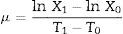Presence of the relatively new sulfonylurea herbicide monosulfuron-ester at 0.03–300nmol/L affected the growth of two non-target nitrogen-fixing cyanobacteria (Anabaena flos-aquae and Anabaena azotica) and substantially inhibited in vitro Acetolactate synthase activity, with IC50 of 3.3 and 101.3nmol/L for A. flos-aquae and A. azotica, respectively. Presenting in 30–300nmol/L, it inhibited protein synthesis of the cyanobacteria with less amino acids produced as its concentration increased. Our findings support the view that monosulfuron-ester toxicity in both nitrogen-fixing cyanobacteria is due to its interference with protein metabolism via inhibition of branch-chain amino acid biosynthesis, and particularly Acetolactate synthase activity.
Monosulfuron-ester is a relatively new sulfonylurea herbicide that was developed by the National Pesticide Engineering Research Center in Tianjin, China.1 This herbicide is very effective at post-emergence rates of 30–60nmol/L (active ingredients) in a wide range of crops including corn (Zea mays L.), wheat (Triticum aestivum L.), rice (Oryza sativa L.), and millet (Panicum miliaceum L.).2 The mode of action of sulfonylurea is inhibition of acetolactate synthase (ALS).3 The ALS is present in all plants as well as in bacteria, fungi, and cyanobacteria,4 but not present in mammals. Because of their relatively low toxicity to mammals, high efficacy and environmental safety,5 the use of sulfonylurea herbicides has increased rapidly. However, application of selective herbicides such as sulfonylurea in cropping systems can substantially reduce soil microbial diversity if they are overused or misused.
Cyanobacteria are one of the largest and most important groups of algae on earth. In particular, nitrogen-fixing cyanobacteria are vital photosynthetic microorganisms that contribute to soil fertility by fixing atmospheric nitrogen, releasing small quantities of the major fertilizing product, ammonia, as well as other small nitrogenous polypeptides during active growth,6 and also because they maintain ecosystem stability.7Anabaena azotica Ley and Anabaena flos-aquae (Lyngb) Breb are two of the most common filamentous nitrogen-fixing cyanobacteria in China agricultural fields.8 In general, cyanobacteria are very sensitive to herbicides because they possess many characteristics of higher plants.9 Previous study showed that cyanobacteria had different degrees of sensitivity to herbicides.10 However, the effect of monosulfuron-ester on nitrogen-fixing cyanobacteria that may play a role in enhancing the fertility of agricultural fields has not been reported in the literature. Therefore, the objectives of this research were to determine the effects of a range of monosulfuron-ester concentrations on growth, protein content, in vitro and extractable activity of ALS, and amino acid production of both nitrogen-fixing cyanobacteria. Findings from this work should provide a better understanding of the toxicity and its mode of action of monosulfuron-ester in the two ubiquitous nitrogen-fixing cyanobacteria.
Materials and methodsMonosulfuron-ester of 99.4% purity was synthesized at the National Pesticide Engineering Research Center in Tianjin, China. Monosulfuron-ester was dissolved in the mixture of organic solvent dimethylformamide (DMF, N,N-dimethylformamide, Jianshan Chemicals Ltd., China) and surfactant TritonX-100 (4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol) (Jibishi Gene Technology Ltd., China) to form the herbicide concentrate below the concentration of first effect, i.e., DMF 0.5% (v/v) and TritonX-100 0.005% (v/v). The fresh concentrate was filtered and sterilized and then added to the culture medium to prepare the test solutions of the desired monosulfuron-ester doses.
To measure monosulfuron-ester concentrations in the medium and in algal cells a HPLC analysis procedure was developed employing a Agilent 1100 HPLC (Agilent Inc.) equipped with UV-200 detector, ODS Diamonsi (5μm) column with 250 ×4.6mm. Operating conditions for the HPLC analysis were: mobile phase-methanol/water (containing H3PO4, pH4.0) (53/47, v/v); mobile phase flow rate 1.0mL/min; sample injection volume 10μL; wavelength 236nm; and column temperature 40°C. Under these conditions the resulting calibration curve correlating the monosulfuron-ester concentration to the apex area was y=91302x+26735 (R2=0.9953) with an average recovery of 95–103%.
Cultures of A. azotica (FACHB-181) and A. flos-aquae (FACHB-245) two nitrogen-fixing algal species of the Nostocaceae, were obtained from the Institute of Hydrobiology of the Chinese Academy of Sciences in Wuhan, China. Axenic cultures were grown in a sterilized HB-111 liquid medium (0.075g/LK2HPO4, 0.125g/L MgSO4, 0.1g/L CaCO3, 0.005g/L FeC6H5O7, 0.005g/LC6H8O7, 0.0025g/L MoO3·H2O, 0.01g/L NaOH) at 30±2°C under constant fluorescent light of 36.2μmol/m2/s. The experimental cultures were first grown in 250mL flasks containing 100mL of the medium with 0.5–1 million cells per milliliters under the same conditions. At the exponential growth phase of the algal cultures, small aliquots of the concentrate were added to the culture medium flasks to result in 0.003, 0.03, 0.3, 3, 30, and 300nmol/L of monosulfuron-ester in the test samples. Sterilized water was added instead to the same culture media to produce the control samples. Samples were collected 6d after the herbicide addition to determine the protein and amino acid contents, and the ALS activity.
Growth rates of cyanobacteria were measured by recording light absorbance of the culture at 448nm using a spectrophotometer (Shanghai Biotechnic Ltd.). Standard curves relating Abs448nm with cell numbers were developed for continuous cultures of A. azotica and A. flos-aquae. During the experimental period, samples were withdrawn after the herbicide treatment at 1, 2, 3, 4, 5, and 6 days for growth rate measurements. The growth rate (μ) of the cyanobacteria was calculated using the following Eq. (1).
where X1 and X0 are Abs448nm measured at times T1 and T0. To estimate the cell's dry weight, the cultures were centrifuged, and the solid mass was washed with distilled water three times, dried at 105°C for 8h; the cell's dry weight was the average of three weight measurements.The protein content of the cultures was determined by using bovine serum albumin (BSA) as a standard described by Bradford (1976).11 One-hundred nanomoles of Coomassie Brilliant Blue G-250 was first dissolved in 50mL of 95% ethanol and then 100mL of 85% (w/v) phosphoric acid was added to this solution. The resulting solution was diluted to a final volume of 1L. BSA was diluted in five concentrations (0.2, 0.4, 0.6, 0.8, 1mg/L) and their absorbance at the 595nm wavelength were measured after 2min at 20°C. 5mL liquid sample was taken and centrifuged (8000rpm) 10minutes at 4°C to collect algal cells. Add a small amount of phosphate buffer (pH 7.8), repeated freezing and thawing until the algae fluid became blue, then centrifuged the sample at 8000rmp for 30min at 4°C and the resulting supernatant was used to determine protein content. 0.1mL supernatant was taken to determine the protein content according the regression equation of the straight line of protein relating Abs595nm.
The amino acid components were analyzed using an amino acid automatic analyzer (Hitachi) with an ion exchange column after the cyanobacterial sample was hydrolyzed in 6mol/L HCl at 110±1°C for 24h. The absorbance of the sample was measured at 570nm except 440nm for proline after color development of the ninhydrin reaction at 100°C.
ALS was extracted from 6d cultivated nitrogen-fixing cyanobacteria of the two test species. The cyanobacteria were centrifuged at 10,000×g for 30min at 4°C (Sorvall Centrifuge, DJB Labcare Ltd.) in buffer or medium after cells were sonicated using an ultrasonic cell disruptor (250mv, 3s, 3s) in the dark at 4°C. The protein in the supernatant was precipitated with ammonium sulphate, and then centrifuged at 10,000×g for 30min at 4°Cafter 2h incubation at 0°C. The pellet was dissolved in phosphate buffer (pH=7.5) containing 200mmol/L sodium pyruvate, 5mmol/L MgCl2, 10mmol/L thiamine pyrophosphate (TPP Sigma Company), 200μmol/L flavin adenine dinucleotide (FAD Sigma Company), and 1mol/L dl-dithiothreitol (DTT GL Biochem Ltd.). This enzyme solution was kept in ice/water in the dark. The enzyme solution was dissolved in 50mmol/L phosphate buffer (pH=7.5) containing 20mmol/L sodium pyruvate and 0.5mmol/L MgCl2. A volume of 0.1mL of monosulfuron-ester was added to 0.4mL of enzyme and 0.5mL of enzyme reaction solution. The mixture was subsequently incubated at 37±1°C in a water bath for 1h in the dark. After adding 0.1mL of 3mmol/L sulfuric acid, the mixture was incubated at 60±1°C for 15min to decarboxylate the acetolactate to acetoin. 0.5mL of freshly prepared 5.0% α-naphthol in 2.5mol/L sodium hydroxide and 0.5mL of 0.5% creatine were then added to the mixture and incubated at 60±1°C for 15min for color development. The mixture was then cooled to the room temperature (25°C) in a water bath and absorbance was measured at 525nm using a spectrophotometer. The specific activity of ALS was expressed as nmol of acetoin/nmol of protein/h.12
To determine the extractable ALS activity, 0.1mL of monosulfuron-ester and 0.4mL of the enzyme were substituted with 0.5mL of the enzyme solution extracted from nitrogen-fixing cyanobacteria that had been cultivated in the culture medium containing monosulfuron-ester. Six concentrations of monosulfuron-ester were used as outlined previously and the specific activity of ALS was determined and expressed as described above.
A completely randomized design with three replications was used in all duplicated experiments. Analyses of variance (ANOVA) were performed on the non-transformed data. Significant differences were determined using Duncan's test at p=0.05 level of significance (PROC GLM, SAS Institute, 2001). The values of IC50 were calculated with the program OriginPro7.5SR1 (OriginLab Corporation, USA).
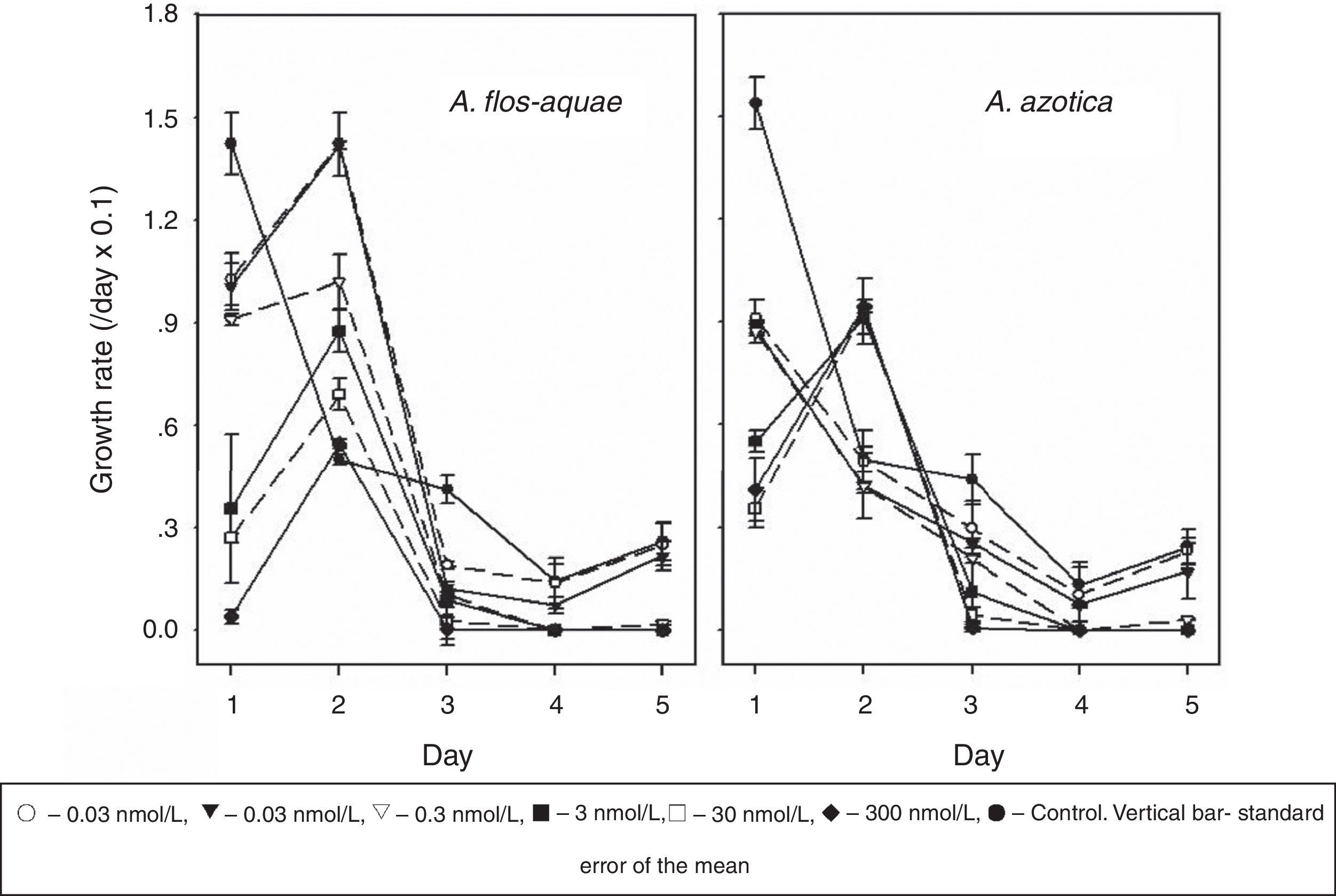
ResultsIn general, the growth rates of A. flos-aquae and A. azotica of the control decreased gradually as the culturing time increased; however, the growth rates of both cyanobacteria treated with monosulfuron-ester (0.03–300nmol/L) exhibited a wave pattern of initial decline followed by gradual increases (Fig. 1).
The growth rates of A. flos-aquae and A. azotica were reduced by 28–97% and 41–73% at 0.03–300nmol/L, after 1 day, respectively, relative to the control; the monosulfuron-ester treatment then transient stimulated the growth on second day. For A. flos-aquae, the growth rates at 0.03, 0.3, 3, 30, and 300nmol/L increased by 185, 184, 104, 75, 38, and 9%, respectively relative to the control treatment and the growth rate increase declined with higher monosulfuron-ester concentration; however, for A. azotica, the growth rates increased by 83–90% at 3–300nmol/L. It can be seen that both cyanobacteria had the different responses to monosulfuron-ester. A. flos-aquae was more sensitivity to this herbicide than A. azotica.
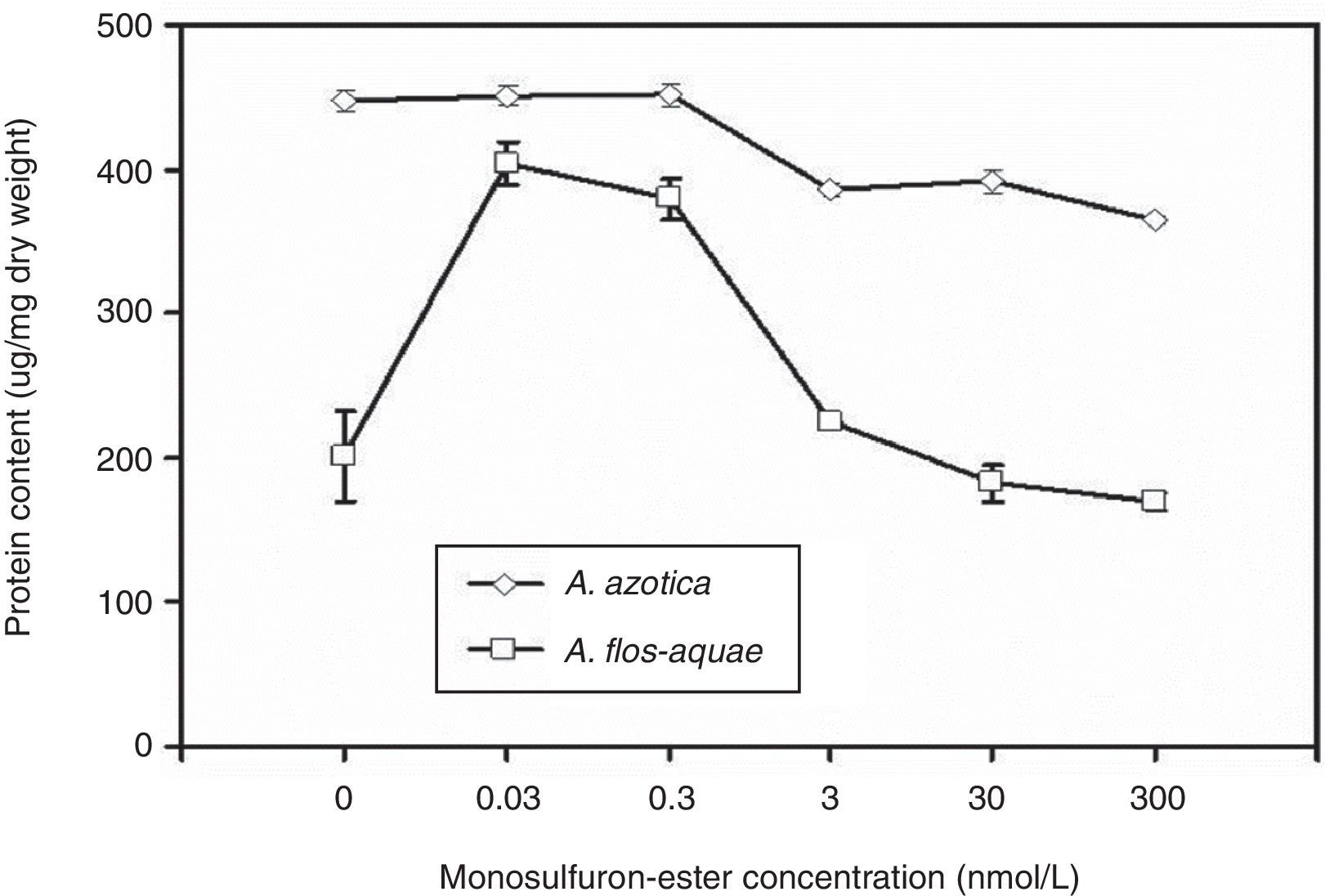
Fig. 2 illustrates the effect of monosulfuron-ester concentration on the protein content in the two cyanobacteria. Monosulfuron-ester applied at low concentrations of 0.03–0.3nmol/L stimulated the production of proteins in both cyanobacteria, but the reverse was observed at higher concentrations (i.e. 30–300nmol/L). The protein content of A. flos-aquae cell cultures treated with monosulfuron-ester at 0.03 and 0.3nmol/L increased 101 and 89%, respectively relative to the control (p<0.05); its protein content decreased sharply as monosulfuron-ester concentration increased, for example, the protein content was 9–16% lower with 30–300nmol/L monosulfuron-ester. The monosulfuron-ester treatment did not markedly have effect on protein content in A. azotica, its protein content increased by only 0.8% with monosulfuron-ester 0.03nmol/L. It is thus clear that A. flos-aquae exhibited greater sensitivity to monosulfuron-ester.
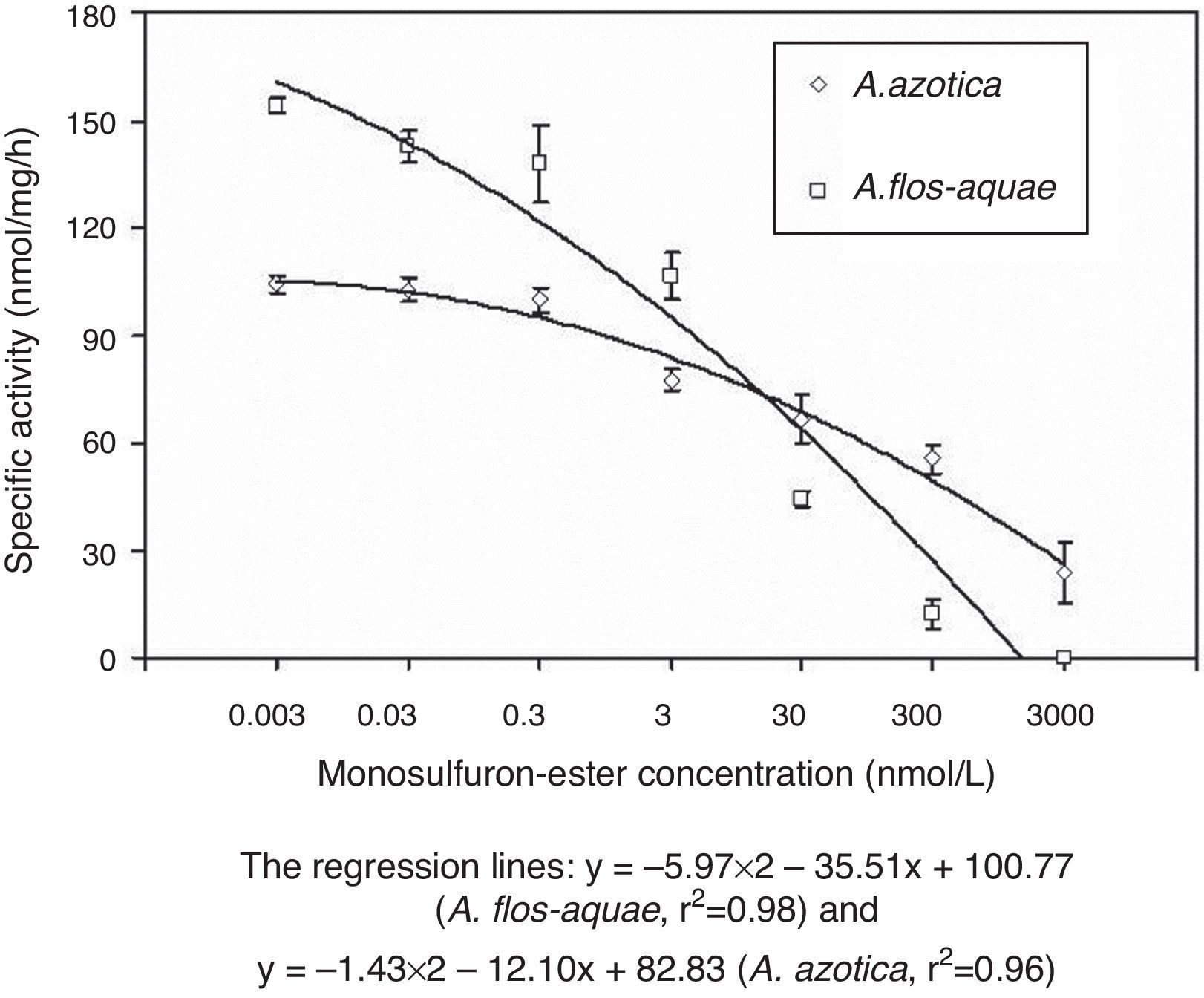
The in vitro ALS activity varied between the two cyanobacterial species following monosulfuron-ester application (Fig. 3). For A. flos-aquae, the activity of ALS was reduced in the range of 0.8–92% as the concentration of monosulfuron-ester increased from 0.003 to 300nmol/L, and there was a significant difference in activity between each concentration (p<0.05). The ALS activity of A. azotica at low monosulfuron-ester concentrations (0.003–0.3nmol/L) did not differ significantly from the same of the control; it declined notably, by 19–75% relative to the control, as the concentration of monosulfuron-ester increased from 3 to 300nmol/L. Calculated IC50 values for A. flos-aquae and A. azotica were 3.3 and 101.3nmol/L respectively. These results indicate that monosulfuron-ester does inhibit ALS activity in the two nitrogen-fixing cyanobacteria, particularly at higher concentrations.
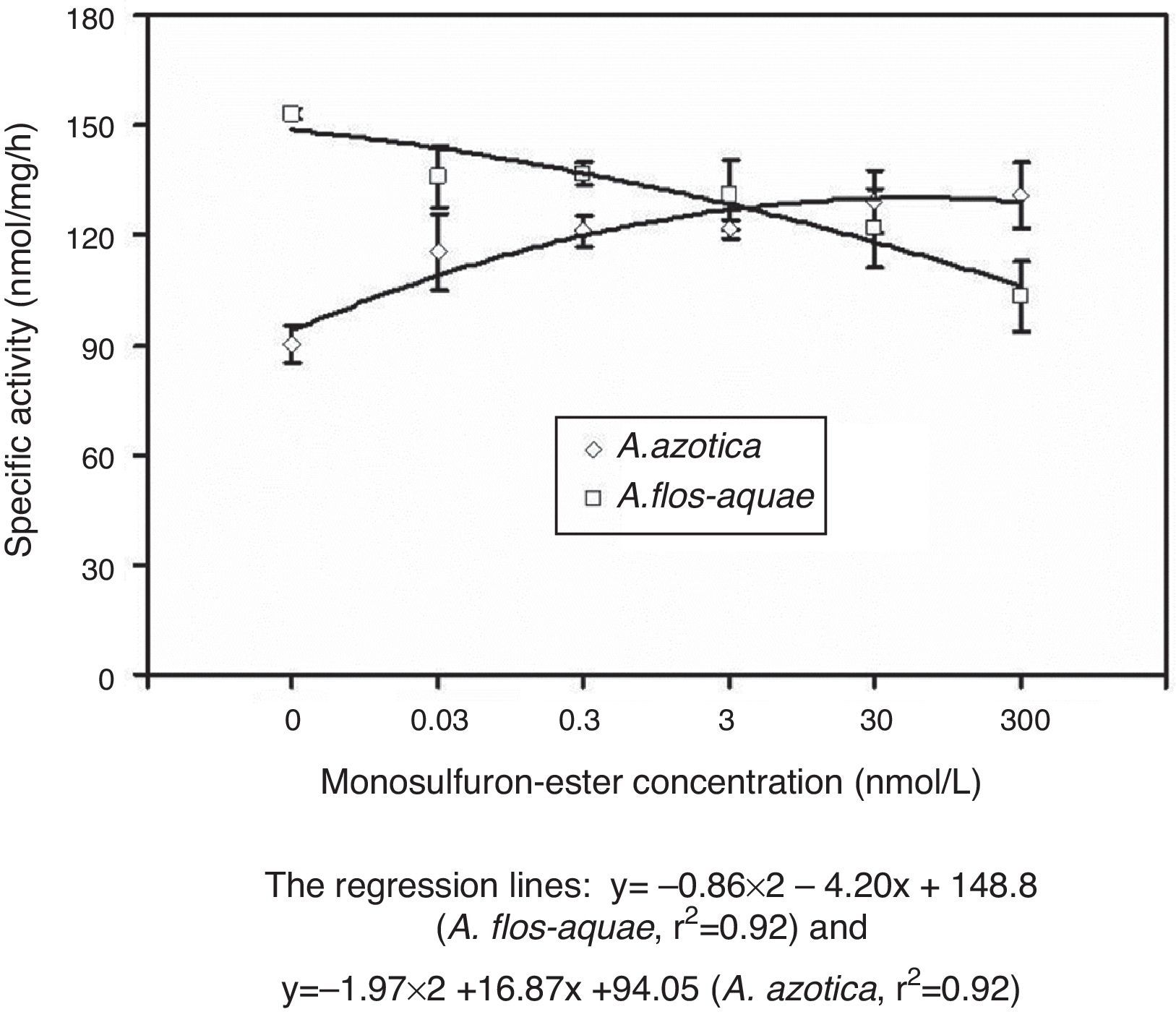
Interestingly, the extractable ALS activity in the two cyanobacteria responded differently to monosulfuron-ester in 0.03–300nmol/L (Fig. 4); relative to the control, the extractable ALS activity in A. azotica increased 28–45% while the same in A. flos-aquae declined by 11–33%. There was a further evidence that A. flos-aquae were more sensitive to this herbicide than A. azotica.
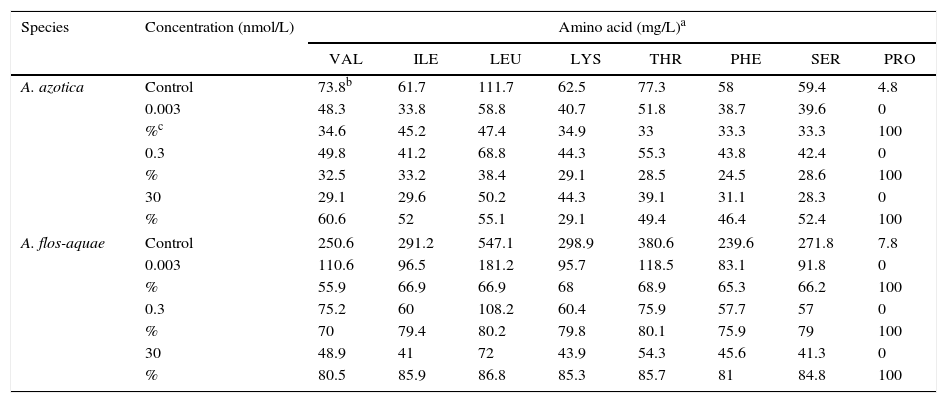
Table 1 presents the effect of monosulfuron-ester concentration on amino acids produced in the two cyanobacteria. Amino acid production in cells of A. flos-aquae and A. azotica was clearly inhibited by monosulfuron-ester in the characteristic dose-dependent response; namely, increased inhibition of amino acid production with increasing monosulfuron-ester concentration. The quantity of branch-chain amino acids valine, isoleucine, leucine in cells of A. flos-aquae was reduced by 56, 67, and 67% at the 0.003nmol/L, respectively relative to the control, and by 80, 86, and 87% at the 30nmol/L (p<0.05). The valine, isoleucine, leucine content of A. azotica cells was reduced by 35, 45, and 47% at the 0.003nmol/L concentration, and by 61, 52, and 55% at the 30nmol/L concentration. The other amino acids content of A. azotica and A. flos-aquae cells such as glycine, alanine, phenylalanine, serine, threonine, methionine, glutarnine, asparticacid, lysine, and arginine were respectively reduced 8–55% and 67–90% at monosulfuron-ester concentrations 0.003 and 30nmol/L, and proline, tyrosine, and histidine were below detection at 30nmol/L. Again A. flos-aquae was the more sensitive of the two.
Effect of monosulfuron-ester concentration on amino acids produced.
| Species | Concentration (nmol/L) | Amino acid (mg/L)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VAL | ILE | LEU | LYS | THR | PHE | SER | PRO | ||
| A. azotica | Control | 73.8b | 61.7 | 111.7 | 62.5 | 77.3 | 58 | 59.4 | 4.8 |
| 0.003 | 48.3 | 33.8 | 58.8 | 40.7 | 51.8 | 38.7 | 39.6 | 0 | |
| %c | 34.6 | 45.2 | 47.4 | 34.9 | 33 | 33.3 | 33.3 | 100 | |
| 0.3 | 49.8 | 41.2 | 68.8 | 44.3 | 55.3 | 43.8 | 42.4 | 0 | |
| % | 32.5 | 33.2 | 38.4 | 29.1 | 28.5 | 24.5 | 28.6 | 100 | |
| 30 | 29.1 | 29.6 | 50.2 | 44.3 | 39.1 | 31.1 | 28.3 | 0 | |
| % | 60.6 | 52 | 55.1 | 29.1 | 49.4 | 46.4 | 52.4 | 100 | |
| A. flos-aquae | Control | 250.6 | 291.2 | 547.1 | 298.9 | 380.6 | 239.6 | 271.8 | 7.8 |
| 0.003 | 110.6 | 96.5 | 181.2 | 95.7 | 118.5 | 83.1 | 91.8 | 0 | |
| % | 55.9 | 66.9 | 66.9 | 68 | 68.9 | 65.3 | 66.2 | 100 | |
| 0.3 | 75.2 | 60 | 108.2 | 60.4 | 75.9 | 57.7 | 57 | 0 | |
| % | 70 | 79.4 | 80.2 | 79.8 | 80.1 | 75.9 | 79 | 100 | |
| 30 | 48.9 | 41 | 72 | 43.9 | 54.3 | 45.6 | 41.3 | 0 | |
| % | 80.5 | 85.9 | 86.8 | 85.3 | 85.7 | 81 | 84.8 | 100 | |
| Species | Concentration (nmol/L) | Amino acid (mg/L)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HIS | TYR | ARG | ALA | MET | ASP | GLU | GLY | ||
| A. azotica | Control | 12.7 | 13.5 | 81.5 | 132.4 | 31.3 | 138.6 | 147.4 | 68.8 |
| 0.003 | 0 | 7.4 | 75.3 | 98.5 | 24 | 122.1 | 110.7 | 51.9 | |
| %c | 100 | 45.2 | 7.6 | 25.6 | 23.3 | 11.9 | 24.9 | 24.6 | |
| 0.3 | 0 | 0 | 70.7 | 94.7 | 18.9 | 120.8 | 104.5 | 52.1 | |
| % | 100 | 100 | 13.3 | 28.5 | 39.6 | 12.8 | 29.1 | 24.3 | |
| 30 | 0 | 0 | 36.8 | 60.9 | 7 | 76.2 | 68.9 | 37 | |
| % | 100 | 100 | 54.8 | 54 | 77.6 | 45 | 53.3 | 46.2 | |
| A. flos-aquae | Control | 87.6 | 173 | 492.1 | 608.3 | 147.9 | 676.1 | 727 | 329.7 |
| 0.003 | 21.8 | 46.6 | 151.1 | 201.9 | 47.7 | 215.6 | 226.7 | 107.8 | |
| % | 75.1 | 73.1 | 69.3 | 66.8 | 67.7 | 68.1 | 68.8 | 67.3 | |
| 0.3 | 11.9 | 13.8 | 85 | 131.6 | 29.5 | 139.8 | 145.9 | 69.2 | |
| % | 86.4 | 92 | 82.7 | 78.4 | 80.1 | 79.3 | 79.9 | 79 | |
| 30 | 0 | 0 | 58 | 91 | 14.9 | 104.2 | 106.2 | 50.7 | |
| % | 100 | 100 | 88.2 | 85 | 89.9 | 84.6 | 85.4 | 84.6 | |
The transient stimulative effect of monosulfuron-ester on algal growth on the second day of this study is similar to the findings of Shen et al. (2005) for butachlor and acetochlor on several Anabaena species.7 Our result that the corresponding growth rates of the A. flos-aquae and A. azotica exposed to different concentrations of monosulfuron-ester exhibited wave patterns was similar to the report of Shen et al. (2011).13 Hormesis, the stimulative effect of a toxin at sub-toxic concentrations, following the application of other herbicides and allelochemicals has been documented.14
The study has evaluated the effects of herbicides on metabolic pathways of protein synthesis in non-target organisms.15 Our findings at low monosulfuron-ester concentrations (0.03–3nmol/L) indicate that the protein content of cells from the two cyanobacteria increased substantially. The increase may have resulted from stimulation in protein synthesis via increased production of RNA.16 Alternatively, at lower monosulfuron-ester concentrations, cyanobacterial cells may have assimilated more organic carbon and nitrogen, which interfered with herbicide uptake, due to the formation of an inactive herbicide complex with organic carbon and nitrogen, and thus reduced the herbicide’ toxicity; the bacteria may also degrade the herbicide into hydrate of carbon and amino acids metabolites resulting in a lower toxicity.17 The lower protein contents of both cyanobacteria subjected to monosulfuron-ester at high concentrations (30–300nmol/L) may be related to increase of protease activity18; it may also be the result of inhibition of the ALS activity which interfered with production of the three branched-chain amino acids (valine, leucine, and isoleucine) essential precursors of proteins.12
The mode of action of the sulfonylurea is inhibition of ALS, which is responsible for catalyzing the biosynthesis of the branch-chain amino acids valine, leucine, and isoleucine.3 Shen et al. (2009) reported that monosulfuron inhibits vitro ALS activity in three nitrogen-fixing cyanobacteria at higher concentrations.19 Our study is similar to her results that the ALS activity was more reduced as the concentration monosulfuron-ester increased; IC50 values of 3.3 and 101.3nmol/L for A. flos-aquae and A. azotica, respectively, have provided further evidences of the negative impact of this herbicide on growth of these cyanobacterial species. The strong inhibition of ALS activity found in both species in vitro confirms that the toxic effects of monosulfuron-ester is indeed of inhibition to ALS as is the case with other sulfonylurea herbicides.20 Shen et al. (2009) also pointed out that monosulfuron stimulated the extractable ALS activity in A. azotica, but the specific activity in A. flos-aquae decreased at high concentration of monosulfuron.19 In our study, with monosulfuron-ester present at 0.03–300nmol/L, the extractable ALS activity in A. azotica increased by 28–45%, while the specific activity of A. flos-aquae decreased by 3–11%. It is possible that differences in specific activity between in vitro and in vivo responses of the two cyanobacteria were due to the presences of different levels of herbicide detoxifying enzymes (e.g. glutathione S-transferase (GST) and mixed-function oxidase (MFO)).21 Other possible reasons for the markedly different effects between the cyanobacterial species on the extractable ALS specific activity at the same monosulfuron-ester concentration include absorption of the herbicide and the different degrees of ALS inhibition between the species.
That A. flos-aquae exhibited greater sensitivity to monosulfuron-ester than A. azotica may be related to its greater capacity for taking up the herbicide. Such different responses may be due to different biological properties of the cyanobacteria22 and may be exploited to select for cyanobacteria that are more resistant to monosulfuron-ester in the cropping systems. For instance, the culturing of beneficial nitrogen-fixing cyanobacteria in paddy fields as ‘bio-fertilizers’ is a highly desirable option for increasing rice yield and preventing any negative effects of this cropping system on the environment.10
ConclusionApplication of monosulfuron-ester at 0.03–300nmol/L affected the growth rates of A. flos-aquae and A. azotica in the wave patterns, and had an inhibitory effect on protein synthesis at higher concentrations (30–300nmol/L). The production of amino acids was reduced with increasing monosulfuron-ester concentration. Monosulfuron-ester substantially inhibited in vitro Acetolactate synthase (ALS) activity as evidenced by the IC50 values of 3.3 and 101.3nmol/L for A. flos-aquae and A. azotica, respectively. The results show that the normal agricultural use of monosulfuron-ester at post-emergence rates of 30–60nmol/L in rice fields will likely be toxic to the both ubiquitous nitrogen-fixing cyanobacteria; the monosulfuron-ester toxicity was due to its interference with protein metabolism via inhibition of branch-chain amino acid biosynthesis, especially the ALS activity. We also suggest that in rice cropping systems where the new sulfonylurea herbicide monosulfuron-ester is frequently applied, the beneficial nitrogen-fixing cyanobacteria, A. azotica, may be employed as “biofertilizers” since it is more resistant to the herbicide than A. flos-aquae.
Conflicts of interestThe undersigned author(s) state(s) that everyone involved in the publication process (authors, reviewers, editorial board members, and editorial staff) must be free from conflicts of interest that could adversely influence their judgment, objectivity or loyalty to the article and assignments.
Funding for this work was provided by the National Natural Science Foundation of China (21377086).