The aim of this study was to develop a kefir apple-based vinegar and evaluate this fermentation process using new methodology with Biospeckle Laser. Brazilian kefir grains were inoculated in apple must for vinegar production. In this study, the microbial community present in kefir, and correspondent vinegar, was investigated using Matrix Assisted Laser Desorption/Ionization – Time of Flight Mass Spectrometry (MALDI-TOF MS) technique. Saccharomyces cerevisiae, Lactobacillus paracasei, Lactobacillus plantarum, Acetobacter pasteurianus and Acetobacter syzygii were the microbial species identified. S. cerevisiae, L. plantarum, A. pasteurianus and A. syzygii were found in smaller quantities at the beginning of the alcoholic fermentation, but were found throughout the alcoholic and acetic fermentation. Kefir grains were able to utilize apple must as substrate to produce ethanol, and acetic acid. Acetate, volatile alcohols and aldehydes in the vinegar-based kefir were also produced. The yield of acetic acid in the kefir vinegars was ∼79%. The acetic acid concentration was ∼41gL−1, reaching the required standard for the Brazilian legislation accepts it as vinegar (4.0% acetic acid). Kefir vinegar showed good acceptance in the sensory analysis. The technology proposed here is novel by the application of immobilized-cell biomass (kefir grains) providing a mixed inocula and eliminating the use of centrifuge at the end of the fermentative process. This step will save energy demand and investment. This is the first study to produce apple vinegar using kefir grains.
Apple (Malus spp.) is probably the oldest fruit known to man and is favored by millions of people around the globe.10 In Brazil, some of these apple fruits are consumed by local populations, but the majority is wasted during harvest because of the high production per tree, the short shelf life of the fresh fruit, and the lack of use of these fruits as processed products.1,22 Fruits wine and/or vinegar production, could be a good solution because they allow products to be maintained in alcohol or acetic acid.1,9 Although the number of studies of fruit wines1,5,7,21 and fruit vinegars9,10,12 has recently increased, no study has focused on the production of apple kefir vinegar.
Kefir is a culture employed to produce beverages, such as the traditional Russian beverage also named “kefir” which is from milk, and has low alcohol content.23,13–17 The kefir is a mixed culture of various yeast species of the genus Kluyveromyces, Candida, Saccharomyces and lactic acid from bacteria of the genus Lactobacillus combined in a matrix of proteins and polysaccharide ‘kefiran’, which are formed during cell growth under aerobic conditions.23 The grains of kefir are irregularly shaped, with yellowish-white color, and hard granules, which resemble miniature cauliflower blossoms.15 In Brazil, the grains of kefir are used in private household,15 and they are added to different types of milk, such as cow, goat or sheep, coconut, rice and soy milk.21,23 The grains are responsible for the fermentation that produce lactic acid, acetic acid, CO2, alcohol (ethyl alcohol) and aromatic compounds. These compounds provide kefir's unique sensory characteristics: fizzy, acid taste, tart and refreshing flavor.15 The beverage contains vitamins, minerals and essential amino acids that help the body with healing and maintenance functions and contains easily digestible complete proteins.15
An optical technique to evaluate the biological activity of kefir grains was used, the Laser Biospeckle. The technique defines when a laser beam is scattered by a biological sample; the scattered waves generated in the illuminated sample create the speckle pattern that changes its image in accordance with the changes in the monitored material. Thus, the surface appears to be covered with tiny bright dots that fluctuate in a seemingly random way as for a boiling liquid.3,8
The aim of this study was to develop an apple vinegar-based kefir and study this fermentation process using new method Biospeckle Laser to evaluate the biological activity of kefir grains.
Materials and methodsBrazilian milk kefir grains (Stock-culture of the Microbial Fermentation Laboratory of the Federal University of Lavras, Brazil) were used in the experiments. The apple fruits were obtained from Lavras city market, Minas Gerais State in Brazil. These apple fruits, which fail to meet the quality standards required for marketing, were washed in clean water to remove residues. The pulp was extracted using an automatic depulping machine (ITAMETAL 0.5 DS, Itabuna, BA, Brazil). The °Brix was analyzed, and the juice was divided into three 500mL Erlenmeyer flasks to start batch fermentation.
The process of vinegar productionAlcoholic fermentationThe must was inoculated with kefir grains in a proportion of 10% (w/v). All fermentations were performed in triplicate. The flasks were incubated statically at a temperature of 28°C and fermentation was monitored daily to observe the end of the fermentation (stability of the consumption of sugars – Brix). The fermentation must was filtered by kitassato filters (0.5μm). The kefir grains were recovered. The alcoholic must was used for apple kefir vinegar production. Microbiological analysis (grains and must), measurement of Brix, reducing sugar analysis and pH 11 were carried out during fermentation.
To determine fermentation performance were calculated the substrate conversion factors in ethanol (Yp/s), substrate conversion in glycerol (Yg/s), substrate conversion in acetic acid (Yac/s), ethanol volumetric productivity of ethanol (Qp), biomass productivity (Px) and conversion efficiency (Ef). The total concentration of sugars was calculated considering the conversion for each mole of sucrose (342g) in 1mol of glucose (180g) and 1mol of fructose (180g).
Acetic fermentationThe apple fermented must obtained in the previous section were acetified in 500mL Erlenmeyer flasks, which were controlled temperature of 28°C and agitation of 150rpm. The acetic fermentation was conducted using the following treatments: (1) kefir grains 10% (w/v); (2) kefir grains 20% (w/v). During acetic fermentation, daily samples were taken (6 days) in triplicate for acidity analysis (pH meter) and alcohol (alcoholometer). The acetic fermentation was finish on the sixth day when the vinegar presented alcohol content below 1.0% (v/v).
The yield was calculated as the acetic acid produced in relation to the theoretical yield. The theoretical yield was calculated as the amount of ethanol converted to acetic acid, in which 1.0g of ethanol yields 1.304g of acetic acid.5 The ethanol, methanol, higher alcohols were analyzed according to Magalhães et al.15 using a Jasco chromatograph equipped with a refractive index (RI) detector (Jasco 830-RI, Madrid, Spain). A Chrompack column (30cm×6.5mm) at 60°C, using 5mM sulphuric acid as the eluent, at a flow rate of 0.5mL/min. Glycerol, organic acids (lactic, acetic, tartaric, malic, citric and succinic acid) and carbohydrates (glucose, sucrose and fructose) contents were quantified according to Puerari et al.21 using a Jasco chromatograph equipped with a refractive index (RI) detector (Jasco 830-RI, Madrid, Spain) and UV–visible detector (Jasco 870-UV-visible). A Chrompack column (300×6.5mm) at 60°C, using 5mM sulphuric acid as the eluent, at a flow rate of 0.5mL/min and a sample volume of 20L was used. All the samples were examined in triplicate.
Microbiological analysisYeast, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) counts were performed on Yeast Extract Peptone Glucose [YEPG: 1% yeast extract, 2% peptone (Himedia, Mumbai, India), 2% glucose at pH 3.5, containing 100mgL−1 chloramphenicol (Sigma, St. Louis, Missouri, USA), and 2% agar (Merck, Darmstadt, Germany)] for yeast, De Man Rogosa Sharpe agar [MRS (Merck), containing 0.4% (v/v) nystatin (Merck)] for LAB counting, Glucose Yeast Extract [GYC: 5% glucose (Merck), 1g yeast extract (Merck), 3g calcium carbonate (Merck) for AAB, and 2g agar (Merck) per liter, pH 5.6].
Morphological and biochemical characterizationA total of 358 isolates were subjected to macroscopic and micro-morphological analyses and biochemical assays. Yeast strains were characterized by determining spore formation and by testing fermentation on different carbon sources (glucose, fructose, and sucrose), as described elsewhere.15,17
All bacterial strains were characterized morphologically and biochemically via Gram staining, catalase reaction, motility test, sporulation, and oxidase activity. Bacterial cells were grown on MRS medium and underwent fermentation tests using different carbon sources: glucose, maltose, mannitol, and sorbitol. Furthermore, the bacterial cells were grown on GYC medium. Strains that were gram-negative and oxidase-negative were assessed using the Bactray I and II kits for cluster analysis. For gram-negative but oxidase-positive strains, the Bactray III kit was used.
MALDI-TOF sample preparation, measurement, and data analysisBased on the results from the morphological and biochemical characterization, strains were selected for MALDI-TOF MS analysis.11 Cells were grown on plates using specific culture medium for each taxonomic group, as described above. Cells were incubated at 28°C for 18h and then approximately 7.11logCFU/mL (107 cells) of each strain were aseptically transferred to microtubes. Subsequently, 3μL of organic solution (water/acetonitrile/trifluoro-acetic acid, 50:47.5:2.5) was added to each microtube containing the bacterial isolates, and 3μL of formic acid/acetonitrile (25:75) was added to yeast isolates.
The microtubes were immediately and vigorously vortexed for 1min and then 1μL of the resulting suspension was transferred to a 96-well MALDI flex target plate (Bruker Daltonics, Bremen, Germany). When the liquid phase was almost evaporated, 1μL of matrix solution [saturated solution of α-cyano-4-hydroxy-cinnamic acid (CHCA) in 50% acetonitrile/2.5% trifluoro-acetic acid] was added and the solution was gently mixed.11
An Escherichia coli K12 colony was obtained from the Public Portuguese Culture Collection Micoteca da Universidade do Minho (MUM, www.micoteca.deb.uminho.pt) and used for in situ extraction of proteins, which were used as standard for the MALDI-TOF MS external calibration. Cells of E. coli K12 were grown on Luria-Bertani medium agar (LB; 1% Bacto-tryptone, 0.5% Bacto-yeast extract, and 1% NaCl per liter) at 37°C for 18h, as previously described by Lima et al.11 Briefly, approximately 1μg of cellular material from a single E. coli K12 colony was transferred from the plate to the MALDI flex plate, and CHCA matrix solution was added followed by gentle mixing. Each MALDI-TOF sample was spotted in triplicate to evaluate reproducibility. Samples were then analyzed in a MALDI-TOF microflex LT spectrometer (Bruker Daltonics, Bremen, Germany), using the MALDI Biotyper 3.0 automatic system. For MALDI-TOF microbial identification, reference strains were from the Culture Collection of Agricultural Microbiology (CCMA-UFLA, https://sites.google.com/site/ccmaufla/).
Assessment of metabolic activity by Biospeckle LaserDaily analyses of kefir grains were done by Biospeckle Laser to observe the biological activity. The grains of kefir were illuminated by a HeNe laser, wavelength of 632nm, and 10mW power, enlarged by a plane concave lens in order to cover the entire sample. The interference patterns formed on them were captured by a CCD camera 640×486 pixels, with a shutter speed of 1/60s and in a rate of 0.08s creating a collection of 128 images. The analysis of the images, from the laser illumination, was performed according to Guedes et al.8 The kefir grains were illuminated by a laser HeNe 17mW 632nm and analyzed by the numerical Speckle Laser method. Organoleptic properties were evaluated individually via the senses, including taste, sight, smell, and touch. Alcoholmeter was used for alcohol, the titration method for acidity and sugars by Fehling's solution
Vinegar treatmentThe kefir apple vinegar was then filtered through diatomaceous earth, bottled and pasteurized at 65°C for 20min. Samples were analyzed by optical microscopy (National Optical 131 Microscope) to evaluate the clarity of the vinegar. Analyses (organoleptic evaluation, real-alcoholic degree, volatile acidity and total sugar) were performed following the “operating manual of beverages and vinegars” as instruction No. 24.
Sensory evaluationThe final vinegar was evaluated in a sensory test. The tasters were asked to indicate how much they liked or disliked each product on a 9-point hedonic scale (9=like extremely; 1=dislike extremely) according to overall acceptability18 by twenty-five trained tasters, both males and females, 25–35 years of age (students and staff of the Biology Department, Federal University of Lavras, Brazil). The evaluation of the appearance, color and odor attributes were also conducted. Randomized, refrigerated (10°C) 10mL samples were served in clear, tulip-shaped glasses with a volume of 50mL. These samples were marked with a three-digit random number and covered with Petri dishes.
Statistical analysesEach fermentation process (alcoholic and acetic) was conducted in duplicate and the mean values±standard deviations are reported. The Tukey's test was performed using Statgraphics Plus for Windows 4.1 software (Statistical Graphics Corp. Software – (Free download)) to evaluate the statistical significance (level of p<0.05).
ResultsMicrobiological analysisMicrobiological analysis is in Fig. 1. LAB counts started with a population of 8.10logCFU/mL (approximately 108 cells) on the second day of fermentation, there was a slight decrease, where the population was 7.55logCFU/mL (107 cells). The largest LAB population was observed on the fourth day, where 8.45logCFU/mL was reached, coming to the end of the fifth day with a population of 8.22logCFU/mL. According to the data presented, the population of LAB performed between 7.73logCFU/mL 107 and 8.522logCFU/mL 108 cells throughout the fermentation. The acetic fermentation was divided into two treatments, one conducted with 10% inoculum (treatment 1) and another 20% (treatment 2). At the beginning of acetic fermentation, the population of LAB was the same for both treatments, 8.22logCFU/mL (108 cells), the second to the fifth day, the population has remained stable, with 108 cells. The final fermentation shows population of 9.75logCFU/mL-(treatment 1) and 10.73logCFU/mL (treatment 2). The initial yeast population was of 4.56logCFU/mL, increasing in third day to 7.97logCFU/mL (107 cells). The population declined in the fourth day to 7.89logCFU/mL. In the treatment 1 of acetic fermentation the yeast population increased to 8.81logCFU/mL. In the sixth day the population decrease to 3.98logCFU/mL. The treatment 2 was similar to treatment 1. AAB were found in both fermentations. At the beginning of the alcoholic fermentation the population was low (4.01logCFU/mL) increasing to 6logCFU/mL (treatment 1) and 9logCFU/mL (treatment 2) in final fermentation. In both acetic fermentation treatments (1 and 2) the population of AAB increased to 109 and 108 cells, respectively.
A total of 358 organisms were isolated, and 31 (9%) were yeasts and 327 (91%) were bacteria (divided into lactic and acetic bacteria's). The isolates were identified by MALDI-TOF MS technique (Fig. 2). According to Fig. 2a, the beginning of fermentation (0h) showed higher population of Lactobacillus paracasei (106CFU/mL). Saccharomyces cerevisiae, Lactobacillus plantarum, Acetobacter pasteurianus and Acetobacter syzygii were found in smaller quantities (105CFU/mL) at the beginning of the alcoholic fermentation. These microorganisms were found throughout the alcoholic and acetic fermentation (10% and 20% kefir). According to Fig. 2b, at the end of 264h (11 days) of fermentation the population of S. cerevisiae, remained the same initial population of 105CFU/mL. The population of L. paracasei and L. plantarum increased, compared to the initial time reaching 108CFU/mL and 107CFU/mL, respectively. The population of A. pasteurianus and A. syzygii had the highest increase 109CFU/mL at the end of acetic fermentation.
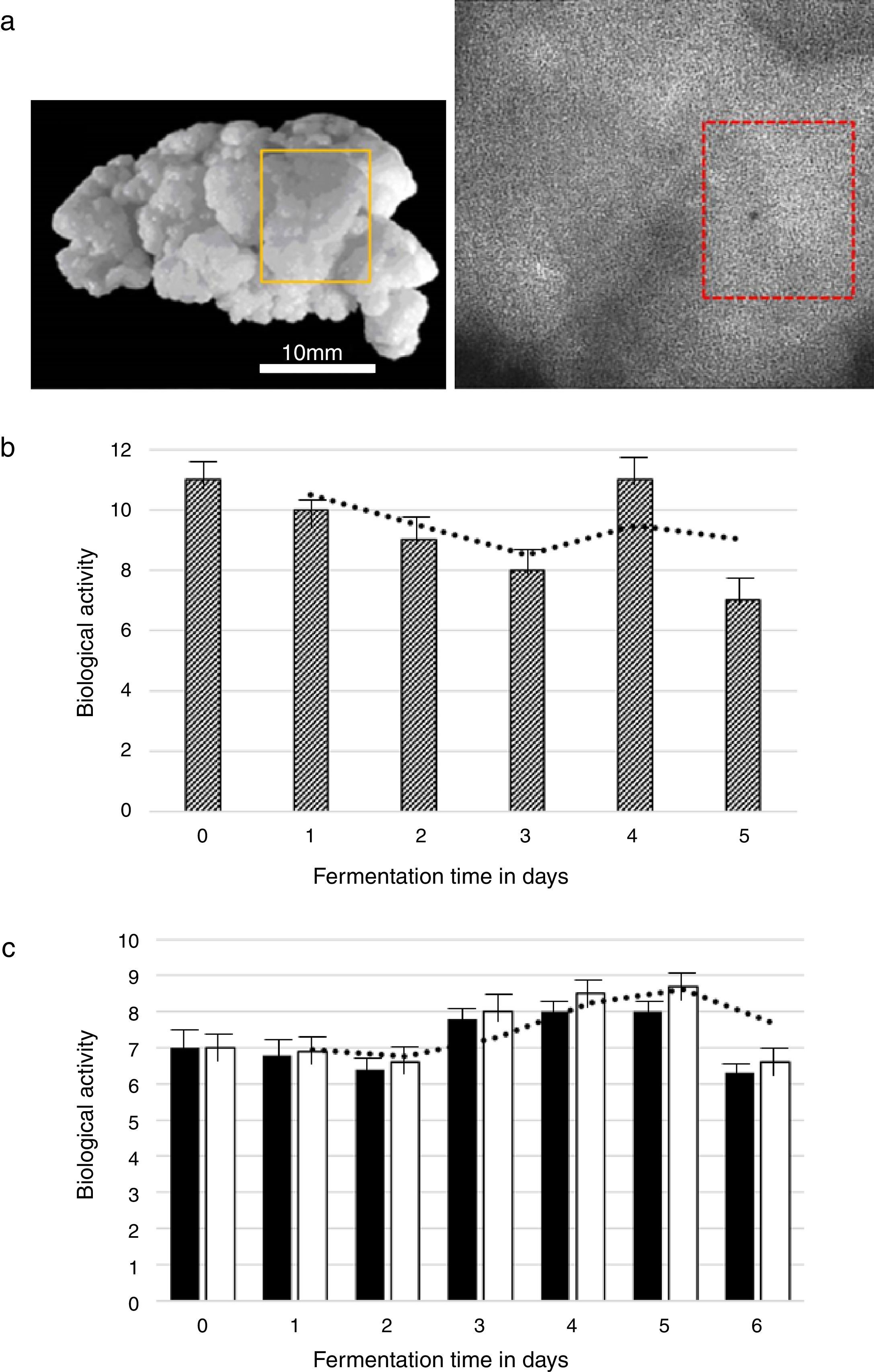
Kefir grains were analyzed daily using Biospeckle Laser and the biological activity value obtained was 14.21. This value was considered the initial fermentation time (0h). The test of sensorial analyses of the grains of kefir can be seen in Fig. 3, where in Fig. 3a the image of the kefir grain is presented with an illustration of a window where the laser was illuminated and the images assembled.
The result of the biological activity test is presented in Fig. 3(b and c). In the alcoholic fermentation the biological activity of the kefir grains decreased in the early days of the fermentation process (Fig. 3b). The decrease in biological activity persisted until the third day of fermentation. On the fourth day the biological activity of kefir grains in apple must increased to 11.51. On the last day of fermentation the biological activity of kefir grains decreased to value 7.12.
The acetic fermentation occurred simultaneously as the alcoholic fermentation. In the acetic fermentation the biological activity of the kefir grains decreased in the early days of the fermentation process for treatment 1 and 2 (Fig. 3c). The decrease in biological activity persisted until the third day of fermentation. On the fifth day, the biological activity of kefir grains increased to 8.01 (treatment 1) and 8.79 (treatment 2). On the last day of fermentation, the biological activity of kefir grains decreased to 6.36 (treatment 1) and 6.77 (treatment 2).
Chemical analysisApple alcoholic must productionThe apple alcoholic must was produced and whereas lactic, acetic, citric, succinic and malic acids were found in the apple alcoholic must (1.37, 0.92, 1.37, 1.71 and 1.00gL−1, respectively) (Table 1). Tartaric, butyric and propionic acids were not detected.
Chemical and physical characteristics of the apple alcoholic must.
| Period of fermentation | Acids (gL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Lactic acid | Citric acid | Tartaric acid | Butyric acid | Succinic acid | Malic acid | Oxalic acid | Propionic acid | |
| 0h | 0.11±0.01a | 0.10±0.01a | 1.99±0.01a | n.d. | n.d. | 0.25±0.01a | 0.11±0.01a | 0.09±0.01a | n.d. |
| 120h | 0.92±0.01b | 1.37±0.01b | 1.37±0.01b | n.d. | n.d. | 1.71±0.01b | 1.00±0.02b | 0.29±0.01b | n.d. |
| Period of fermentation | Sugars (gL−1) | |||
|---|---|---|---|---|
| Sucrose | Glucose | Fructose | Reducing sugars | |
| 0h | 92.60±0.11a | 12.60±0.08a | 23.47±0.21a | 36.46±0.31a |
| 120h | 0.09±0.01b | n.d.b | 0.06±0.01b | 0.04±0.01b |
| Period of fermentation | Alcohols (gL−1) | ||
|---|---|---|---|
| Ethanol | Glycerol | Methanol | |
| 0h | n.d.a | n.d.a | n.d. |
| 120h | 96.78±0.41b | 5.10±0.01b | n.d. |
| Period of fermentation | pH | Brix |
|---|---|---|
| 0h | 4.05±0.02a | 13.5±0.02a |
| 120h | 3.82±0.02a | 4.37±0.02b |
The data are the mean values of triplicate measurements±standard deviation. Different letters in the row and column indicate significant differences (p<0.05). n.d., not detected.
The fermentation temperature selected was 28°C and the kefir grains were chosen as the inoculum for the fermentation process. An inoculum of 10% kefir inoculum was sufficient because the ethanol concentration at 120h of fermentation was approximately 97.0g/L (∼12.3GL (Gay Lussac)) (Table 1). Small amounts of sucrose (0.09g/L), fructose (0.06g/L) and reducing sugars (0.04g/L) were detected in the apple alcoholic must. Methanol was not detected in the samples of apple alcoholic must. The decrease in the total soluble solids and the increase in the content of ethanol during the fermentative process are shown in Table 1. The pH value was 3.82 and °Brix was 4.37 in the final alcoholic must. Oxalic acid was detected in the apple alcoholic must before the aerobic phase of acetic fermentation. The concentration of glycerol was low (5.10g/L) (Table 1). The values of the kinetic parameters obtained from the apple alcoholic must are described as ethanol productivity (Yp/s)/g 0.47g, yield glycerol (Yg/s) 0.05g/g, ethanol yield (qp) 0.37g/L/h and fermentation efficiency (Ef) 93.12%.
After the alcoholic fermentative process, the alcoholic must was processed as described in the methodology for the subsequent production of apple vinegar.
Kefir vinegarThe kefir vinegar was produced and lactic, citric, succinic and malic acids were similar in the treatment 1 and treatment 2 (Tables 2 and 3). The yield of acetic acid in the kefir vinegars was ∼79%. The total acetic acid concentration was ∼41.00gL−1, reaching the required standard for the Brazilian legislation accepts it as vinegar (4.0% acetic acid) (Brasil, 20094). In our study, five flavor-active compounds, including three alcohols, one acetate and one aldehyde were identified (Table 4). The values of these compounds were similar for the kefir vinegars in treatment 1 and treatment 2. The kefir vinegars showed the clear appearance and had a good color (pale yellow).
Chemical and physical characteristics of the apple kefir vinegar in treatment 1 (10% kefir inoculum).
| Period of fermentation | Acids (gL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Lactic acid | Citric acid | Tartaric acid | Butyric acid | Succinic acid | Malic acid | Oxalic acid | Propionic acid | |
| 0h | 0.96±0.01a | 1.12±0.01a | 1.39±0.01a | n.d. | n.d. | 5.52±0.01a | 1.07±0.01a | 0.22±0.01a | n.d. |
| 144h | 41.66±0.31b | 1.11±0.01a | 6.67±0.01b | n.d. | n.d. | 5.60±0.01a | 7.02±0.01b | 0.18±0.01a | n.d. |
| Period of fermentation | Alcohols (gL−1) | ||
|---|---|---|---|
| Ethanol | Glycerol | Methanol | |
| 0h | 94.02±0.01a | 5.32±0.01a | n.d. |
| 144h | 1.76±0.01b | 5.36±0.01a | n.d. |
| Period of fermentation | PH |
|---|---|
| 0h | 3.08±0.01a |
| 144h | 2.74±0.11b |
The data are the mean values of triplicate measurements±standard deviation. Different letters in the same column indicate significant differences (p<0.05). n.d., not detected.
Chemical and physical characteristics of the apple kefir vinegar in treatment 2 (20% kefir inoculum).
| Period of fermentation | Acids (gL−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Lactic acid | Citric acid | Tartaric acid | Butyric acid | Succinic acid | Malic acid | Oxalic acid | Propionic acid | |
| 0h | 0.96±0.01a | 1.11±0.01a | 1.42±0.01a | n.d. | n.d. | 4.59±0.01a | 1.01±0.01a | 0.16±0.01a | n.d. |
| 144h | 40.06±0.31b | 1.09±0.01a | 5.27±0.01b | n.d. | n.d. | 4.90±0.01a | 6.04±0.01b | 0.11±0.01a | n.d. |
| Period of fermentation | Alcohols (gL−1) | ||
|---|---|---|---|
| Ethanol | Glycerol | Methanol | |
| 0h | 94.02±0.01a | 4.82±0.01a | n.d. |
| 144h | 2.16±0.01b | 4.76±0.01a | n.d. |
| Period of fermentation | PH |
|---|---|
| 0h | 3.09±0.01a |
| 144h | 2.42±0.11b |
The data are the mean values of triplicate measurements±standard deviation. Different letters in the same column indicate significant differences (p<0.05). n.d., not detected.
Volatile compounds in the kefir vinegar identified by GC-FID.
| Compounds | Final concentration (mgL−1) | |
|---|---|---|
| Treatment 1a | Treatment 2b | |
| Aldehyde | ||
| Acetaldehyde | 21.33±0.24a | 20.33±0.54a |
| Alcohols | ||
| 1-Hexanol | 9.01±0.06a | 9.38±0.56a |
| 2-Methyl-1butanol | 7.01±0.06a | 7.38±0.56a |
| 3-Methyl-1-butanol | 12.76±10.08a | 12.23±12.68a |
| Acetate | ||
| Ethyl acetate | 9.24±0.32a | 9.94±0.45a |
The data are the mean values of triplicate measurements±standard deviation. Different letters in the same line indicate significant differences (p<0.05).
Sensory analyses were conducted to kefir vinegar (10% and 20% of kefir grain inoculum) and the commercial apple vinegar. Using the check-all-that-apply (CATA) testing, the vinegars were subjected to sensory evaluation to assess their acceptance and preference. Vinegars at 10%, 20% and commercial had good acceptance (scores 7.1, 7.4 and 7.2, respectively). Consumers classified vinegars in “liked slightly”. Vinegars were evaluated as “sour, acetic acid, limpid and translucent appearance” (Fig. 4). No significant differences observed between all evaluated vinegars. The apple kefir vinegars produced were well accepted by the evaluators (95% of consumers would buy kefir vinegar).
DiscussionThe apple pulp was processed to obtain a fermentable must from which the alcoholic must was produced. The inoculum for the fermentation process (kefir grains) was sufficient because the ethanol concentration at 120h of fermentation was approximately 97.0gL−1 (∼12.3GL (Gay Lussac)). These results are comparable to that obtained by Dias et al.,5 who found a value of 89.5gL−1 when fermenting cocoa pulp with an initial 22 °Brix.
Small amounts of sucrose, glucose, fructose and reducing sugars were detected in the final alcoholic must of apples. The rapid decrease in the sugar content and increase in the concentration of ethanol during an inoculated fermentation (74.78gL−1) was also observed by Domizio et al.6 during fermentation of grape must under controlled temperature conditions. Nurgel et al.19 found that fermentation of non-pasteurized grape using the selected yeast (6.7logCFU/mL) was completed in 6 days, while indigenous fermentation lasted 10 days. These authors also found that the pH values of the fermenters were similar (approximately 3.08).
Acetic acid was formed during the fermentation of apple alcoholic must. Acetic acid provides a pleasant taste and inhibits the development of undesirable or pathogenic microorganisms, due to the high acidity. During the fermentation process, the citric acid concentration was almost the same as at 0h; the citric acid concentration decreased of 1.99–1.37g/L. It is possible that citric acid was metabolized by S. cerevisiae (present in kefir grains) as a carbon and energy source; which have the ability to ferment/assimilate this organic acid, causing the increase in the pH value.15 Citric and malic acids are commonly found in fermented fruit beverages, where they act as preservatives with antimicrobial properties.20 The organic acids produced by yeast and bacterial species contribute to the refreshing flavor, unique aroma and the texture, in addition to controlling the growth of food-spoilage microorganisms.2 The concentration of glycerol in the apple alcoholic must was low (5.10g/L). This value was consistent with the value of less than ∼10.0g/L suggested by Dias et al.5 to confer the characteristic body and texture of the beverage. Glycerol is the main secondary product of alcoholic fermentations by S. cerevisiae, which is present in the kefir inoculum in this study. The glycerol concentration of approximately 5.1g/L was close to the values of 6 and 10g/L suggested by Vogt et al.24 to confer the characteristic body and texture of the beverage.
A total of 358 organisms were isolated, and 31 were yeasts and 327 were bacteria (divided into lactic and acetic bacteria). The isolates were identified by MALDI-TOF MS technique (Fig. 2). The population were L. paracasei, S. cerevisiae, L. plantarum, A. pasteurianus and A. syzygii. These microorganisms are commonly found in kefir grains.23,13–17 Rapid diagnosis of microorganisms is decisive to guarantee adequate identification in samples. Culture methods are precise and sensitive, but rather slow. New resources are available to enable faster diagnosis, and the most promising is MALDI-TOF MS technology applied to microbiological diagnosis. Times as fast as 10–15min to etiological diagnosis are possible after a positive blood culture result.20
Kefir grains were also analyzed by the Biospeckle Laser technique. The results presented the activity of the kefir grains during fermentation (Fig. 3), where it was possible to observe the sensitivity of the technique to follow the expected activity. The proposed technique is simple, relatively cheap and fast, easy to implement, and requires only a laser and standard digital imaging processing hardware components.8 The application of Biospeckle Laser method to the kefir viability detection was one of objectives of this work, but the optical technique could also be applied to characterize the kefir grains in other types of processes. Biospeckle Laser technique showed an efficient tool for monitoring and quantifying biological activity of kefir grains, showing the viability time of these grains in a fermentation process, which demonstrates the technique potential for evaluating and monitoring of kefir grains in production of kefir vinegars in industrial scale.
Volatile compounds are important contributors to the flavors of beverages, and are responsible for different desirable sensory characteristics.14–16 Previous studies have shown that the formation of volatile higher alcohols and esters during kefir fermentation is influenced by the composition of the medium.15 Five flavor-active compounds, including three alcohols, one acetate and one aldehyde, were identified by gas chromatography coupled with flame ionization detection (GC-FID) in kefir vinegars. The concentration these compounds were particularly high (Table 4) in comparative with study of15 showing the biochemical changes and volatile compound formations during the production of novel whey-based kefir beverages and traditional milk kefir. The values of these compounds were similar for the kefir vinegars in treatment 1 and treatment 2; i.e., fermentation with 10% and 20% inoculum kefir produced similar quantities of volatiles. The higher alcohols identified during kefir vinegar fermentations were 2-methyl-1-butanol (active amyl alcohol) and 3-methyl-1-butanol (isoamyl alcohol). The volatile higher alcohol identified, 2-methyl-1-butanol is produced during the catabolism of the branched chain amino acid (BCAA) isoleucine, or is synthesized de novo during the biosynthesis of the AAB.1 3-methyl-1-butanol has a positive influence on the aroma of the fermented beverage. On the contrary, increased concentration of these alcohols, having a volatile description of “coconut-like”, “harsh” and “pungent”, can contribute negatively to the product aroma.1 Only one ester, characterized by fruity attributes, namely ethyl acetate was detected in kefir vinegar. Kourkoutas et al.,10 showed that yeasts were capable of producing ethyl acetate from fermentation process in a wide range of concentrations (from traces to 95mgL−1). According to these authors, such concentrations are typical of fermented beverages. Acetaldehyde, which imparts nutty and pungent aromas, was also found in kefir vinegar.
The taste of the filtered vinegar was fruity and a nice smell was noted. Various organic acids in vinegar are important for imparting a suitable taste and flavor. The total acetic acid concentration in the apple vinegar produced using kefir grains inoculum was ∼41.0g/L, which is approximately two times the concentration in onion vinegar (∼25.0g/L) produced using cell-free of Acetobacter aceti.9 The kefir grains were highly satisfactory for acetic fermentation using apple alcoholic must as the medium. This system achieved higher acetic acid productivity than that of fermentations by free cells. Our results indicated that the use of kefir grains might be one of the best fermenting strategies employed to overcome substrate limitation and achieve high product yield.
The period for the oxidation of ethanol to yield acetic acid was 140h. According to the stoichiometry of reaction that converts ethanol to acetic acid, 1.0g of ethanol can provide 1.304g of acetic acid.12 In industries, the conversion of 1.0g ethanol to 1.0g of acetic acid (yield of 76.6%) is considered economic.12 The production was favorable, reaching values of ∼79.0% yield. Therefore, we can conclude that the evaporation of volatile compounds was low, which might be attributed to using appropriate aeration and thermal conditions. Methanol was not detected in the apple kefir vinegar and the glycerol concentration remained low in both treatments.
Based on the analyses of the vinegar and the limits required by law,9 the final product of kefir vinegar has an acceptable acetic acid level, of approximately 5.0% (w/v), and an ethanol concentration of less than 1.0% (v/v). The kefir vinegar proved to be a very promising product.
ConclusionsVinegar could be successfully produced from apple alcoholic must using kefir grains. This is the first study to produce kefir vinegar. The chemical analyses revealed that kefir vinegar has high contents of organic acids, which add functional value to the vinegar. Kefir vinegar had a good performance during the acetylation (∼79%), reaching the required standard for the Brazilian legislation accepts it as vinegar (4.0% acetic acid).4 Microorganisms were found previously described in kefir grains and the microbiota remained standardized during the fermentation process. There was no microbial contamination during the fermentation process. A new methodology to measure the metabolic activity of kefir grains by Biospeckle Laser was presented. This fact facilitates the microbiological control over a fermentation process. Kefir grains showed efficiency in the production of apple vinegar. Kefir vinegar was well accepted by sensory analysis.
The novel technology proposed use of kefir grains for the production of vinegar was successfully done. The key point for industrial application of the proposed technology is the promotion of fermentation by an immobilized-cell biomass (kefir grains) that eliminate the use of centrifugal separators, which have a high energy demand and require high industrial investment.
Conflicts of interestNo conflict of interest to declare.
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for financial support and scholarships.