Apple is one of the most important temperate fruit to Brazil economy, and the use of synthetic chemicals has been the main method for reducing postharvest diseases, such as the blue mold, caused by Penicillium expansum. This work intends to evaluate the practical utilization of chitosan for blue mold control. For this purpose, fruits were treated in a preventive and curative way, immersing the fruits in chitosan solution (5 or 10mgmL−1), or adding a single drop of this solution (10mgmL−1) directly into the injuries. The eradicative effect of the polysaccharide was also evaluated in vitro and in vivo. Chitosan did not show a curative effect against the blue mold, and its eradicative effect was only evidenced on the higher concentration (10mgmL−1). On the other hand, preventively, without the addition of adjuvants, chitosan reduced blue mold incidence in fruits by 24% and 93%, through the immersion or the single drop methods, respectively. Thus, it was found that, for long scale utilization, some improvements in the physico-chemical properties of the chitosan are needed, since it was only capable to prevent the infection by P. expansum when directly added on the fruit injury.
Apple (Malus domestica Borkh) is one of the most important temperate fruit for the Brazilian economy, particularly to the southern states. The fruit production of the country in 2013–2014 was 1,377,393tons, in which 633,197tons were obtained only in Santa Catarina, representing 46% of the total production.1
Among the diseases occurring in apple culture, blue mold caused by Penicillium expansum is the main disease responsible for losses of the fruit in different producing areas.2,3 Prior to the use of cold chambers with controlled atmosphere technology, this fungus was responsible for up to 90% of the postharvest loss in apple.3 Even today, it may cause several damages to fruit particularly during prolonged storage period.4
The contamination of fruit occurs primarily in the field and the onset of symptoms usually occurs during the storage,5 when every symptomatic fruit can potentially infect 12–15 healthy fruit.6,7 Despite the aggressiveness of P. expansum, the fungus is unable to penetrate the fruit through the cuticle, and thus, an injury is needed in order to cause the infection.7
In order to reduce the postharvest losses, the disinfection of fruit is generally carried out using sodium hypochlorite solution or by chemical control methods,2 by using maturation inhibitors and systemic and protective fungicides.8,9 However, the use of fungicides, besides making the production more expensive, may cause the selection of the isolates resistant to the active ingredients. In addition, because of the public concern over synthetic chemical residues on foods, it becomes necessary to seek alternative measures for the control of phytopathogens.
In this regard, chitosan, a polymer of N-acetyl-glucosamine present in crustacean shells, has shown antifungal activity against a wide variety of fungi, leading to the reduction of rot incidence in strawberry,10 grape,11 and citrus.12
In vitro, chitosan shows an antimicrobial effect modifying the germ tube, reducing the diameter of the colony, and/or impeding spore germination.13,14 In the pathosystem of apple against P. expansum, chitosan was proved to be effective in controlling the disease when applied preventively, directly on the injuries of the fruit.15
On the other hand, there are few reports regarding the curative or eradicative properties of chitosan against the blue mold of apples, which could restore the quality of the previously infected fruit or reduce the inoculum present in the water used for washing the fruit. Moreover, the protective effect is questionable because of the fact that in most studies on apples, chitosan-containing products were applied in the form of drops on the wounds and not by fruit immersion.
The aim of this study was to evaluate the antifungal activity of chitosan against P. expansum; verify the feasibility of using this polysaccharide in packing houses; and compare the protective, curative, and eradicative effects of chitosan on the blue mold of apple cv. Fuji.
Materials and methodsFruitApples cv. Fuji, category 1, were provided by COOPERSERRA (São Joaquim, SC, Brasil). The fruit were stored in a cold chamber at 4±1°C; selected with respect to uniform size and color and absence of defects; and were then randomly divided into groups. Fruit were disinfected with 0.5% (v/v) sodium hypochlorite for 3min, washed with tap water, and air dried at room temperature before the experiments.
PathogenP. expansum was isolated from an infected apple, supplied by Dr. Rosa Maria Valdebenito Sanhueza, and stored in the collection of the Laboratório de Fitopatologia of the Universidade Federal de Santa Catarina (UFSC), and assigned code MANE 138. The isolate was cultured on potato dextrose agar (Himedia, Mumbai, India) under a photoperiod of 12-h light at 25±1°C. Spore suspension was prepared by flooding 10-day-old cultures of P. expansum with sterile distilled water or with 4% apple juice for in vivo and in vitro experiments, respectively. The concentrations of the spore suspensions were adjusted using a hemocytometer.
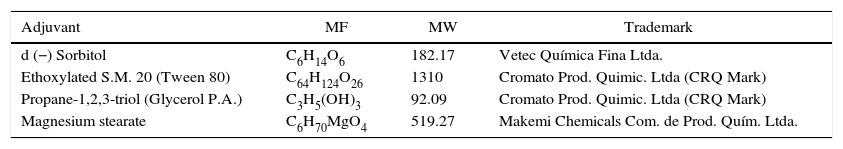
Chitosan and adjuvantsChitosan (Golden-Shell Biochemical, China) with high density (0.8gcm−3) and 90% deacetylation was obtained from Professor Luiz Henrique Beirão (UFSC). Before the experiments, the polysaccharide was dissolved in 0.05N HCl and the pH of each solution was adjusted to 5.6 using 2M NaOH. The adjuvants used in the experiments are shown in Table 1.
Formula (MF), molecular weight (MW) and trademark of the adjuvants used.
| Adjuvant | MF | MW | Trademark |
|---|---|---|---|
| d (−) Sorbitol | C6H14O6 | 182.17 | Vetec Química Fina Ltda. |
| Ethoxylated S.M. 20 (Tween 80) | C64H124O26 | 1310 | Cromato Prod. Quimic. Ltda (CRQ Mark) |
| Propane-1,2,3-triol (Glycerol P.A.) | C3H5(OH)3 | 92.09 | Cromato Prod. Quimic. Ltda (CRQ Mark) |
| Magnesium stearate | C6H70MgO4 | 519.27 | Makemi Chemicals Com. de Prod. Quím. Ltda. |
To assess the effects of chitosan on spore germination and germ tube elongation, 30μL of P. expansum spore suspension (1×105sporesmL−1) and 30μL of chitosan at different concentrations (0, 1.0, 2.5, 5.0, and 10mgmL−1) were mixed and plated on microscope-excavated slides. Subsequently, the slides were placed inside Petri dishes and incubated under high humidity at 25°C for 12h. Approximately, 100 spores of P. expansum were measured for germination rate and 20 for germ tube length per treatment using a light microscope (Leica DM500, Leica, Germany). Each treatment was done in quadruplicates and the experimental plot consisted of a microscope-excavated slide.
Control of blue moldTo evaluate the eradicative effect of chitosan on P. expansum, fruit were wounded (5-mm deep and 1-mm wide) with a sterile nail at equator region and immersed in a mixture containing spore suspension and polysaccharide for 3min. In this experiment, the contact time between spores and chitosan before the immersion of fruit as well as the concentrations of inoculum and chitosan were evaluated. In the first experiment, a suspension containing 1×105sporesmL−1 remained in contact with the polysaccharide (5mgmL−1) for 15min, 2h, 4h, or 8h before the immersion of fruit. In the second experiment, two different concentrations of P. expansum were evaluated, i.e., 1×104 and 1×105sporesmL−1 in contact with chitosan (5mgmL−1) for 8h. In a third experiment, some adjuvants (such as 0.1% Tween 80, 0.3% magnesium stearate, and 4.5% glycerol) were added to the chitosan suspension (10mgmL−1) and the time of contact (8 and 18h) of these suspensions with 1×104sporesmL−1 of P. expansum were evaluated. As the control, fruit were immersed only in spore suspension.
The protective and curative effects of chitosan were evaluated simultaneously. To evaluate the protective effect, fruit were wounded (5-mm deep and 1-mm wide) with a sterile nail at equator region. Following the immersion of fruit in chitosan suspensions (with or without the addition of adjuvants) for 3min, they were air dried at room temperature and the inoculation was performed with a single drop (20μL) of P. expansum suspension (1×105sporesmL−1) applied directly to the injuries. To evaluate the curative effect, wounded fruit (5-mm deep and 1-mm wide) were inoculated with a single drop (20μL) of P. expansum suspension (1×105sporesmL−1), and after the drops dried, the fruit were immersed in chitosan suspensions (with or without the addition of adjuvants).
Initially, the protective and curative effects of chitosan were evaluated by immersing the fruit in distilled water; chitosan (10mgmL−1); chitosan (10mgmL−1) containing 0.1% Tween 80 and 0.3% magnesium stearate; and chitosan (10mgmL−1) containing 0.1% Tween 80, 0.3% magnesium stearate, and 4.5% glycerol. All fruit used in the experiment were inoculated at the same time. Subsequently, the efficiency of chitosan (10mgmL−1) was compared by applying it in a form of a single drop (20μL directly to the wounds) or by the immersion of the fruit into the chitosan suspensions, in the preventive and curative method, by applying spore suspension of P. expansum at 1×105sporesmL−1. As the control, fruit were immersed in distilled water and inoculated.
In vivo experiments were conducted in a completely randomized design with four replications per treatment, each replication consisting of four fruit. The treated fruit were placed in 400mm×270mm×133mm plastic boxes with sterile water to maintain a high relative humidity and were stored at 25±1°C in dark. Decay severity of apple fruit caused by P. expansum was determined at 12 days after inoculation by measuring the lesion diameter. Moreover, the disease incidence (%) was measured at the end of each experiment by the ratio between the number of symptomatic injuries and the number of total injuries in the fruit, in each treatment.
Results and discussionThe blue mold caused by P. expansum is one of the main diseases in apple postharvest. The fungus is very aggressive and is able to survive even at low temperatures, leading to several economic losses.7
There are many fungicides registered for the control of postharvest decay. However, with the eminent risk of the persistence of synthetic chemical residues in fruit, alternative ways to control fungal diseases are being evaluated.14 Currently, studies regarding chitosan for the control of postharvest diseases and food preservation have gained attention because of the antifungal properties, biofilm formation, and biodegradability of this polysaccharide.16,17
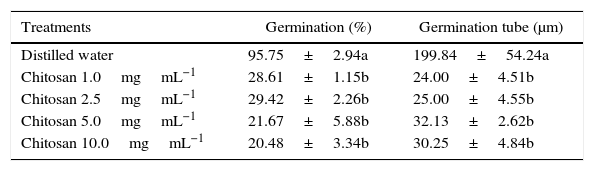
In the present study, when tested in vitro at different concentrations (1–10mgmL−1), chitosan reduced the germination of P. expansum by up to 78% and the length of the germ tube by 86%, comparing with the control (Table 2).
Effect of chitosan concentrations on germination and germ tube length of P. expansum spores.
| Treatments | Germination (%) | Germination tube (μm) |
|---|---|---|
| Distilled water | 95.75±2.94a | 199.84±54.24a |
| Chitosan 1.0mgmL−1 | 28.61±1.15b | 24.00±4.51b |
| Chitosan 2.5mgmL−1 | 29.42±2.26b | 25.00±4.55b |
| Chitosan 5.0mgmL−1 | 21.67±5.88b | 32.13±2.62b |
| Chitosan 10.0mgmL−1 | 20.48±3.34b | 30.25±4.84b |
Means±standard deviation at the columns, followed by the same letter do not differ by the Tukey test at 5% of significance.
Previous studies have shown that chitosan at 1% (10mgmL−1) reduces the mycelial growth of R. stolonifer,18 whereas that at 0.05mgmL−1 caused morphological changes in the germinative tube and reduced the germination of Colletotrichum acutatum.19 Besides the antifungal effect, it was reported that chitosan may also delay the onset of infection caused by B. cinerea and R. stolonifer during fruit storage.11,13,20
Considering the in vitro inhibition of P. expansum spore germination, a high reduction of blue mold on apples treated eradicatively was expected. However, fruit immersed in chitosan suspension (5mgmL−1) containing 1×105sporesmL−1 of P. expansum showed no reduction in the incidence and severity of disease (Fig. 1), even when the concentration of the spore suspension was reduced to 1×104sporesmL−1 (data not shown).
Eradicative effect of chitosan (C) on P. expansum evaluated through the incidence (%) and severity (cm) of blue mold, 12 days after the inoculation. The fruit were inoculated after different contact times (15min, 2h, 4h or 8h) between the spore suspension (1×105sporesmL−1) and chitosan (5mgmL−1). In the control-treatment (spores in water – W), the fruit were immersed in spore suspension without chitosan. Bars (mean and standard deviation) followed by the same letter do not differ by the Tukey's test at 5% significance.
Studies have described that higher the concentration of P. expansum spores in water, higher is the percentage of apples that can be infected, where an amount of at least 1×103sporemL−1 are needed to cause an infection.21,22 Thus, if the concentration used was not effective to reduce the incidence, the remaining spores were sufficient to infect the apples, leading to the disease.
When the concentration of chitosan was increased to 10mgmL−1, 40% and 47% reductions in the incidence and severity of blue mold, respectively, were observed after 8h of contact with the inoculum (Fig. 2). However, even with a longer contact time (18h) between spores and chitosan and the addition of adjuvants, the disease-control efficiency was not increased (Fig. 2). These results were consistent with other findings showing that the inhibition of fungi by chitosan is highly correlated with the polysaccharide concentration and the amount of spores in the suspension.13
Eradicative effect of chitosan (C) on P. expansum evaluated through the incidence (%) and severity (cm) of blue mold, 12 days after the inoculation. The fruit were inoculated after different contact times (8h or 18h) between the spore suspension (1×104sporesmL−1) and chitosan (10mgmL−1), with or without adjuvants. In the control-treatment (spores in water – W), the fruit were immersed in spore suspension without chitosan. The adjuvants, magnesium stearate (MS), glycerol (G), and Tween (T), were added. Bars (mean and standard deviation) followed by the same letter do not differ by Tukey's test at 5% significance. * The incidences of blue mold on fruits (%), given in parentheses, were significantly different compared to the respective control.
When the fruits arrive in a packing house, they are subjected to a selection process followed by washing. Active chlorine (150ppm) is commonly used for the elimination of microorganisms in water or on fruit surfaces.23 Chlorine has an eradicative effect on the spores of the fungus, but it does not have protective or curative effect on the blue mold. An effective control method for such cases that can be used in a simplified form inside the packing house would be an interesting alternative for the postharvest decay control.
Considering this, chitosan could aid in eliminating the spores present in the wash water and also in reducing disease in a curative and preventive mode, acting on infected fruits and/or preventing infections for those who suffered injuries during the stages of processing and storage. However, when the fruit were treated in a curative way, no significant reduction in the incidence and the severity of blue mold on apples was observed (Figs. 3 and 4).
Preventive (Prev.) and curative (Curat.) effects of chitosan (C) (10mgmL−1), with or without adjuvants, on the incidence (%) and severity (cm) of blue mold of apple. Control treatment (W), magnesium stearate (MS), glycerol (G), Tween (T). Bars (mean and standard deviation) followed by the same letter do not differ by Tukey's test at 5% significance. * The incidences of blue mold on fruits (%), given in parentheses, were significantly different compared to the control.
Preventive (Prev.) and curative (Curat.) effects of the chitosan (C) (10mgmL−1) on the incidence (%) and severity (cm) of blue mold of P. expansum. The apples were treated by the application of drops onto the wound or by fruit immersion in a suspension of chitosan. Control treatment (immersion in water – W). Bars (mean and standard deviation) followed by the same letter do not differ by Tukey's test at 5% significance. * The incidences of blue mold on fruits (%), given in parentheses, were significantly different compared to control.
On the other hand, chitosan could prevent the infection caused by P. expansum at the injury sites by forming a protective biofilm around the fruit. In this study, fruit treated preventively by immersion showed an average reduction of 24% and 45% in the incidence and severity of disease, respectively (Figs. 3 and 4). Higher levels of the protection in apples were obtained by dipping the fruit preventively in a chitosan solution (5mgmL−1), resulting in 66% and 95% reduction in the incidence and severity of disease caused by P. expansum.24
On the other hand, chitosan had a higher curative effect than a protective effect against gray mold caused by B. cinerea on grapes.11 However once P. expansum is a very aggressive fungus, it is unlikely that a product would provide an efficient control once fruit colonization is established.
Comparing the modes of application, a higher level of control of blue mold was observed when chitosan (10mgmL−1) was applied preventively and directly to the wounds in the form of a single drop, showing a 93% and 87% reduction in the incidence and severity of disease, respectively, as against 24% and 45% using the immersion mode (Fig. 4). It was found that the polysaccharide (1–10mgmL−1) was effective in controlling blue mold on apples in a preventive way even when applied in the form of a single drop.15 In addition, chitosan could protect tomato fruit against B. cinerea and P. expansum when applied preventively in the form a single drop to the wounds of the fruit at a concentration of 5 and 10mgmL−1.20
We, therefore, suggest that the high level of control of blue mold, provided by chitosan applied in the form of a single drop, could only be avoiding the penetration of the fungus. In this case, chitosan would be acting as a physical barrier, forming a protective biofilm on the injuries.
In order to increase the efficiency of chitosan against blue mold using the fruit immersion method, several additives that improve the physico-chemical properties of chitosan biofilm were tested such as Tween 80 (surfactant), glycerol (plasticizer), and magnesium stearate (lubricant).
However, in the experiments involving the addition of these adjuvants, no increase in the efficiency of polysaccharide for the control of blue mold was found after the immersion of fruit in chitosan suspensions. As opposed to this, the chitosan formulation (2.5mgmL−1) containing adjuvants such as 0.75% glycerol, 0.1% magnesium stearate and 0.1% Tween 80 was effective to reduce the severity of blue mold by 44.5%.24In vitro tests showed that the adjuvants tested did not affect the germination of P. expansum, in general (data not shown).
In conclusion, the present study shows that chitosan does not offer a curative effect for the blue mold and that its eradicative effect is not expressive and depends on the concentrations of spores in the apple wash water. The highest level of decay control was observed with the preventive application of chitosan in the form of a single drop. This suggests that if chitosan reaches the injuries, it is capable of controlling the disease, although that may not always happen after the immersion of fruit. Thus, additional studies are needed to facilitate the practical use of chitosan for postharvest decay control in apples.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank to the CNPq for granting a scholarship to JCD and to Professor Dr. Luiz Henrique Beirão for providing the chitosan used in this study.