Lignocellulose-derived inhibitors have negative effects on the ethanol fermentation capacity of Saccharomyces cerevisiae. In this study, the effects of eight typical inhibitors, including weak acids, furans, and phenols, on glucose and xylose co-fermentation of the recombinant xylose-fermenting flocculating industrial S. cerevisiae strain NAPX37 were evaluated by batch fermentation. Inhibition on glucose fermentation, not that on xylose fermentation, correlated with delayed cell growth. The weak acids and the phenols showed additive effects. The effect of inhibitors on glucose fermentation was as follows (from strongest to weakest): vanillin>phenol>syringaldehyde>5-HMF>furfural>levulinic acid>acetic acid>formic acid. The effect of inhibitors on xylose fermentation was as follows (from strongest to weakest): phenol>vanillin>syringaldehyde>furfural>5-HMF>formic acid>levulinic acid>acetic acid. The NAPX37 strain showed substantial tolerance to typical inhibitors and showed good fermentation characteristics, when a medium with inhibitor cocktail or rape straw hydrolysate was used. This research provides important clues for inhibitors tolerance of recombinant industrial xylose-fermenting S. cerevisiae.
In recent years, the production of ethanol for fuel purpose has increased rapidly.1,2 Lignocellulosic biomass, such as agricultural or forestry residues, is regarded as the most promising material for fuel ethanol production because it is both abundant and renewable. Saccharomyces cerevisiae is commonly used for the production of fuel ethanol due to its rapid hexose sugar consumption and excellent ethanol tolerance. Wild S. cerevisiae strains cannot ferment xylose, which is the second most abundant sugar in lignocellulosic hydrolysates. Heterologous expression of xylose reductase (XR) and xylitol dehydrogenase (XDH), or xylose isomerase (XI), along with overexpression of xylulokinase (XK), confers the ability to utilize xylose.3,4 However, various toxic compounds generated during the pretreatment process of lignocellulosic biomass inhibit cell growth and ethanol production.1,2,5,6 The inhibitory compounds present in the hydrolysates can be classified into weak acids (primarily acetic, formic, and levulinic acids), furan derivatives (furfural and 5-hydroxymethyl-2-furaldehyde (5-HMF)), and phenolic compounds (syringaldehyde, vanillin, and other phenols). Acetic acid is formed by the de-acetylation of hemicelluloses, whereas formic and levulinic acids are degradation products of 5-HMF.1,7 Furfural and 5-HMF are products of the dehydration of pentose and hexose, respectively.1,7 Phenolic compounds are formed during lignin breakdown and the degradation of carbohydrate during acid hydrolysis.8 The levels of inhibitory compounds present in hydrolysates depend on the type of biomass and the pretreatment method.9
Previous studies have suggested that S. cerevisiae strains show different tolerances to inhibitors when different carbon sources are used.5,6,10–12 Strains show better tolerance to toxic compounds when glucose is used as the sole carbon source, than when xylose is used.5,6,10–12 Because glucose and xylose are both present in lignocellulosic hydrolysates, it is essential to study the inhibitor tolerance of strains during co-fermentation of glucose and xylose. However, studies about the effects of inhibitors on glucose and xylose co-fermentation are limited.13 Most of the studies on the inhibitor tolerance of S. cerevisiae have been conducted under the conditions using glucose or xylose as the sole sugar, and most of them have focused on the effect of one or a limited number of inhibitors on the laboratory strains.10,14–18 The study on the additive effects of inhibitors is very limited.14 In addition, only limited studies have investigated the inhibitor tolerance of industrial strains. Due to the distinct metabolic backgrounds of the laboratory strains and the industrial strains, industrial strains generally exhibit better inhibitor tolerance than laboratory strains, as reflected in cell growth, sugar consumption, and ethanol yield.2,14,16,18 The results obtained in laboratory strains may not be applicable to industrial strains.
In our previous study, a xylose-fermenting flocculating industrial S. cerevisiae strain, named NAPX37, was genetically engineered from the flocculating industrial strain KF-719,20 via heterologous expression of the genes encoding XR and XDH.21 The batch and continuous fermentation study suggested that the strain NAPX37 had excellent xylose fermentation capacity. Meanwhile, the strain also displayed good inhibitor tolerance during the fermentation using xylose as the sole sugar.21–23 However, the response of the recombinant strain to various inhibitors in different carbon source may distinct. To further evaluate the industrial application potential of the strain NAPX37, the tolerance to inhibitors, including acetic acid, formic acid, levulinic acid, furfural, 5-HMF, syringaldehyde, vanillin, phenol, and their mixtures, of the strain during glucose and xylose co-fermentation was systematically evaluated via batch fermentation. The results of the present study could also provide a reference point for optimizing the ethanol production process and for engineering industrial strains of S. cerevisiae with improved capacity for lignocellulosic bioethanol production.
Materials and methodsStrain and mediumThe recombinant xylose-utilizing industrial S. cerevisiae strain NAPX37 was used21 in this study. Yeast strain was routinely cultivated at 30°C in 2% YPD medium (20g/L peptone, 10g/L yeast extract, and 20g/L glucose) with 2% agar. For pre-cultivation, 2% or 5% YPD (20g/L peptone, 10g/L yeast extract, and 20 or 50g/L glucose) were used. Batch fermentation was performed using 10% YPDX (20g/L peptone, 10g/L yeast extract, 60g/L glucose, and 40g/L xylose, pH 5). Inhibitors dissolved in distilled water were filter-sterilized, and then quantitatively added to the cooled, sterilized fermentation medium.
Effects of inhibitors on glucose and xylose co-fermentationAfter activation on 2% YPD plate for 24h, yeast cells were pre-cultured under aerobic conditions at 30°C for 16h in 5% YPD medium. Ten milliliter pre-cultivation broth was inoculated into a 300mL flask with 90mL fermentation medium containing specific concentrations of inhibitors (initial cell concentration was approximately 0.2g dry cell weight/L). Fermentation was performed under microaerobic conditions at 35°C for 48h with an agitating speed of 200rpm using a HS-6DN magnetic stirrer (AS ONE, Japan). Five milliliter broth was sampled periodically to analyze cell density and the concentrations of sugar, ethanol, and by-products. All fermentation experiments were repeated twice.
Since the concentrations of inhibitors varied in different lignocellulosic hydrolysates, we set the same type inhibitors at the same concentrations, but different type inhibitors with different concentration ranges, based on the inhibitor concentrations reported in literatures.11,16–18,24–27 For evaluating the effects of single inhibitors, each of the weak acids was set at 40, 80, 120, and 160mM, and each of the furans or phenols was set at 5, 10, 20, and 30mM. For investigating the additive effects, we had considered the inhibitors concentration in real lignocellulosic hydrolysates, and selected five typical inhibitors: 20mM acetic acid, 10mM formic acid, 10mM levulinic acid, 5mM furfural, and 1mM vanillin. Five sets of fermentation were performed and the inhibitor types 1 to 5 were added. To evaluate the effects of the admixture of inhibitors on ethanol fermentation, 23.31mM acetic acid, 8.69mM formic acid, 5.21mM levulinic acid, 6.24mM furfural, 4.76mM HMF, 0.22mM syringaldehyde, 0.26mM vanillin, and 0.07mM phenol were mixed and added into the fermentation medium.
Fermentation of lignocellulosic hydrolysateLignocellulosic hydrolysate of rape straw was prepared by diluted sulfuric acid treatment, as described previously.28 Main inhibitors in the hydrolysate were acetic acid, formic acid, furfural, and 5-HMF, and the concentrations were 64.45mM, 1.06mM, 7.29mM, and 7.61mM, respectively. Besides, the concentration of total phenolic compounds was 0.3g/L. Glucose and xylose in the hydrolysate were adjusted to approximately 60g/L and 40g/L, respectively. Other sugars in the hydrolysate were very limited, 0.92g/L of mannose, 1.46g/L of arabinose, and 1.13g/L of galactose. The pH of the hydrolysate was adjusted to 5 before fermentation. The fermentation was performed under microaerobic conditions at 35°C for 48h with an agitating speed of 200rpm using a HS-6DN magnetic stirrer (AS ONE, Japan).
Analytical methodFermentation broth was centrifuged at 9000×g for 3min. The cell pellet was washed twice with distilled water and then dispersed in 50mM EDTA to measure the OD660. The supernatant was used to analyze the concentrations of sugars and products. Glucose and xylose concentrations were determined using a LC-10AD VP HPLC (Shimadzu, Japan) equipped with a fluorescence detector (RF-10AXL) under the following conditions: column, Shimpack ISA-07/S2504 (4mm i.d.×25cmL); temperature, 65°C; eluants, 0.1M borate buffer (pH 8.0) (buffer A) and 0.4M borate buffer (pH 9.0) (buffer B). Eluants were used at a flow rate of 0.6mL/min with an gradient from 100% buffer A (0% buffer B) to 0% buffer A (100% buffer B) at a changing rate of 2%/min.20 Ethanol concentration was measured using a GC 353B gas chromatograph (GL sciences, Japan) equipped with a FID detector and a TC-1 capillary column (0.25mm i.d.×60mL; d.f.: 0.25μm). The GC was run with a 50°C oven temperature and 180°C injection and detector temperatures using He as the carrier gas and H2 as the flaming gas. Isopropanol was used as the internal standard.20 Xylitol concentration was assayed using a SCL-10A VP HPLC (Shimadzu, Japan) equipped with an AMINEX HPX-87H column (300×7.8mm) (Bio-Rad, USA) and a RID-10A refractive index detector (Shimadzu). The HPLC system was operated at 65°C, with a mobile phase of 5mM H2SO4 at a flow rate of 0.6mL/min.22 Furfural, 5-HMF, acetic acid, and formic acid concentrations were determined using a LC-10AD VP HPLC (Shimadzu, Japan) under the following conditions: temperature, 65_C; mobile phase, 0.5mM H2SO4 at a flow rate of 0.8mL/min.28 The concentration of the total phenolic compounds was determined using a Folin–Ciocalteu assay as described by Guo et al.,29 with minor modification. One millliter of the hydrolysate was mixed with 5mL of distilled water, 1.5mL of Folin–Ciocalteu reagent (Sigma–Aldrich), and 2mL of 10% (w/v) Na2CO3. The mixture was diluted to 25mL, and then incubated for 30min at 30°C. The absorbance was measured at 765nm by using a spectrophotometer (Shimadzu, Japan). Gallic acid was used as the standard.
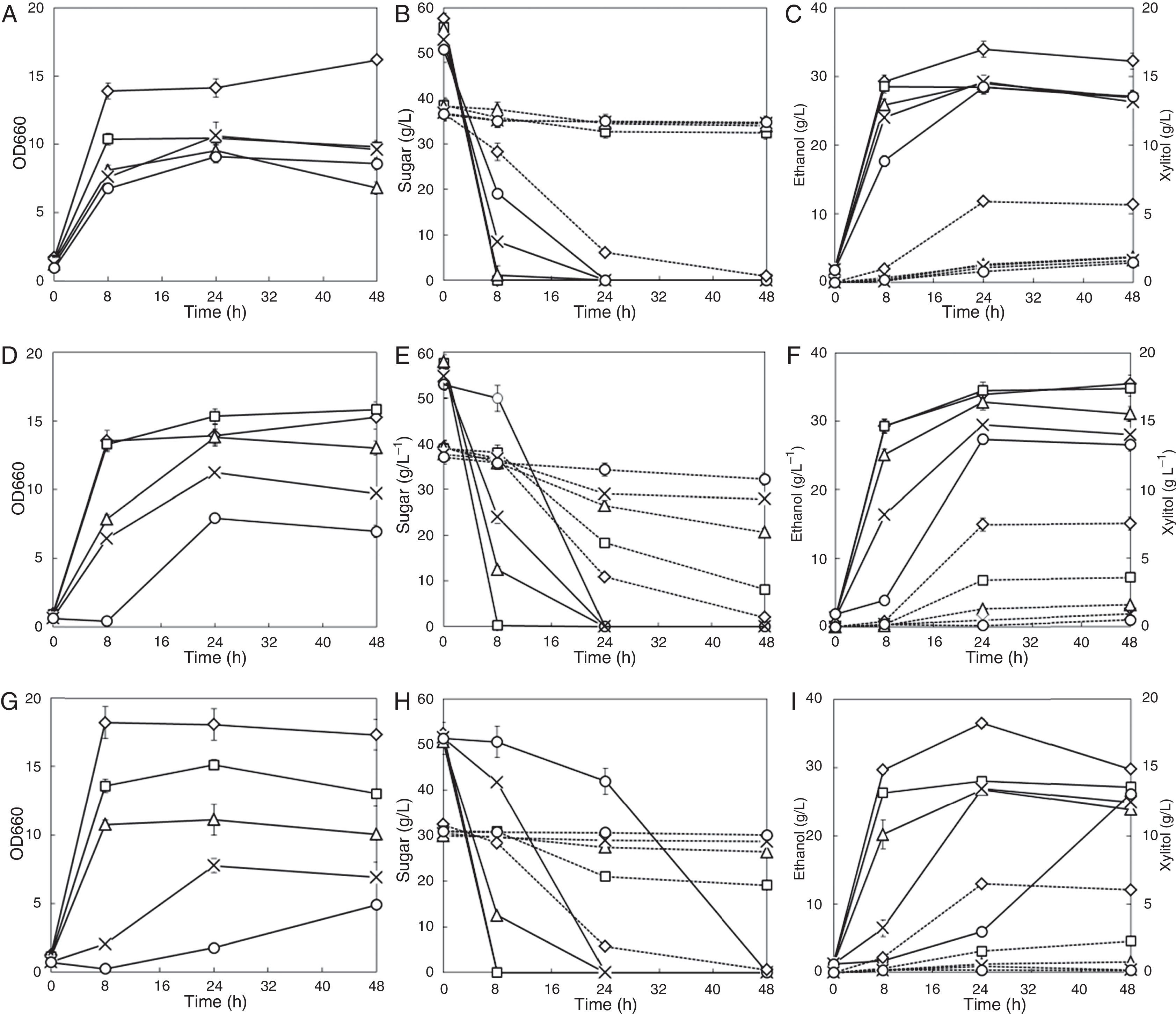
ResultsEffects of single inhibitors on glucose and xylose co-fermentationThe effects of weak acids are shown in Fig. 1. Addition of 40mM acetic acid weakly inhibited cell growth, but the same concentrations of formic acid and levulinic acid decreased cell growth by 25.4% and 25.4%, respectively, during the first 8h of fermentation. As the concentration of weak acids increased, both the cell growth rate and the maximum cell density decreased; this effect was most striking upon the addition of levulinic acid (Fig. 1G). Cell growth did not increase further after 24h of fermentation, upon the addition of 120mM levulinic acid; adding 160mM levulinic acid strongly delayed cell growth (Fig. 1G). Glucose was completely consumed within 8h when the concentration of weak acids was 40mM. The glucose consumption rate decreased by 19.1% and 27.8%, when the concentrations of acetic acid and levulinic acid were increased to 80mM, respectively. However, the same concentration of formic acid did not inhibit glucose fermentation (Fig. 1B, E, H). Increasing the weak acids concentration further to 120mM reduced the glucose consumption rate even more. Adding 160mM acetic acid or levulinic acid slowed glucose fermentation markedly, which was attributable to the very low cell growth under such high-acid conditions (Fig. 1E, H). Among the three weak acids, levulinic acid inhibited glucose fermentation the most, and formic acid had the weakest effect. However, formic acid showed strongest inhibition effect during xylose fermentation stage. The xylose consumption rate in the first 24h decreased by 80.9% when 40mM formic acid existed compared to the inhibitor-free control, while addition of 40mM acetic acid and 40mM levulinic acid reduced the xylose consumption rate by 28% and 63%, respectively. The xylose consumption was negligible when 80mM formic acid was present; the xylose consumption rate was reduced by 55.5% and 90.8% when 80mM acetic acid and 80mM levulinic acid were present, respectively. Although high concentrations of weak acids slowed glucose fermentation, it did not affect the ethanol yield in the first 8h which is the main period of glucose consumption. The presence of acetic acid or levulinic acid reduced the xylitol yield, which partly contributed to the increased ethanol yields.
Effects of weak acids on ethanol fermentation by the strain NAPX37. (A–C) Formic acid; (D–F) acetic acid; (G–I) levulinic acid; symbols: diamonds—0mM, squares—40mM, triangles—80mM, multiplication signs—120mM, circles—160mM; solid line—OD660, glucose and ethanol concentration; dotted line—xylose and xylitol concentration. Values represent the averages of duplicate experiments±SD.
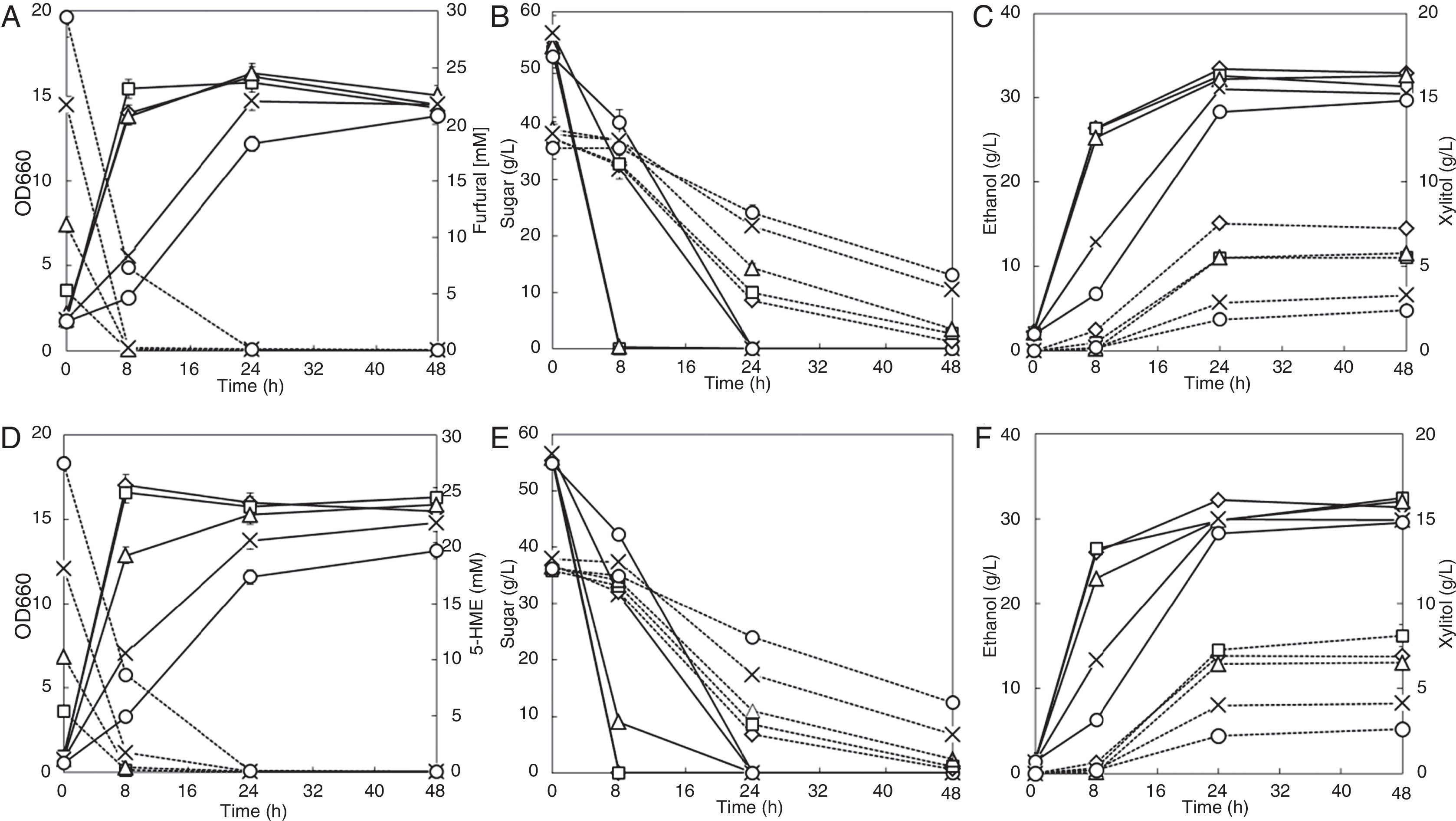
Furfural and 5-HMF showed similar inhibitory effects on cell growth and fermentation (Fig. 2). Cell growth and the maximum cell density decreased when 20mM or 30mM of furfural or 5-HMF was present, which leading to decreased glucose and xylose consumption rate. The xylose consumption rates decreased by 43.3% and 60.0% when 20mM and 30mM furfural existed, respectively, and decreased by 31.8% and 60.0% when 20mM and 30mM 5-HMF were present, respectively, compared to that of the inhibitor-free control. High concentration of furans delayed sugar fermentation perhaps because of the longer time needed for the reduction of furfural and 5-HMF by the strain. The time for the reduction of 30mM furfural or 5-HMF was delayed to 24h (Fig. 2A, D). When 5–20mM furfural or 5-HMF was present, the ethanol yields during glucose-fermentation stage (the first 8h) were slightly higher than that without inhibitors. Five millimolar or 10mM of 5-HMF increased the xylitol yield and reduced the ethanol yield. However, when 20mM or 30mM 5-HMF was present, the ethanol yield increased with reduced xylitol yield.
Effects of furans on ethanol fermentation by the strain NAPX37. (A–C) Furfural; (D–F) HMF; symbols: diamonds—0mM, squares—5mM, triangles—10mM, multiplication signs—20mM, circles—30mM; solid line—OD660, glucose and ethanol concentration; dotted line—furfural, HMF, xylose and xylitol concentration. Values represent the averages of duplicate experiments±SD.
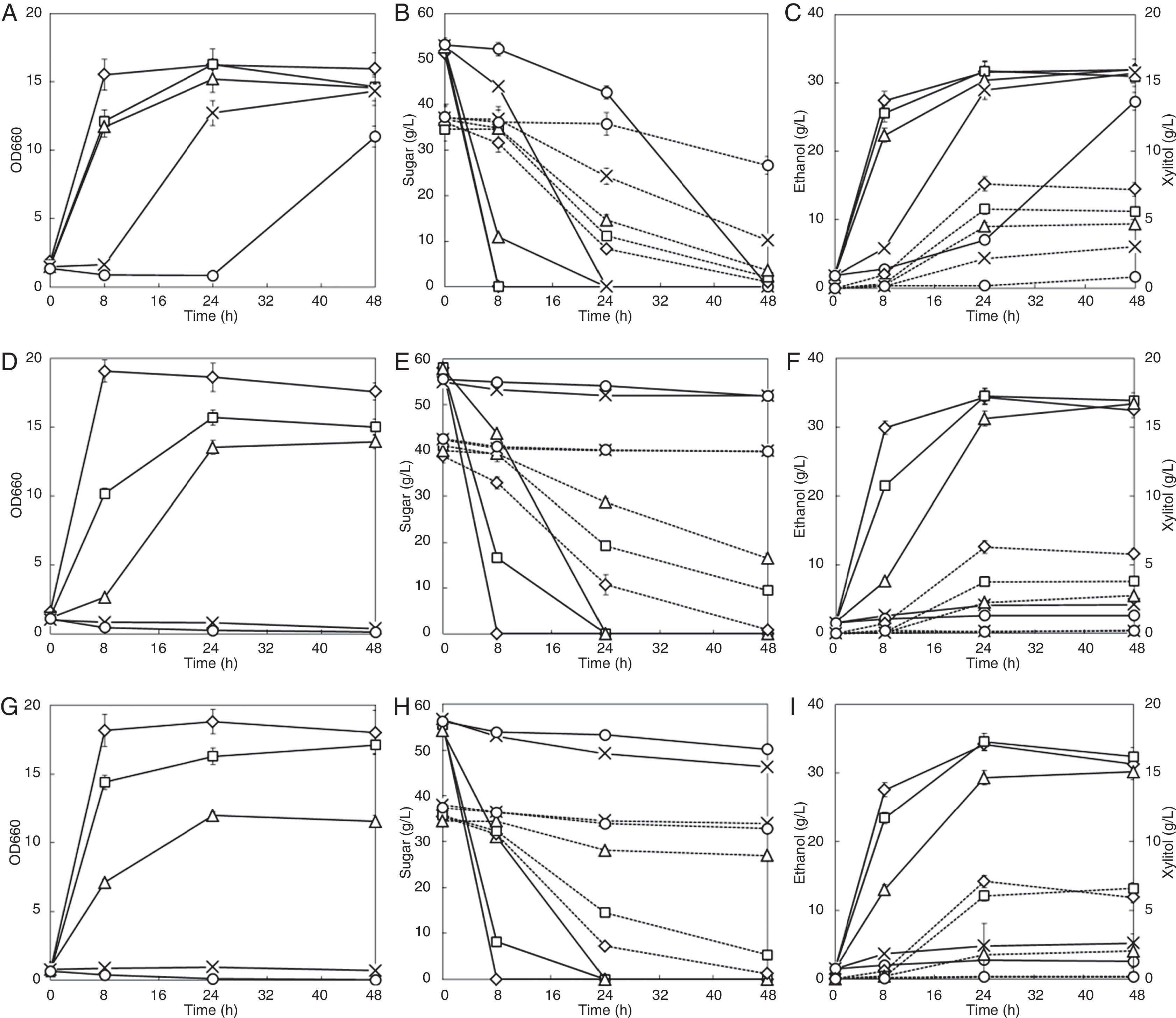
Three phenols showed different inhibitory effect on cell growth and fermentation (Fig. 3). Addition of 20mM and 30mM syringaldehyde significantly delayed cell growth, but the growth recovered after 8h and 24h, respectively. The cell growth was completely inhibited and no growth was observed even after 48h of cultivation when adding 20mM or 30mM vanillin or phenol. The glucose consumption rate did not decrease when adding 5mM syringaldehyde, but reduced by 28.6% and 15.7% when adding 5mM vanillin and 5mM phenol respectively. Adding 10mM vanillin and 10mM phenol severely reduced the glucose consumption rate by 75.6% and 58.6%, respectively. Adding 5mM or 10mM syringaldehyde reduced the xylose consumption rate in the first 24h by approximately 20% (Fig. 3B); however, adding 20mM syringaldehyde reduced it by 54.2%. On the other hand, 5mM or 10mM vanillin or phenol strongly reduced the xylose consumption rate; it reduced by 59.9% and 77.4% when 10mM vanillin and phenol was added respectively. The xylitol yield decreased with increasing concentrations of syringaldehyde and vanillin, which partially contributed to the increased ethanol yields. However, similar to 5-HMF, phenol increased the xylitol yield. Although vanillin showed the strongest inhibitory effect on glucose fermentation, phenol had the strongest inhibition on xylose fermentation, among the three phenols studied, during glucose and xylose co-fermentation.
Effects of phenolics on ethanol fermentation by the strain NAPX37. (A–C) Syringaldehyde; (D–F) vanillin; (G–I) phenol; symbols: diamonds—0mM, squares—5mM, triangles—10mM, multiplication signs—20mM, circles—30mM; solid line—OD660, glucose and ethanol concentration; dotted line—xylose and xylitol concentration. Values represent the averages of duplicate experiments±SD.
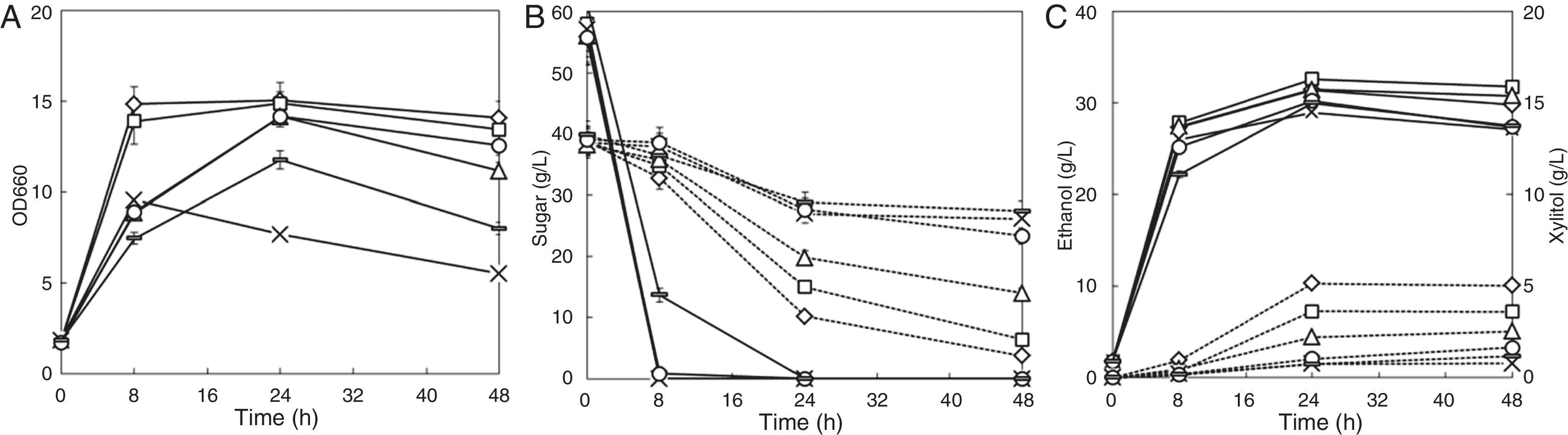
Five typical inhibitors (acetic acid, formic acid, levulinic acid, furfural, and vanillin) were selected to investigate the additive effects of inhibitors on glucose and xylose co-fermentation. As shown in Fig. 4, adding 20mM acetic acid slowed xylose fermentation; the xylose consumption rate in the first 24h decreased from 1.193g/L/h to 0.985g/L/h, and further decreased to 0.766g/L/h when 10mM formic acid was further added. Further addition of 10mM levulinic acid significantly reduced the xylose consumption rate to 0.483g/L/h. These results indicated a strong additive effect of the three weak acids. The ethanol yield from glucose and from the total sugar increased with increase in the total concentration of the three acids. Adding 5mM furfural, along with the three weak acids, did not affect xylose fermentation; however, the ethanol yield from both glucose and xylose improved, suggesting that the presence of a low concentration of furfural may be favorable for fermentation. Further addition of 1mM vanillin further reduced both the glucose and xylose consumption rates. The glucose was even not fully utilized during the first 8h of fermentation (Fig. 4); the xylose consumption rate was reduced to 0.460g/L/h, and the ethanol yield in the first 24h of fermentation decreased from 0.424 to 0.389g/g consumed sugar. These results suggest that, vanillin, even with relatively low concentration which does not show inhibitory effect when it present alone, showed strong additive inhibitory effect with other inhibitors.
Additive effects of the inhibitors on ethanol fermentation by the strain NAPX37. Symbols: diamonds —without inhibitor, squares—20mM acetic acid, triangles—20mM acetic acid and 10mM formic acid; multiplication signs—20mM acetic acid, 10mM formic acid and 10mM levulinic acid, circles—20mM acetic acid, 10mM formic acid, 10mM levulinic acid, 5mM furfural, rectangles—20mM acetic acid, 10mM formic acid, 10mM levulinic acid, 5mM furfural, 1mM vanillin; solid line—OD660, glucose and ethanol concentration; dotted line—xylose and xylitol concentration. Values represent the averages of duplicate experiments±SD.
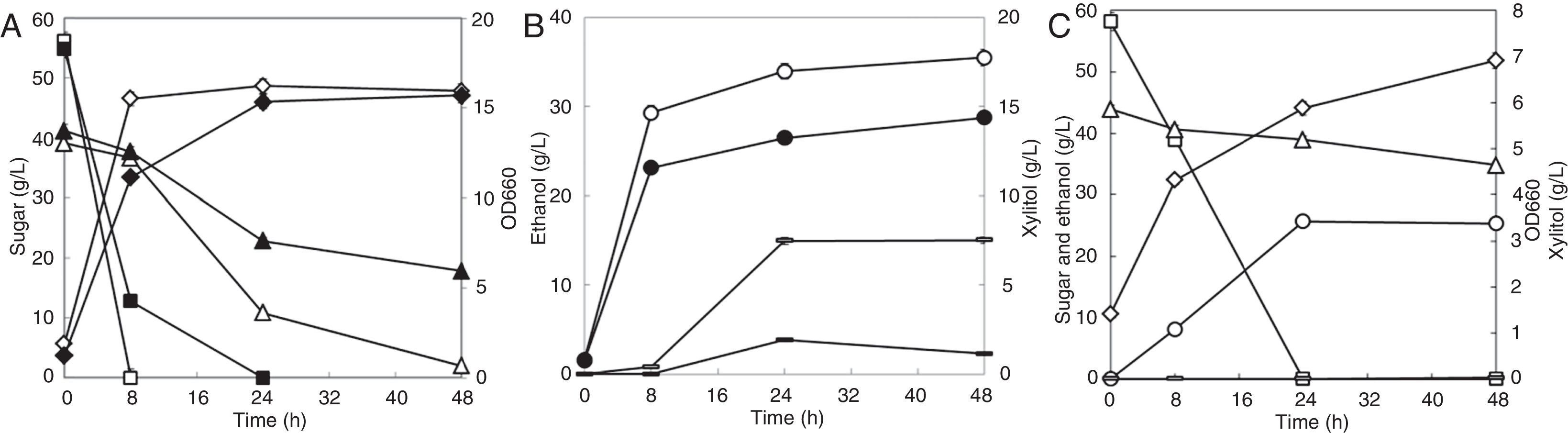
YPDX medium containing an inhibitor cocktail including all the eight inhibitors, including three weak acids at a total concentration of 37.21mM, two furfurals at a total concentration of 11mM, and three phenols at a total concentration of 0.55mM, was used for batch fermentation. As shown in Fig. 5A and B, cell growth was delayed and the glucose consumption rate reduced from 7.025g/L/h to 5.251g/L/h. The xylose consumption rate also reduced from 1.177g/L/h to 0.763g/L/h during the first 24h of fermentation. The ethanol yield from glucose did not change; however, the ethanol yield from total sugar in the first 24h increased from 0.384 to 0.401, partly owing to the reduced xylitol yield.
Ethanol fermentation using medium containing mixed inhibitors (A, B) or rape straw hydrolysate (C) by S. cerevisiae strain NAPX37. Symbols: diamonds—OD660, squares—glucose, triangles—xylose, circles—ethanol, rectangles—xylitol; open symbols in (A) and (B)—without inhibitors; filled symbols in (A) and (B)—with mixed inhibitors. Values represent the averages of duplicate experiments±SD.
As shown in Fig. 5C, the consumption of both glucose and xylose was strongly inhibited when the rape straw hydrolysate prepared by diluted acid pretreatment was used for fermentation. The cell growth was strongly inhibited. The OD660 at 24h was only 5.0, and it increased to 7.0 at 48h. Compared to that of the inhibitor-free control, the glucose consumption rate decreased by 65.7%, though glucose was depleted within 24h. The xylose consumption rate was relatively low, and most of the xylose was unutilized in 48h. The ethanol yield in the first 24h of fermentation was 0.408, similar to the ethanol yield when using YPDX medium with the inhibitor cocktail. Compared to the fermentation results when adding single inhibitors or mixed inhibitors, the unsatisfactory fermentation result of the rape straw hydrolysate was unexpected. In spite of those inhibitors we detected, some other compounds in the hydrolysate might play strong additive inhibitory effects on cell growth and fermentation.
DiscussionTo date, although there are several reports on the effects of inhibitors on ethanol fermentation by S. cerevisiae, most of them only focus on the effects of inhibitors on single sugar (glucose or xylose) fermentation.10,11 In lignocellulosic hydrolysate, glucose and xylose are the main sugars present; hence, a thorough understanding of the effects of inhibitors on glucose and xylose co-fermentation is essential. Moreover, most of the published studies have investigated the effects of single inhibitors, such as acetic acid, formic acid, HMF, and furfural,5,10,11,24–26 on ethanol fermentation, rarely focusing on phenols and levulinic acid, let alone the additive effects of these inhibitors. However, the inhibitory mechanisms of different inhibitors are different.1,16,30 It is crucial to systematically investigate the effects of inhibitors on ethanol fermentation, which would facilitate genetic modification to improve inhibitor tolerance.
In the present study, the effects of eight inhibitors, as well as their additive effects, on glucose and xylose co-fermentation were systematically evaluated by using batch fermentation. The results suggest that during glucose-fermentation stage, there was a strong positive correlation between cell growth and glucose fermentation. However, in the xylose-fermentation stage, no clear correlation between xylose consumption rate and cell growth or cell density was observed. This suggests that the inhibitors acted directly on xylose metabolism, and not on cell growth, during the glucose and xylose co-fermentation. Inhibitors affected glucose and xylose fermentation differently; all eight inhibitors affected xylose fermentation much stronger than glucose fermentation. In the range of inhibitor concentrations that permitted cell growth, glucose was completely depleted in 24h (Figs. 1–3). Helle et al.13 and Casey et al.5 also reported that xylose fermentation is more sensitive to acetic acid than glucose fermentation, during mixed sugar fermentation by XR/XDH-based recombinant strains.
Among the three weak acids, levulinic acid inhibited cell growth most strongly, leading to the slowest glucose fermentation. The strain required 48h to completely ferment the glucose when 160mM of levulinic acid was present (Fig. 1). Formic acid did not significantly inhibit glucose fermentation, even at a concentration of 160mM (Fig. 1). However, xylose fermentation was very sensitive to formic acid, and it was almost totally inhibited in the presence of 40mM formic acid during glucose and xylose co-fermentation (Fig. 1). The pK values of formic acid (3.75), levulinic acid (4.66), and acetic acid (4.75), might determine their inhibitory effects on xylose fermentation; the lower the pK value, the stronger the inhibition.1 However, when xylose is the sole sugar used for fermentation by the strain NAPX37, the inhibitory effect of formic acid was the least among the three weak acids and xylose could be fermented even when the formic acid was high of 120mM,21 this result also indicated that the response of the xylose-fermenting strain to various inhibitors in different carbon source may distinct. What's more, the differences in pK values of the weak acids do not explain their effects on glucose fermentation, which seem to be correlated with their molecular weight: the higher the molecular weight, the stronger the inhibition.
The strain showed considerable tolerance to furfural and 5-HMF. Cell growth and sugar fermentation were not markedly repressed in the presence of 10mM furfural or 10mM 5-HMF; however, 20mM or 30mM of the furans significantly repressed cell growth and delayed both glucose and xylose fermentation. 5-HMF inhibited glucose fermentation slightly stronger than furfural, whereas the opposite was observed during xylose-fermentation stage. The strain metabolized 5-HMF more slowly than furfural (Fig. 2), which might result in slower glucose fermentation. Furfural and 5-HMF caused similar inhibitory effects on xylose fermentation. However, when xylose was used as sole sugar, the inhibitory effects of furfural and 5-HMF were distinct and 5-HMF showed a weaker inhibitory effect than furfural.21
Phenolic compounds have been suggested to exert considerable inhibitory effect on fermentation, and even very low concentrations of phenolic compounds have a strong inhibitory effect.1 Kumari and Pramanik31 investigated the effect of vanillin on glucose and xylose co-fermentation and reported that ethanol fermentation was significantly affected by the addition of about 4.9mM vanillin. Strain NAPX37 showed substantial tolerance to all the three phenols examined (Fig. 3). The inhibitory effects of these three phenols on glucose and xylose fermentation are different. Vanillin inhibited glucose fermentation the most, followed by phenol and then syringaldehyde. During xylose-fermentation stage, phenol showed stronger inhibitory effect than vanillin. The xylose consumption rate with 10mM vanillin was 1.75-fold higher than that with 10mM phenol. The strain showed excellent tolerance to syringaldehyde; the xylose consumption rate with 20mM syringaldehyde was 0.533g/L/h, which was approximately 50% of the uninhibited rate. These results indicate that low molecular weight phenols inhibit xylose fermentation more strongly than high molecular-weight phenols, which is consistent with the results of a previous report.32
Because hydrolysates typically contain multiple inhibitors, it is crucial to investigate their additive effects. The additive effect of the three weak acids was significant, although they did not show obvious inhibitory effects when present individually at low concentrations. Addition of 1mM vanillin alone did not affect cell growth and fermentation; however, it produced apparent inhibition when other inhibitors were co-present (Fig. 4), which suggests that there are additive effects between weak acids and phenols. Addition of 5mM furfural did not increase the inhibition, suggesting the absence of additive effects between weak acids and furans at low furan concentrations. This is similar to previously reported observations.27 These results also indicate that it is important to consider not only the individual concentration of each inhibitor but also the total constitution of the inhibitors, during modification and screen inhibitor tolerant strains through metabolic engineering, such as evolutionary engineering.
Ethanol yield is very important for reducing production costs. In most cases, inhibitors delayed glucose fermentation, but did not negatively affect the ethanol yield from glucose. Increasing the concentrations of most inhibitors reduced the rate of xylose consumption, which decreased the xylitol yield, suggesting an increased ethanol yield from xylose. To some extent, the reduced xylose fermentation rate favors ethanol production. We compared the yields of ethanol and xylitol using small and large cell inoculum. A larger inoculum size resulted in a higher rate of xylose fermentation and higher xylitol yield but a lower ethanol yield (data not shown). However, there were exceptions, such as upon the addition of 5-HMF or phenol, which either caused no change or only slightly increased xylitol yield, even xylose fermentation was significantly delayed. Because the inhibitory mechanisms of these inhibitors might be different,1 different strategies should be considered to improve tolerance to these inhibitors.
Although the strain NAPX37 showed better inhibitor tolerance than some other xylose-fermenting strains, while using hydrolysate for fermentation,33,34 the ethanol fermentation is not ideal when fermenting the rape straw hydrolysate with relatively high total concentration of inhibitors in the present study. Xylose fermentation was relatively slow, although glucose was depleted within 24h. No xylitol was detected throughout the hydrolysate fermentation, even though approximately 10g/L xylose was consumed (Fig. 5). Similar phenomena have been reported in literatures.35–37 This may be attributed to some compounds, such as furfural, in the hydrolysate serving as electron acceptors to regenerate NAD+, which then reduced the accumulation of xylitol.38,39 Therefore, it appears that xylitol accumulation is not a major concern for strains harboring the XR-XDH pathway when fermenting hydrolysate. The most important work is to improve inhibitor tolerance for xylose fermentation. Due to the very different xylose fermentation efficiency when using synthetic medium and rap straw hydrolysate with similar main inhibitors, further studies should be conducted to reveal the inhibitory mechanism of inhibitors in hydrolysate and efforts should be made for the improvement of the tolerance of yeast strains to all inhibitors in the hydrolysate, including not only those that are well studied.
ConclusionIn this study, the inhibitor tolerance of the flocculating industrial xylose-fermenting strain NAPX37 of S. cerevisiae was studied. Eight typical inhibitors (three weak acids, two furans, and three phenols) were selected for evaluation using batch fermentation. The results suggest that cell growth affects glucose fermentation and that inhibition of glucose fermentation correlates with delayed cell growth. Xylose fermentation did not correlate with cell growth, indicating that different types of inhibitors operate via different mechanisms. Additive effects among weak acids and phenols were observed. Compared to other strains of the same type, the strain NAPX37 showed considerable tolerance to typical inhibitors and good fermentation characteristics, when fermentation either in medium containing inhibitor cocktail, or in hydrolysate.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (31170093) and the Talent Project for Science and Technology Innovation of Sichuan Province (2017RZ0021).