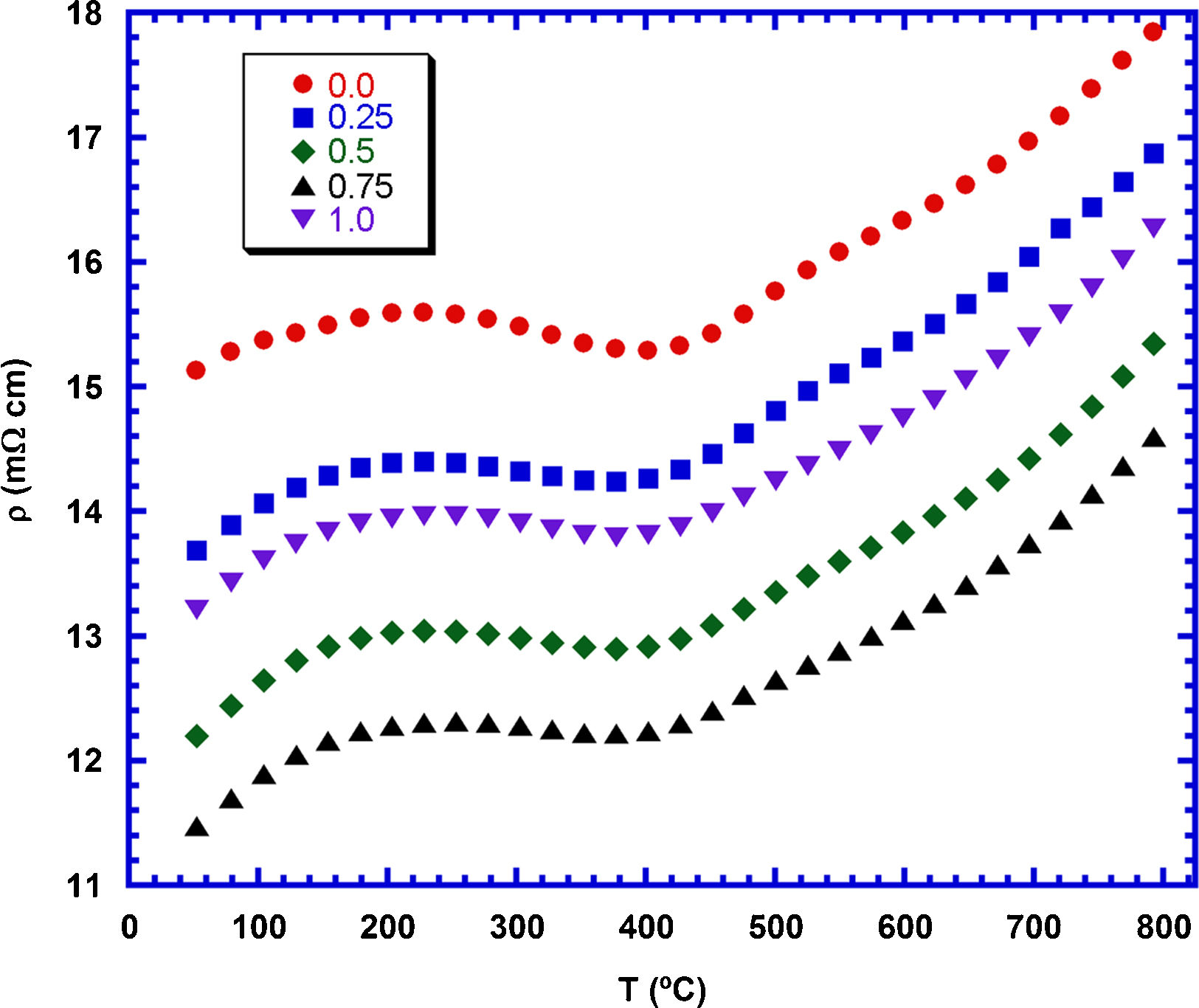
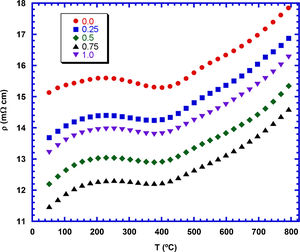
The electrical resistivity evolution with temperature and the TiC content is shown in Fig. 6. As it can be observed in the graph, all samples display the same behaviour in the whole measured temperature range. On the other hand, TiC addition promotes a decrease of electrical resistivity, when compared with the samples without additions. It can be associated to the slight increase of grain sizes which leads to the opposite effect produced by TiC nanoparticles, which can scatter charge carriers. Moreover, the low electrical resistivity values of TiC material [33] can help to reduce electrical resistivity in the grain boundaries, as it has been reported for the addition of other conducting materials [50,51]. Moreover, electrical resistivity decreases when the TiC content is increased up to 0.75wt.%, increasing for further addition. This increase for the highest TiC containing samples can be explained by the agglomeration of TiC nanoparticles, which can limit the charge carrier mobility due to the very large amount of grain boundaries in these regions. The lowest measured resistivity values at 800°C (14.5mΩcm for the 0.75wt.% TiC samples) are around the best values obtained for Ca3Co4O9 samples sintered and textured by spark plasma sintering (15–18mΩcm) [52] and about 15% lower than the pure Ca3Co4O9 samples prepared in this work. On the other hand, they are higher than the reported for fully dense materials prepared through alternative methods (10mΩcm) [53].
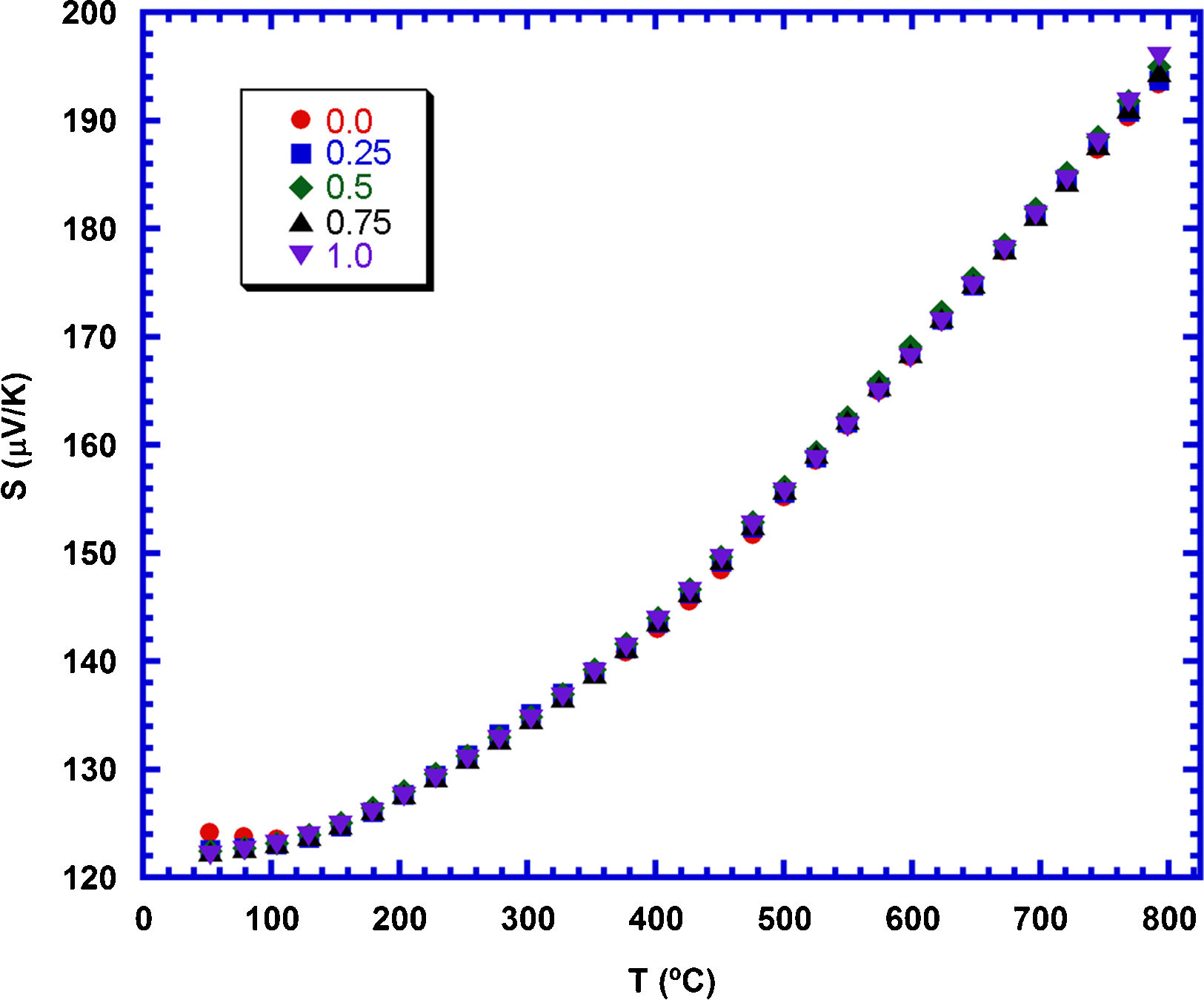
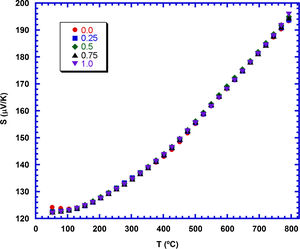
Fig. 7 shows the variation of S with temperature, as a function of the TiC nanoparticles addition. The graph clearly shows that S is positive at all temperatures, confirming a conduction mechanism mainly produced by holes. The values of the Seebeck coefficient increase with temperature, and display the same vales for all the samples. This behaviour can be explained when considering the very small contribution of TiC to the S values of these samples [33]. Consequently, the total S values, which have been measured in these samples are only due to the Ca3Co4O9 contribution, and it is the same for all of them. The highest S value has been determined at 800°C (around 195μV/K) is higher than the best values reported for pure Ca3Co4O9 samples sintered and textured by spark plasma sintering (170–175μV/K) [52]. On the other hand, it is lower than the measured in highly dense materials prepared through alternative methods (205μV/K) [53].
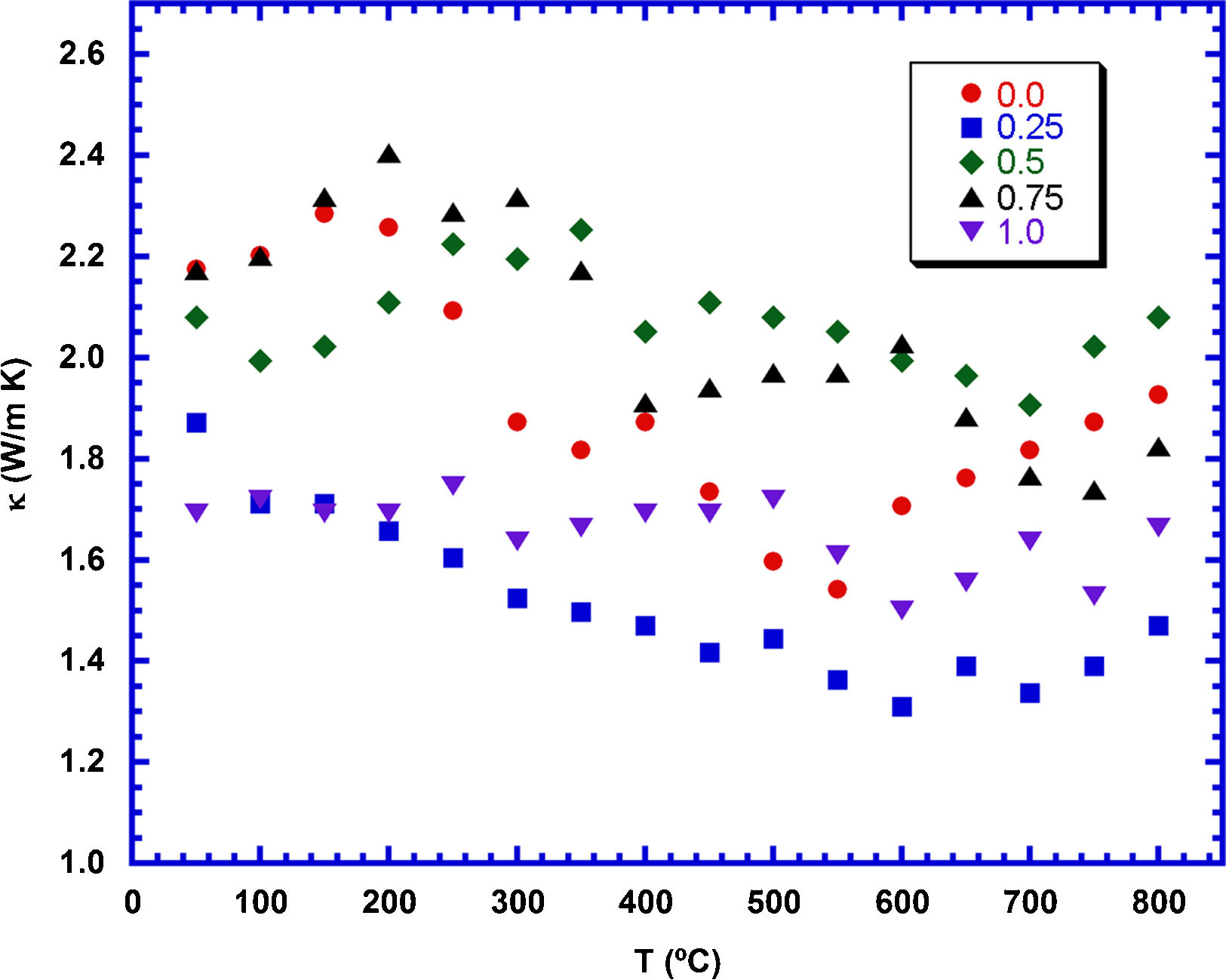
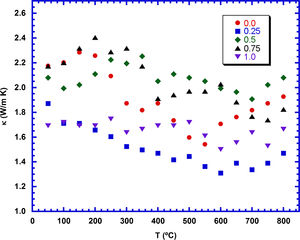
The temperature dependence of the thermal conductivity, as a function of TiC content, is plotted in Fig. 8. As it can be observed in the graph, in spite of the irregular variation with temperature, thermal conductivity shows a tendency to decrease when the temperature is raised. On the other hand, the samples do not show a regular evolution with the TiC content, and only the 0.25wt.% TiC added sample displays a clear lower value than the other samples, in the whole measured temperature range. The low values obtained in these samples are consistent with their high amount of porosity. The minimum value measured in these samples at 800°C (1.45W/Km) is much lower than the reported in hot-pressed samples (2.2, 3, and 4.7W/Km) [54–56], measured along the ab-plane, or sintered through Spark Plasma Sintering (SPS, 2.1W/Km) [57]. On the other hand, it is higher than the measured in classically sintered materials (0.9W/Km) [55] with much lower density (60% of the theoretical) than the samples presented in this work (>70%).
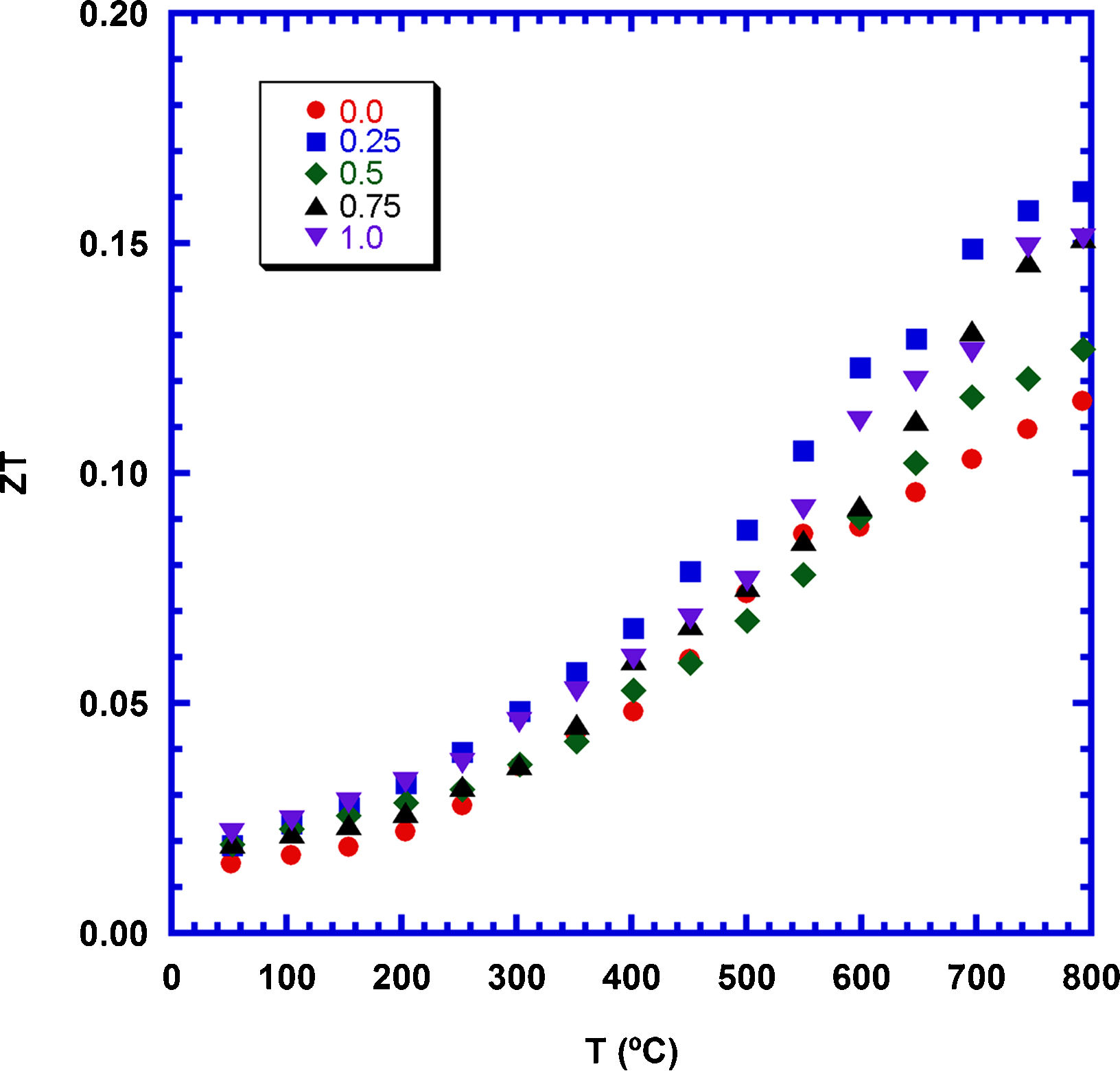
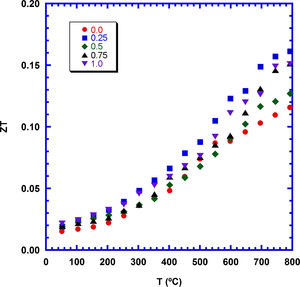
The samples performances have been calculated through ZT, and presented in Fig. 9 as a function of temperature and TiC content. As it can be observed in the plot, at 800°C the highest increase is obtained for the 0.25wt.%TiC (0.16), which is ∼40% higher than the determined in pure ones. Moreover, it is in the order of the reported for textured samples (0.18, and 0.16 [54,55]), or sintered through SPS (0.15 [57]). On the other hand, it is lower than the measured in Sr-doped textured materials (0.29 [56]), but much higher than the reported for classically sintered materials (0.08 [55]). It is important to highlight that the results presented in this work have been obtained through a very simple and scalable method without the need of long processes or expensive equipment.
Finally, thermal expansion coefficient has been determined as a very important parameter when considering these materials for integration in power generation modules. The mean values determined in all samples, as a function of TiC content, are displayed in Table 2. As it can be deduced from these data, linear thermal expansion coefficient for the pure sample is 10.3410−6K−1, and it is decreased when TiC is added. Moreover, it is further decreased when the TiC content is increased, reaching the minimum values (9.5710−6K−1) for the highest TiC addition (about 10% decrease, when compared to the pure Ca3Co4O9 one), and lower than the measured in pure and Bi-doped Ca3Co4O9 (between 10.610−6K−1 and 12.810−6K−1) [46]. Unfortunately, in the best of our knowledge there are not many data available for this compound, but they are in the order of the typically reported for the pure and doped Bi2AE2CoxOy compounds (AE=Ca, Sr, and Ba, and x=1.7, 1.8, and 2, respectively) [58].
All these data clearly point out that the addition of foreign compounds to the Ca3Co4O9 are useful, not only to improve thermoelectric performances without drastically modifying mechanical ones, but also to tune the thermal expansion coefficient to make it fitting with the one of the different components used to build the thermoelectric modules.
ConclusionsThis work has shown that Ca3Co4O9+x wt.% TiC (x=0, 0.25, 0.5, 0.75, and 1.0) polycrystalline ceramics can be successfully prepared using the classical solid state method. XRD data showed that single Ca3Co4O9 phase has been obtained in all samples, while TiC has not been detected due to its low amount. Microstructural observations have confirmed that both phases have not reacted and TiC does not influence sintering process, in agreement with density characterization. On the other hand, electrical resistivity is decreased with TiC addition, reaching the lowest values for the 0.75wt.% addition, while Seebeck has not been modified in all samples. Thermal conductivity tends to decrease when the temperature is raised in all cases, and the lowest values have been achieved in the 0.25wt.% TiC samples, which led to an increase of about 40% in ZT values at 800°C in these samples, when compared with the pure ones. Finally, linear thermal expansion coefficient decreases when TiC content increases. These last data are of the main importance, when considering these materials to build a power generation thermoelectric module, to match the thermal expansion coefficients of all components.
The authors wish to thank the Gobierno de Aragón-FEDER (Research Group T 54-17R), and the Spanish MINECO-FEDER (Project MAT2017-82183-C3-1-R) for financial support. Authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación-SAI, Universidad de Zaragoza.