Spore counts, species composition and richness of arbuscular mycorrhizal fungi, and soil glomalin contents were evaluated in a soil contaminated with Zn, Cu, Cd and Pb after rehabilitation by partial replacement of the contaminated soil with non-contaminated soil, and by Eucalyptus camaldulensis planting with and without Brachiaria decumbens sowing. These rehabilitation procedures were compared with soils from contaminated non-rehabilitated area and non-contaminated adjacent soils. Arbuscular mycorrhizal fungi communities attributes were assessed by direct field sampling, trap culture technique, and by glomalin contents estimate. Arbuscular mycorrhizal fungi was markedly favored by rehabilitation, and a total of 15 arbuscular mycorrhizal fungi morphotypes were detected in the studied area. Species from the Glomus and Acaulospora genera were the most common mycorrhizal fungi. Number of spores was increased by as much as 300-fold, and species richness almost doubled in areas rehabilitated by planting Eucalyptus in rows and sowing B. decumbens in inter-rows. Contents of heavy metals in the soil were negatively correlated with both species richness and glomalin contents. Introduction of B. decumbens together with Eucalyptus causes enrichment of arbuscular mycorrhizal fungi species and a more balanced community of arbuscular mycorrhizal fungi spores in contaminated soil.
Arbuscular mycorrhizal fungi (AMF – Phylum Glomeromycota) are important components of the soil microbiota that contribute to the diversification and stability of natural ecosystems.1 AMF are obligate mutualistic symbionts that colonize the roots of more than 80% of plant families, and which establish an association known as arbuscular mycorrhiza.2 Several studies have reported on the abundance and occurrence of AMF in contaminated soils,3–6 and have highlighted the high resistance of AMF isolates to several types of stress, including water stress, soil acidification, disaggregation, and absence or scarcity of vegetation cover.
Mining activities produce wastes which may contain heavy metals as contaminants. These residues are generally deposited on the ground, and often occupy large extensions. In order to prevent chemical elements contained in these residues to be exposed to leaching processes, or to the action of the winds, rehabilitation programs are usually employed in these areas through the establishment of vegetation cover. However, plants often have very limited development in contaminated areas. Due to their beneficial effects on plant growth under stressed conditions, AMF have been used to enhance rehabilitation of contaminated soils through phytoremediation.7 Besides, mycorrhizal plants and their associated microbiota can regulate absorption, transformation and removal of soil pollutants.8 The contribution of AMF to plant growth in soils contaminated with heavy metals is related to several factors, including diversity, abundance, persistence, and efficiency of AMF populations, which might vary between different locations, and are related to the environmental variables and to the presence of vegetation.9 The insoluble glycoprotein glomalin, which is abundantly produced by the hyphae of some AMF, is known for accumulating in the soil where it can retain large amounts of heavy metals.10 It has been suggested that soil glomalin content is related to changes associated with land use and rehabilitation of degraded soils.11 These changes in vegetation cover may directly influence AMF communities and the quantity and quality of compounds produced by them, such as glomalin.4 Therefore, abundance and richness of AMF or glomalin content may be useful indicators of rehabilitation. Studies showed that these attributes of AMF are inversely related to concentration of heavy metals in the soil.3 Therefore, improved knowledge on the dynamics of these fungi in contaminated rehabilitated soils is highly relevant, considering that AMF may contribute to revegetation and phytostabilization.12 The aim of this study was to evaluate the occurrence and AMF community composition in soils from areas contaminated with zinc (Zn), cadmium (Cd), copper (Cu), and lead (Pb), and subject to rehabilitation.
Materials and methodsStudy areas and soil samplingSoil samples were collected in the summer of 2009 (February) from contaminated sites under rehabilitation, in a Zn smelter unit of the Metal Mining Company (Companhia Mineira de Metais – CMM), currently known as Votorantim Metal Company (CMV), which is located in the municipality of Três Marias (Minas Gerais – MG – Brazil). Soil in the area was classified as anthropogenic, and the regional climate corresponds to type Aw of the Köppen classification system, namely savanna, with dry winter.13 The annual average temperature varies between 19°C and 22°C, and the annual average rainfall varies between 1100 and 1420mm. The areas are located at an average elevation of 539m in a flat relief with original cerrado vegetation.
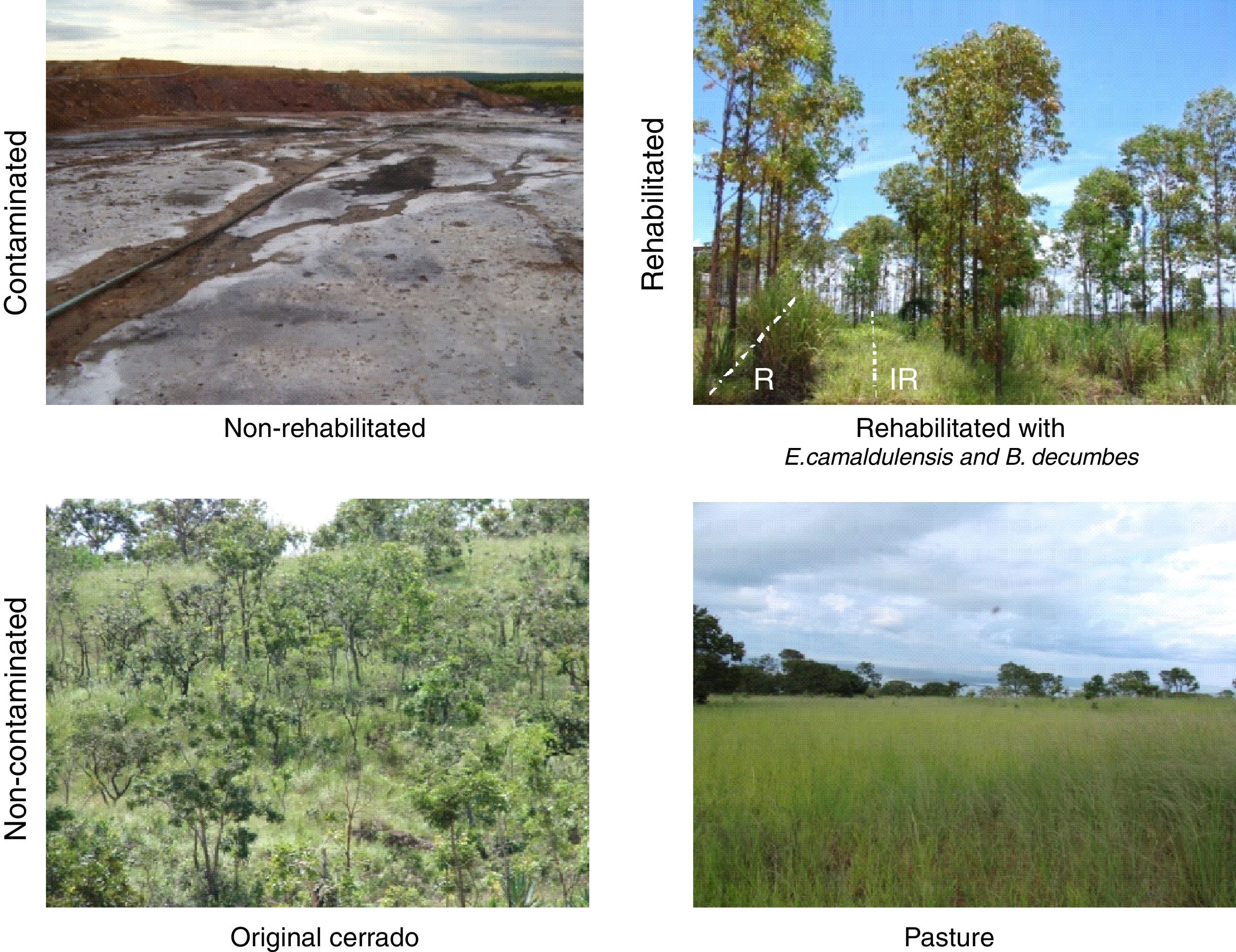
By the year of 2001/2002, two sites in the dumping area designated as industrial waste deposit (System 1 – 18°11′25.70″ S and 45°14′10.90″ W) and ustulation (System 2 – 18°11′10.50″ S and 45°14′8.00″ W), both contaminated by waste disposal, were sampled, and revealed high content of heavy metals: Zn=17,167 and 1844mgkg−1; Cu=882 and 145mgkg−1; Cd=584 and 29mgkg−1; and Pb=573 and 278mgkg−1, respectively. Two pilot rehabilitation programs were set up by the research team from the Department of Soil Science of the Federal University of Lavras (UFLA) and by the CMV staff. Rehabilitation strategy project included excavation of the contaminated soil and its replacement with non-contaminated soil. Eucalyptus camaldulensis seedlings were planted in rows in 1m×1m×10m trenches, where non-contaminated soil replaced the contaminated one. Space inter rows received a 20cm layer of non-contaminated soil, in which Brachiaria decumbens were planted or not (Fig. 1). The soil used to replace (row/trenches) or cover (inter rows) the contaminated one was taken from a Cerrado area adjacent to CMV. Before replacement, soil was subjected to liming, and received organic matter (cow manure) and fertilizers, in order to ensure adequate plant growth.14 For the assessment of AMF, 10 composite soil samples (5 sub-samples each) were taken at 0–20cm depth (one composite sample per sampling point. The distance between sampling points was 200m), from rows and inter-rows of both rehabilitation programs. For comparison, non-contaminated samples were also taken from Cerrado (C – 18°10′55.80″ S and 45°13′38.50″ W) and grass pasture (P – 18°11′28.30″ S and 45°13′55.20″ W), as well as from an adjacent non-rehabilitated area (NR – 18°11′09.50″ S and 45°14′34.80″ W) (Table 1). Five composite samples were taken from the reference areas (C, P and NR).
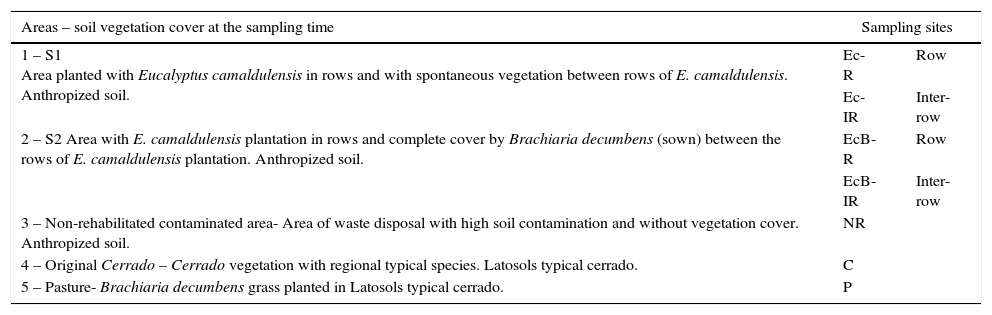
Characterization of soil sampling sites in areas contaminated with heavy metals after the use of the rehabilitation systems [S1 (Ec-R and Ec-IR) and S2 (EcB-R and EcB-IR)], in non-rehabilitated contaminated area (NR,) and in non-contaminated areas [Cerrado (C) and Pasture (P)].
| Areas – soil vegetation cover at the sampling time | Sampling sites | |
|---|---|---|
| 1 – S1 Area planted with Eucalyptus camaldulensis in rows and with spontaneous vegetation between rows of E. camaldulensis. Anthropized soil. | Ec-R | Row |
| Ec-IR | Inter-row | |
| 2 – S2 Area with E. camaldulensis plantation in rows and complete cover by Brachiaria decumbens (sown) between the rows of E. camaldulensis plantation. Anthropized soil. | EcB-R | Row |
| EcB-IR | Inter-row | |
| 3 – Non-rehabilitated contaminated area- Area of waste disposal with high soil contamination and without vegetation cover. Anthropized soil. | NR | |
| 4 – Original Cerrado – Cerrado vegetation with regional typical species. Latosols typical cerrado. | C | |
| 5 – Pasture- Brachiaria decumbens grass planted in Latosols typical cerrado. | P | |
Chemical analyses of soil samples were carried out in the Laboratory of Soil Science of UFLA. Heavy metal contents, including Zn, Cu, Cd, and Pb, were extracted by aqua regia solution, as recommended by the State Council on Environmental Policy (Conselho Estadual de Politica Ambiental – COPAM).15 Total heavy metal content was determined using the USEPA 3051 method, and soluble heavy metal content was determined by the Mehlich-1 method.
Occurrence and richness of arbuscular mycorrhizal fungi (AMF)The occurrence of AMF was assessed by direct extraction of spores in 50mL of soil, using the wet sieving technique,16 followed by centrifugation in 50% sucrose solution. Spores were counted and mounted on slides with polyvinyl lactoglycerol and Melzer's reagent. After that, phenotypic characteristics were observed in the compound microscope for taxonomic classification, according to Schenck and Pérez,17 to the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM) (http://www.invam.caf.wvu.edu), and to the Department of Plant Pathology of the University of Agriculture, Szczecin, Poland (http://www.agro.ar.szczecin.pl/∼jblaszkowski/). Traps were established in triplicates, by placing 50g of soil samples with root pieces, and mixed with 450g sterilized soil (121°C, for 1h, with intervals of 24h between two autoclavings), in 1kg pots. Brachiaria decumbes seeds were surface disinfected with 0.5% sodium hypochlorite for 15min, and their dormancy was interrupted by immersing them in concentrated sulfuric acid. In each pot, 5–10 Brachiaria seeds were sown. After germination, seedlings were trimmed, remaining three plants per pot. Once a month, pots were watered as needed and fertilized with 20mL per pot of Hoagland's solution.18 At the end of ten months growing cycle, in a greenhouse, 50g soil was sampled from each pot for AMF spore extraction, counting and identification, as described above.
The frequency of occurrence (FO) of each AMF species detected in the direct field soil samples and in trap culture was calculated by using the equation Fi=Ji/K, where Fi=frequency of the species I; Ji=number of soil samples in which species i occurred; and K=total number of soil samples.
Extractable glomalin contentsSoil glomalin was classified as “soil glomalin related protein” (SGRP). Two fractions of SGRP (easily extractable glomalin and total glomalin) were distinguished depending on the extraction conditions and on the quantification method.19,20 Quantification for both fractions was carried out using the Bradford's method,21 and therefore was denominated “Bradford-reactive soil protein” (BRSP) and “easily extractable Bradford-reactive soil protein” (EE-BRSP). For EE-BRSP extraction, 20mmol−1 sodium citrate at pH 7.0 was used, while total glomalin (BRSP) was extracted by 50mmol−1 sodium citrate at pH 8.0, followed by cycles of autoclaving (121°C for 1h) and centrifugation (3300rpm for 30min). Coomassie Brilliant Blue G-250 and bovine serum albumin (BSA) were used to generate the standard curve. Readings were carried out on a spectrophotometer at 595nm (values were expressed in mgg−1 soil).
Statistical analysisDifferences in spores abundance and in EE-BSRP and BRSP contents were evaluated by means of analysis of variance, using the Scott–Knott test (5%) using the SISVAR statistical software.22 Pearson correlations between metals concentrations in soil and species diversity, EE-BRSP and BRSP were carried out by the t test (5%), using the SAEG statistical program.
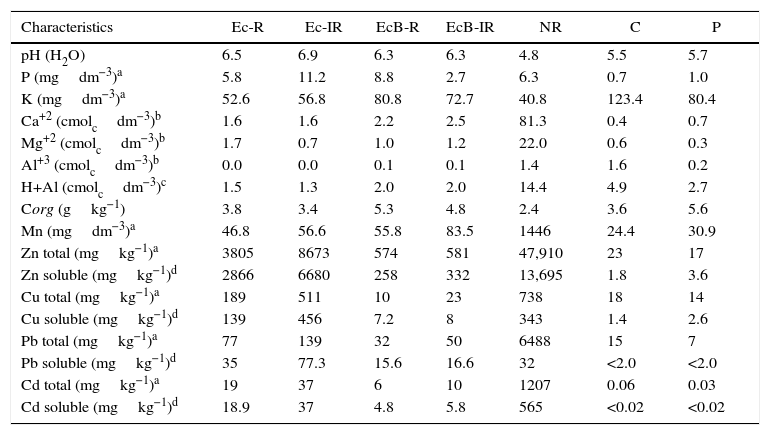
ResultsChemical characteristics of the soilIn general, the reference sites (C and P) presented acid and low fertility soils while the contaminated sites presented higher pH, Ca and Mg due to lime application that was used in order to reduce the activity of metal ions in the soil. Chemical analysis of soil samples revealed variable contents of metals, being very high in contaminated samples and very low in non-contaminated reference soils (Table 2). NR site exhibited high contents of heavy metals compared to the control sites C and P, especially for Zn and Cd (Table 2). NR site presented the highest concentration of all the metals elements, followed by rehabilitated sites. Among the rehabilitated sites, the one planted with Eucalyptus (EcB-R) and Brachiaria (EcB-IR) presented much lower content of all metals.
Chemical characteristics of the soil in areas contaminated with heavy metals after the use of rehabilitation systems [S1 (Ec-R and Ec-IR) and S2 (EcB-R and EcB-IR)], in non-rehabilitated contaminated area (NR), and in non-contaminated areas [Cerrado (C) and Pasture (P)].
| Characteristics | Ec-R | Ec-IR | EcB-R | EcB-IR | NR | C | P |
|---|---|---|---|---|---|---|---|
| pH (H2O) | 6.5 | 6.9 | 6.3 | 6.3 | 4.8 | 5.5 | 5.7 |
| P (mgdm−3)a | 5.8 | 11.2 | 8.8 | 2.7 | 6.3 | 0.7 | 1.0 |
| K (mgdm−3)a | 52.6 | 56.8 | 80.8 | 72.7 | 40.8 | 123.4 | 80.4 |
| Ca+2 (cmolcdm−3)b | 1.6 | 1.6 | 2.2 | 2.5 | 81.3 | 0.4 | 0.7 |
| Mg+2 (cmolcdm−3)b | 1.7 | 0.7 | 1.0 | 1.2 | 22.0 | 0.6 | 0.3 |
| Al+3 (cmolcdm−3)b | 0.0 | 0.0 | 0.1 | 0.1 | 1.4 | 1.6 | 0.2 |
| H+Al (cmolcdm−3)c | 1.5 | 1.3 | 2.0 | 2.0 | 14.4 | 4.9 | 2.7 |
| Corg (gkg−1) | 3.8 | 3.4 | 5.3 | 4.8 | 2.4 | 3.6 | 5.6 |
| Mn (mgdm−3)a | 46.8 | 56.6 | 55.8 | 83.5 | 1446 | 24.4 | 30.9 |
| Zn total (mgkg−1)a | 3805 | 8673 | 574 | 581 | 47,910 | 23 | 17 |
| Zn soluble (mgkg−1)d | 2866 | 6680 | 258 | 332 | 13,695 | 1.8 | 3.6 |
| Cu total (mgkg−1)a | 189 | 511 | 10 | 23 | 738 | 18 | 14 |
| Cu soluble (mgkg−1)d | 139 | 456 | 7.2 | 8 | 343 | 1.4 | 2.6 |
| Pb total (mgkg−1)a | 77 | 139 | 32 | 50 | 6488 | 15 | 7 |
| Pb soluble (mgkg−1)d | 35 | 77.3 | 15.6 | 16.6 | 32 | <2.0 | <2.0 |
| Cd total (mgkg−1)a | 19 | 37 | 6 | 10 | 1207 | 0.06 | 0.03 |
| Cd soluble (mgkg−1)d | 18.9 | 37 | 4.8 | 5.8 | 565 | <0.02 | <0.02 |
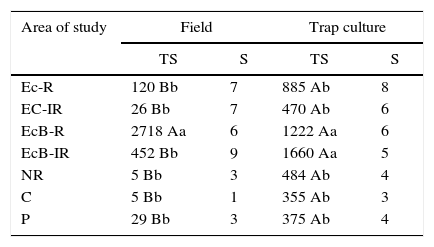
Spore counts were higher in trap cultures than in field samples (Table 3). A total of 5451 spores were found in trap cultures, and 3355 spores were obtained in the direct field sampling technique. The highest (p<0.05) spore number in the field was found in EcB-R, followed by EcB-IR. In NR and C sites, sporulation and species richness were very low in field samples. In trap cultures, sporulation was higher in plots revegetated with Eucalyptus and Brachiaria (EcB-IR), and much higher than in direct field samples from NR and non-contaminated soils. Similar trend was found for species richness. These results indicate that trap culture was more appropriate to detect modifications in AMF community induced by soil rehabilitation. The use of trap cultures allowed detecting significantly higher total spore abundance values for all the sampled and assessed sites, when compared to direct field sampling, except for EcB-R, where there was no significant difference (p<0.05) between the techniques. Spore abundance values detected in soil samples from EcB-R and EcB-IR by using the trap culture technique were 1222 and 1660, respectively, which is significantly different from the spore abundance values of the other investigated sites. Among the direct field samples, spore number was remarkable in the EcB-R site (2718 spores) (Table 3).
Total number of spores (TS) and species richness (S) of AMF detected in the direct field soil samples (50ml soil) and in trap culture pots (50ml soil) corresponding to contaminated and rehabilitated areas (Ec-R, Ec-IR, EcB-R, and EcB-IR), to non-rehabilitated areas (NR), and to non-contaminated areas [Cerrado (C) and Pasture (P)].
| Area of study | Field | Trap culture | ||
|---|---|---|---|---|
| TS | S | TS | S | |
| Ec-R | 120 Bb | 7 | 885 Ab | 8 |
| EC-IR | 26 Bb | 7 | 470 Ab | 6 |
| EcB-R | 2718 Aa | 6 | 1222 Aa | 6 |
| EcB-IR | 452 Bb | 9 | 1660 Aa | 5 |
| NR | 5 Bb | 3 | 484 Ab | 4 |
| C | 5 Bb | 1 | 355 Ab | 3 |
| P | 29 Bb | 3 | 375 Ab | 4 |
Uppercase letters=comparisons of TS within rows; lowercase letters=comparisons within columns. Values followed by the same letter do not differ by the Scott–Knott test (P<0.05)
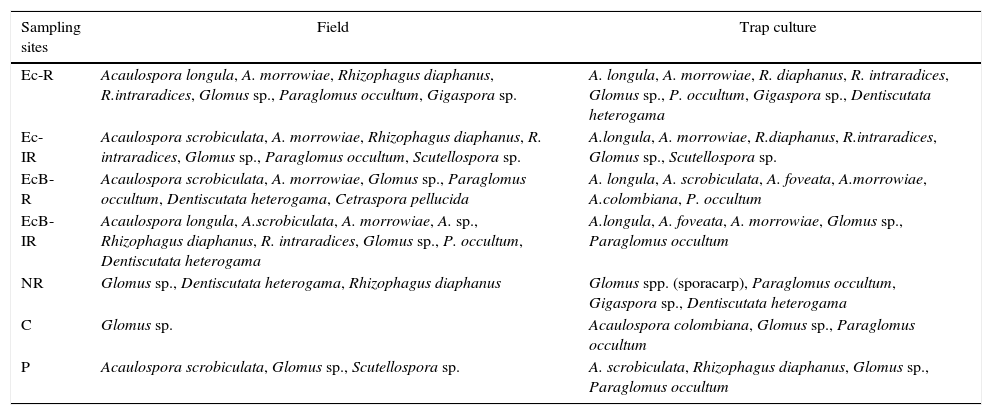
Considering all the samples analyzed by both techniques, a total of 15 morphotypes were detected. These morphotypes corresponded to eight genera (Acaulospora, Glomus, Rhizophagus, Paraglomus, Gigaspora, Dentiscutata, Scutellospora and Cetraspora), and six families (Acaulosporaceae, Glomeraceae, Paraglomeraceae, Dentiscutataceae, Racocetraceae and Gigasporaceae), and most of the morphotypes (66.6%) belonged to the Acaulosporaceae and Glomeraceae families (Table 4). The species Acaulospora foveata, Acaulospora colombiana, and Glomus sp. (sporocarp) were detected only in trap culture pots, whereas Cetraspora pellucida and Acaulospora sp. were detected only in the field samples.
Species of arbuscular mycorrhizal fungi (AMF) detected in the direct field soil samples and in trap culture pots corresponding to contaminated and rehabilitated areas (Ec-R, Ec-IR, EcB-R, and EcB-IR), to non-rehabilitated area (NR), and to non-contaminated areas [Cerrado (C) and Pasture (P)].
| Sampling sites | Field | Trap culture |
|---|---|---|
| Ec-R | Acaulospora longula, A. morrowiae, Rhizophagus diaphanus, R.intraradices, Glomus sp., Paraglomus occultum, Gigaspora sp. | A. longula, A. morrowiae, R. diaphanus, R. intraradices, Glomus sp., P. occultum, Gigaspora sp., Dentiscutata heterogama |
| Ec-IR | Acaulospora scrobiculata, A. morrowiae, Rhizophagus diaphanus, R. intraradices, Glomus sp., Paraglomus occultum, Scutellospora sp. | A.longula, A. morrowiae, R.diaphanus, R.intraradices, Glomus sp., Scutellospora sp. |
| EcB-R | Acaulospora scrobiculata, A. morrowiae, Glomus sp., Paraglomus occultum, Dentiscutata heterogama, Cetraspora pellucida | A. longula, A. scrobiculata, A. foveata, A.morrowiae, A.colombiana, P. occultum |
| EcB-IR | Acaulospora longula, A.scrobiculata, A. morrowiae, A. sp., Rhizophagus diaphanus, R. intraradices, Glomus sp., P. occultum, Dentiscutata heterogama | A.longula, A. foveata, A. morrowiae, Glomus sp., Paraglomus occultum |
| NR | Glomus sp., Dentiscutata heterogama, Rhizophagus diaphanus | Glomus spp. (sporacarp), Paraglomus occultum, Gigaspora sp., Dentiscutata heterogama |
| C | Glomus sp. | Acaulospora colombiana, Glomus sp., Paraglomus occultum |
| P | Acaulospora scrobiculata, Glomus sp., Scutellospora sp. | A. scrobiculata, Rhizophagus diaphanus, Glomus sp., Paraglomus occultum |
Both direct field sampling and trap culture technique presented similar results in regard to species richness. Twelve AMF species were detected in direct field samples, and a high number of species richness (9) was found in samples from the EcB-IR site (Table 4). Trap culture technique allowed detecting 13 AMF species, and the greatest species richness (8) occurred in the Ec-R site. In trap cultures from Cerrado, and in NR site, AMF richness was very low.
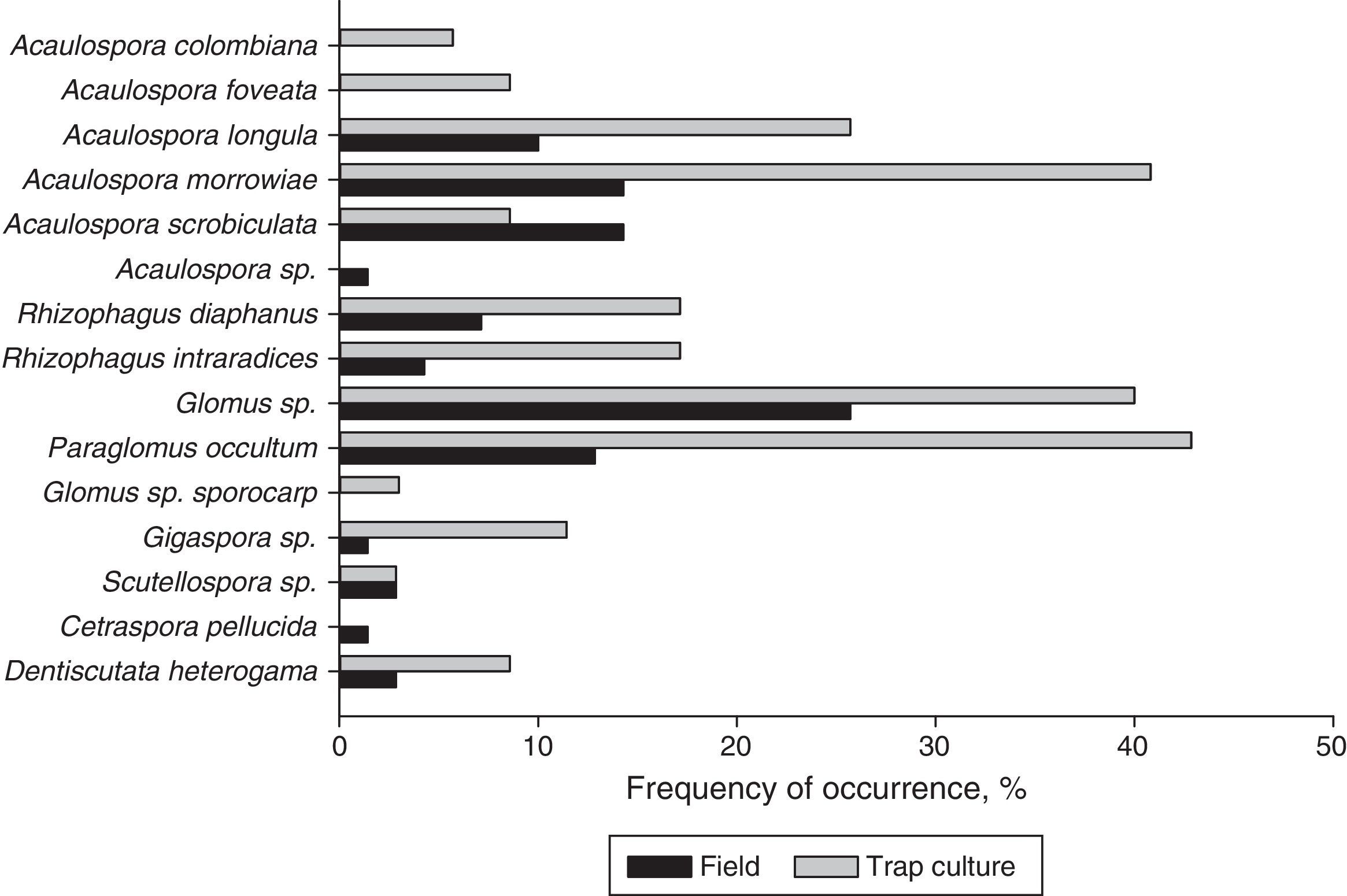
The only species common to all the studied sites was Glomus sp., which is considered a generalist species. Acaulospora sp. and Glomus sp. (sporocarp) were detected in only one sampling site, and thus were rated as exclusive species (Table 4). Glomus sp. presented the highest frequency of occurrence (40%) in the assessment of the direct field samples (Fig. 2). Among the AMF species detected by the trap culture technique, Paraglomus occultum presented the highest frequency of occurrence (42.9%); however, in the direct field samples, its frequency of occurrence was 12.9% (Fig. 2). Species richness was affected by the contents of heavy metals in the soil. Linear correlations were: Zn (y=7.5651−0.00009x; R2=−0.8717), Cu (y=7.8614−0.005x; R2=−0.72531), Pb (y=7.2995−0.0007x, R2=−0.8677), and Cd (y=7.3148−0.0036x; R2=−0.9630) (p<0.05).
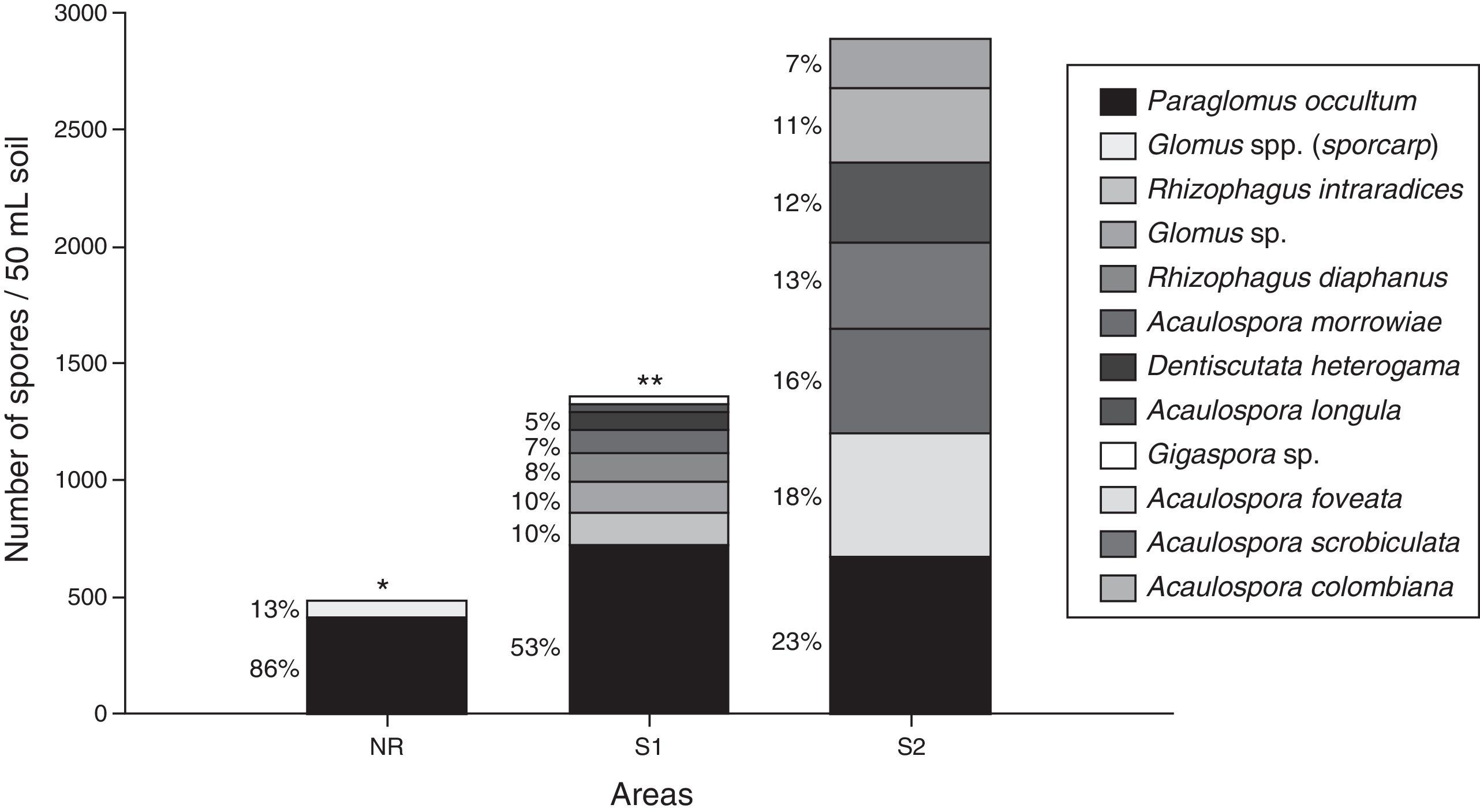
Analysis of fungal community structure in non-rehabilitated and rehabilitated sites, as revealed by trap cultures, shows increase in AMF (spore numbers) and species richness (Fig. 3). Soil replacement and Eucalyptus planting was successful in revegetating the area and in increasing AMF. However, when B. decumbens was simultaneously sown in the inter-row, the benefits to increase AMF were much higher. Besides increasing the number of spores, the community was more balanced, with less species dominance, when compared to non-rehabilitated site, or only with Eucalyptus.
Number and proportion (%) of each AMF species recovered by trap culture for the following areas: Non-rehabilitated contaminated area (NR); System with eucalyptus planted in rows and without brachiaria planted in inter-rows (S1), and System with eucalyptus planted in rows and with brachiaria planted in inter-rows (S2). Spores total amount was 484 for NR, 1355 do S1, and 2882 for S2. * AMF with low proportion in the soil in NR: Dentiscutata heterogama and Gigaspora sp.; ** AMF with low proportion in the soil in System 1: Acaulospora longula, Gigaspora sp. and Scutellospora sp.
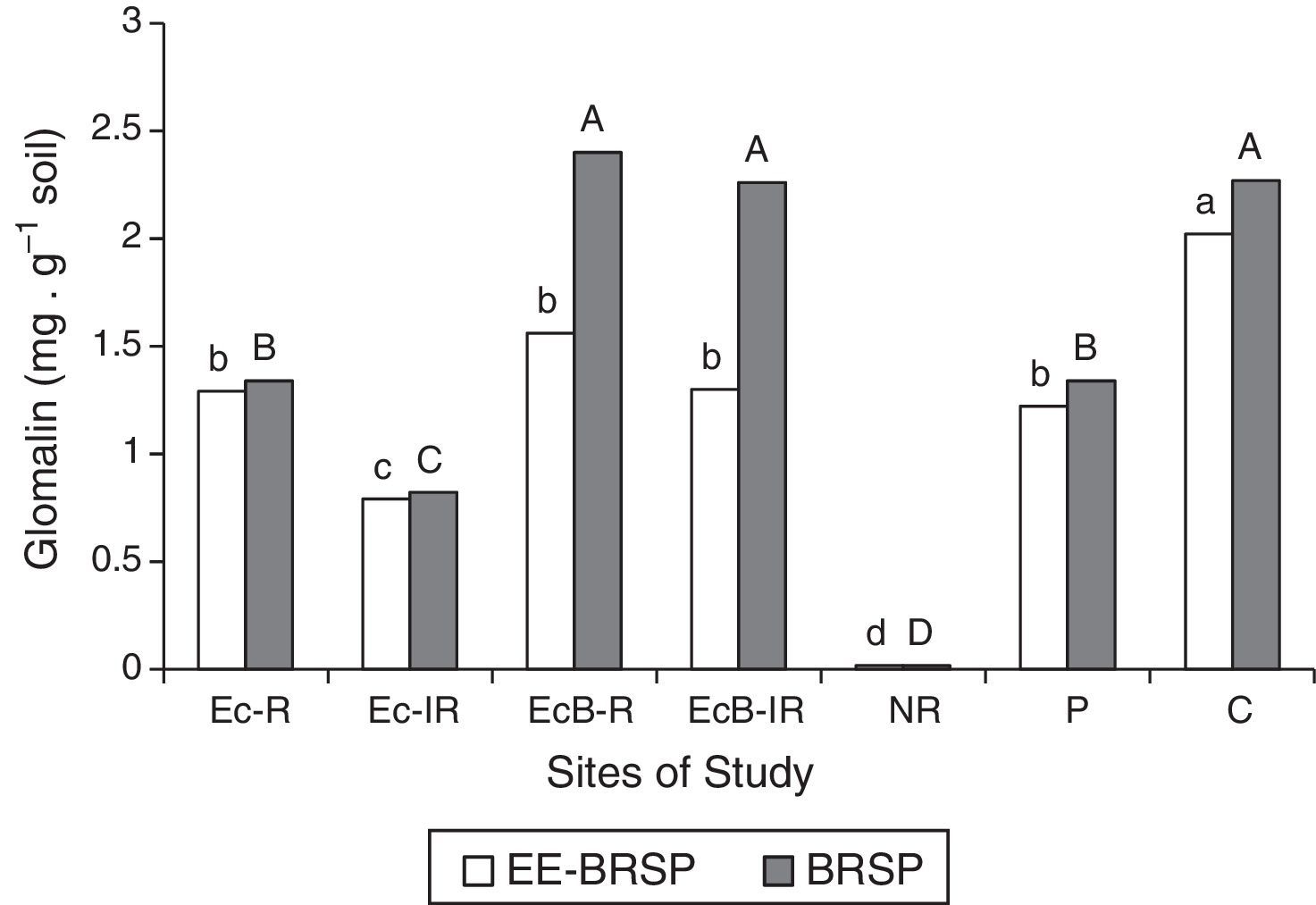
Revegetation increased glomalin contents, which was very low in the non-rehabilitated site (Fig. 4). Glomalin was higher (p<0.05) in plots with Eucalyptus and Brachiaria, where spore counts was also high. EE-BRSP content of the direct field samples significantly decreased (p<0.05) in the following order: C>EcB-R, EcB-IR, Ec-R and P>Ec-IR>NR. Regarding the BRSP contents of the direct field samples, EcB-R, EcB-IR, and C sites did not differ from each other and had the highest values, followed by Ec-R and P, which were also similar to each other. NR site exhibited the lowest BRSP (Fig. 4).
Total BRSP and easily extractable glomalin (EE-BRSP) contents in samples from the direct field soil samples corresponding to the contaminated and rehabilitated areas (Ec-R, Ec-IR, EcB-R, and EcB-IR), to the non-rehabilitated area (NR), and to the non-contaminated areas [Cerrado (C) and Pasture (P)]. Uppercase letters – comparison of BRSP values; lowercase letters – comparison of EE-BRSP values. Bars with the same letter do not differ by the Scott–Knott test (p<0.05).
Glomalin contents also negatively correlated (p<0.05) with heavy metals in the soil. EE-BRSP values were linearly reduced with concentrations of Zn (y=1.3463−0.00003x; R2=−0.92731), Cu (y=1.5345−0.0019x; R2=−0.94244); Pb (y=1.2511−0.0002x; R2=−0.97267) and Cd (y=1.2565−0.001x; R2=−0.96303). A similar trend was observed for BRSP, whose values were linearly reduced with contents of Zn (y=1.8846−0.00004x; R2=−0.84895), Cu (y=2.2659−00031x; R2=−0.97891), Pb (y=1.7287−0.0003x; R2=−0.76431), and Cd (y=1.7371−0.0014x; R2=−0.76948) (p<0.05).
DiscussionReplacement of the contaminated soil with tree plantation in rows with or without B. decumbens sowing in the inter-rows allowed successful rehabilitation of the soil. These rehabilitation programs promote reduction of total and soluble contaminant metals (Zn, Cu, Pb, and Cd) in the soil. Compared to the concentrations found at the beginning of the rehabilitation process, these metals were reduced by 90%, and allowed the establishment and the growth of the introduced plants. Decrease in metal contents varied according to the element, and were higher in rows than in inter-rows due to the amount of soil replaced and to the growth of new seedlings. However, Zn and Cd concentrations exceeded a bit the limits set by COPAM for the agricultural use of soil (450 and 38mgkg−1 of dry soil of Zn and Cd, respectively).15 In turn, NR site presented the highest concentration of heavy metals. Thus, it demonstrated that rehabilitation by soil excavation and replacement with non-contaminated soil was effective to create an adequate environment for plant initial growth. As shown in the Fig. 1, plants were able to develop well in the contaminated area, and consequently showed continuous growth which promoted AMF multiplication. In fact, it was found a total a 15 different AMF morphotypes in the area, and most of these species had been previously reported in studies of AMF occurrence and ecology in these sites before rehabilitation.3 However, differently from these authors, in rehabilitated area, it was found Acaulospora longula and Rhizophagus diaphanus, which indicates that rehabilitation allowed sporulation of other species unable to sporulate in the non-rehabilitated soils.
Disregarding the control sites (P and C), 15 AMF species were found in the present study. This number was similar to, or higher than the ones reported by other studies carried out in heavy metals contaminated soils. Study carried in India found only four AMF species in a soil contaminated with heavy metals (Cu, Zn, Cr, Cd and Pb) collected from a tannery treatment plant.23 In other studies carried in bauxite mining areas in Brazil, and in sewage sludge deposition areas that were contaminated with Zn, Cd, Cu, Ni and Pb in Germany, researchers found six AMF species.4,24 Fifteen species were detected in soils contaminated with Zn, Cu, Pb, Ni, and Cd in India.25 The present results showed that revegetation with Eucalyptus and Brachiaria associated with replacement of contaminated soil by uncontaminated soil may contributed to a continuous revegetation process of the area.
It was found predominance of species from the Glomus and Acaulospora genera, which confirms that they are better adapted to stressful conditions of soil contaminated with heavy metals.26 In fact, Glomus sp. was a generalist species since it was the only one found in all the sites. The species P. occultum was also considered a generalist, since it was detected by the trap culture technique in most plots. As pointed out by other authors,27–29Acaulospora and Glomus genera are well adapted to excess of heavy metals, and few reports reveal the occurrence of P. occultum in contaminated soils, as observed in the present study. However, Melloni, Siqueira and Moreira4 reported the occurrence of P. occultum in mining soil under rehabilitation. Rhizophagus intraradices recorded in this study had only been previously recorded in soils contaminated with Zn, Cu, Cd and Pb in bauxite mining areas in the north of Brazil and in gypsum mining areas.3,28,30,31
Among the AMF species detected in direct field samples, Acaulospora sp. and C. pelucida were not found in the trap culture pots. In turn, the species A. foveata, A. colombiana, and Glomus sp. (sporocarp) were exclusively detected by this method.
Except for A. colombiana, which was found only in the trap culture from Cerrado, it is suggested that the change in the AMF community structure may be related to alterations in the stress levels. In this study, stress levels were higher in the field (higher heavy metal concentrations), when compared to trap cultures (lower heavy metal concentrations promoted by diluting the original soil in the preparation of traps), which is supported by Rydlová and Vosátka.32 These authors emphasized that AMF may lose their adaptation and stress tolerance when sub-cultured without initial stress.
These results highlight the importance of detecting AMF diversity by multiplying them in trap culture pots, in addition to the survey of spores directly collected from the field, in order to promote access to a larger number of species in studies on diversity and to afford a more complete picture of the biology, ecology, and diversity of AMF communities.33 Nevertheless, several authors suggest that the trap culture method might select for species that easily sporulate, and might omit the ones that colonize roots, although they do not sporulate.34
After the evaluation of the direct field samples, it was found greater AMF species richness in EcB-IR, whereas for the trap cultures, the greatest species richness was detected in Ec-R. Nevertheless, both results show AMF as an indicator of soil rehabilitation, since the AMF species richness found in the rehabilitated sites was higher, when compared to NR site, where the concentrations of heavy metals were higher.
The presence of only one Glomus sp. in Cerrado field samples may be related to the absence of, or to the low sporulation of other AMF species at sampling time. However, by using trapping, it was possible to recover two other species (A. colombiana and P. occultum). It should also be considered that sampling occurred in the rainy season, and at this condition, low amount of spores is usually found.35,36 On the other hand, AMF total diversity found in Cerrado was lower, when compared to rehabilitation systems and pasture, possibly because Cerrado is more stable than the other environments regarding the variation in soil characteristics. These conditions could act as selection pressure on fungal communities with reduced sporulation, or produce spores with low capacity to resist adverse conditions.37,38 This is consistent with findings of low spore density in climax ecosystems.39 Thus, although several studies have demonstrated high diversity of AMF in Cerrado, edaphic conditions and vegetation cover might also promote changes in spore density.4
In a study on soils from a bauxite mining area under rehabilitation, it was found that mining affected spore density, which increased after the beginning of rehabilitation.4 The lower spore density in the C and P, might be attributed to the natural stability of these ecosystems, especially due to the constant presence of hosts, and to the absence of abrupt variations in soil fertility. These characteristics might ensure the survival of fungal species with low natural sporulation ability, or which produce spores with poor resistance to adverse conditions.4,40 Similar effects might also be found in areas subjected to agricultural cultivation, such as pasture, which are influenced by soil handling techniques, such as tilling and fertilization, extensive monoculture, and the use of agricultural toxins. According to some authors these handling techniques might reduce the incidence of some AMF and sporulation.40
The number of spores counted in the direct field samples was lower, when compared to the count in the trap culture pots in all the investigated sites. It has been reported that the number of spores in the direct field samples is usually low, and they are parasitized.41 Besides that, usually all of the subcellular structures needed for accurate identification of the species are not intact. Therefore, AMF multiplication system in host plants under controlled conditions is used. In the present study, the use of the trap culture technique allowed the observation that AMF species from sites highly contaminated with heavy metals, such as NR, presented high sporulation capacity under less contaminated conditions, as well as in the presence of a mycotrophic host plant, B. decumbens, which was used as a bait plant in the trap culture pots. In addition, the greater sporulation found in EcB-IR might be associated with the presence of B. decumbens in the inter-row areas, which probably favored sporulation. Studies that evaluated the effect of B. decumbens in revegetation of degraded soils, showed 400% increase in soil spores, which confirmed that this grass species is effective in multiplying AMF.42
Soil restoration system 2 (Table 1) provided better balance between AMF populations, since no predominance of any AMF species was observed (Fig. 3). This result suggests that the soil cultivated with Eucalyptus and Brachiaria in the inter-rows led to the best soil conditions, where microhabitats protected better the soil against sudden disruptions, and showed less competition for nutrients and niches among microorganisms, ensuring low sporulation.28
In the present study, BRSP was related to soil rehabilitation, since NR, where the soil presented lower vegetation cover and higher concentrations of heavy metals, presented lower EE-BRSP and BRSP contents, as suggested for degraded areas in general.11 In conditions with high concentrations of heavy metals, AMF usually presents protective mechanisms, such as greater production and renewal of external mycelium, which contributes to glomalin synthesis.43,44 However, it is worth noting that regardless of the rehabilitation program (systems 1 and 2), EE-BRSP and BRSP values found in the contaminated areas were higher than, or similar to those found by other authors in areas with other type of contaminants. Studies showed values of EE-BRSP that varied from 0.4 to 0.07mggsoil−1, together with an increase in the level of contamination with Cr.45 The high levels of glomalin in Cerrado soil were probably represented by old stocks of this glycoprotein, since its stability and persistence in preserved environments can reach 42 years in tropical soils.46 However, none of the treatments reached BRSP values similar to the 60mggsoil−1 found in forest soils of temperate areas.46
ConclusionThe concentration of heavy metals in the soil of the areas under rehabilitation affected the diversity and the composition of AMF communities. The rehabilitation system 2, which included planting of Eucalyptus in rows and Brachiaria in the inter-rows, presented the most favorable conditions for the development and occurrence of AMF, with potential application in rehabilitation program of contaminated areas. Although at a lesser degree, positive effects were also observed in system 1. It was evident that Glomus sp. is adapted to the stress caused by different concentrations of heavy metals, since it was detected in all the sites contaminated with Zn, Cu, Cd, and Pb. Diversity of AMF and glomalin contents can be considered good indicators of rehabilitation of soils contaminated with Zn, Cu, Pb and Cd.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the Coordination of Higher Education Personnel Training (Capes) and Support Foundation to Research of Minas Gerais (Fapemig), for financial support of this project, and for the fellowships. The authors also thank the National Council of Scientific and Technologic Development (CNPq) for financial support and fellowships.







![Total BRSP and easily extractable glomalin (EE-BRSP) contents in samples from the direct field soil samples corresponding to the contaminated and rehabilitated areas (Ec-R, Ec-IR, EcB-R, and EcB-IR), to the non-rehabilitated area (NR), and to the non-contaminated areas [Cerrado (C) and Pasture (P)]. Uppercase letters – comparison of BRSP values; lowercase letters – comparison of EE-BRSP values. Bars with the same letter do not differ by the Scott–Knott test (p<0.05). Total BRSP and easily extractable glomalin (EE-BRSP) contents in samples from the direct field soil samples corresponding to the contaminated and rehabilitated areas (Ec-R, Ec-IR, EcB-R, and EcB-IR), to the non-rehabilitated area (NR), and to the non-contaminated areas [Cerrado (C) and Pasture (P)]. Uppercase letters – comparison of BRSP values; lowercase letters – comparison of EE-BRSP values. Bars with the same letter do not differ by the Scott–Knott test (p<0.05).](https://static.elsevier.es/multimedia/15178382/0000004700000004/v1_201610040136/S1517838216305251/v1_201610040136/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)