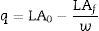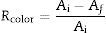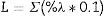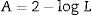Lactic acid, which can be obtained through fermentation, is an interesting compound because it can be utilized in different fields, such as in the food, pharmaceutical and chemical industries as a bio-based molecule for bio-refinery. In addition, lactic acid has recently gained more interest due to the possibility of manufacturing poly(lactic acid), a green polymer that can replace petroleum-derived plastics and be applied in medicine for the regeneration of tissues and in sutures, repairs and implants. One of the great advantages of fermentation is the possibility of using agribusiness wastes to obtain optically pure lactic acid. The conventional batch process of fermentation has some disadvantages such as inhibition by the substrate or the final product. To avoid these problems, this study was focused on improving the production of lactic acid through different feeding strategies using whey, a residue of agribusiness. The downstream process is a significant bottleneck because cost-effective methods of producing high-purity lactic acid are lacking. Thus, the investigation of different methods for the purification of lactic acid was one of the aims of this work. The pH-stat strategy showed the maximum production of lactic acid of 143.7g/L. Following purification of the lactic acid sample, recovery of reducing sugars and protein and color removal were 0.28%, 100% and 100%, respectively.
There is great concern about the amount of waste produced by the use of petroleum-derived plastics. Poly(lactic acid) (PLA) is a green polymer obtained through the polymerization of lactic acid and has been used as a biodegradable plastic source. Some uses for PLA include the improvement of the physical properties in the production of garbage bags, agricultural plastic sheeting, and food packaging.1 As PLA is bioabsorbable, it is also employed in medicine in the regeneration of tissues, sutures, repairs and implants.2
An important characteristic of lactic acid is the optical activity, due to the presence of a chiral carbon in the structure. It is possible to obtain different lactic acid isomers exclusively using fermentation by changing the fermentation conditions. An amorphous polymer is obtained by the polymerization of a racemic mixture of l-(+)- or d-(−)-lactic acid, and a crystalline and stable polymer can be obtained by beginning with optically pure l-(+)-lactic acid or d-(−)-lactic acid to form poly(l-lactic acid) (PLLA) and poly(d-lactic acid) (PDLA), respectively.3,4
Important properties of the polymers, such as crystallinity, can be controlled using different concentrations of the enantiomers. For example, the PLLA when mixed with PDLA increases the melting point of the polymer by 50°C resulting in a highly regular complex with high degrees of crystallinity and thermal stability.5
Optically pure lactic acid can be obtained through fermentation depending on the strain of lactic acid bacteria used. In contrast, the chemical synthesis of always results in a racemic mixture of lactic acid. Furthermore, renewable resources, such as starch and cellulose, can be used to produce lactic acid through fermentation. An additional benefit of using these renewable resources is that they do not produce carbon dioxide as a by-product of fermentation in contrast to oil- and fossil-fuel-based sources.6
Batch fermentation is the method utilized for industrial l-lactic acid production.7 However, the major disadvantage of batch fermentation is that the l-lactic acid concentration and productivity decrease due to inhibition by high substrate concentration. The development of feed techniques can eliminate substrate inhibition promoting an appropriate microbial environment.8 This approach allows control of growth rates, which range from zero to approximately the maximum rate observed in a batch culture, and increases production of lactic acid.7
In the downstream processing, the lactic acid is recovered and purified. However, the cost-effective production of high-purity lactic acid has remained a challenge for decades9 because of the requirement for expensive chemicals, which accounts for 50% of the production cost, and the generation of waste, such as gypsum.10
New techniques have been used to recover lactic acid from the fermentation broth including extraction,11 adsorption12 and membrane separation.13 Membrane separation has been used extensively in the last decades.9 The use of filtration (microfiltration or nanofiltration) has been successful in the separation of cells, proteins and salts from the broth of fermentation.14,15 Beyond these, electrodialysis has been effective in the recovery of lactic acid from fermentation broth due to its rapid treatment, effective removal of non-ionic molecules, concentration of lactic acid and environmentally sustainable process.10
In this study, the improvement of lactic acid production through different fed-batch strategies was investigated, as well as the recovery of the product using alternative methodologies of purification.
Materials and methodsMicroorganismLactobacillus rhamnosus B103 was kindly supplied by Instituto Cubano de Investigaciones de los Derivados de la Cana de Azúgar (ICIDCA). The strain was stored in Man, Rogosa and Sharp (MRS) broth with 20% glycerol (v/v) at −80°C.
Cheese whey and corn steep liquorWhey powder containing 60% (m/V) lactose was obtained from Regina Dairy Ltda, São Paulo (São Paulo, Brazil). Deproteinization was performed by heating the whey powder to 100°C for 15min and then cooling to room temperature. The resulting solution was centrifuged at 10,000×g to remove the solids, and the supernatant was diluted to reach the desired lactose concentration. The feeding medium was prepared by adding more powdered whey to the solution after centrifuging, and then heating and centrifuging were repeated.
Corn steep liquor (CSL) was obtained from Corn Products, Mogi Guaçu (São Paulo, Brazil). The CSL was centrifuged to remove any solid impurities.
Medium compositionThe fermentation medium was adapted from elsewhere16 and comprised 60.00g/L of lactose from whey (approximately 90.00g/L of reducing sugars), 45.00mL/L of CSL, 1.00mL/L of Tween 80 and 0.075g/L of manganese sulfate. The feed medium was composed of 500.00g/L of lactose (from whey) and 7.50% of CSL.
Fermentation and growth conditionsThe inocula were prepared in Erlenmeyer flasks with 150.00mL of MRS medium. The inocula were incubated at 37°C at 150rpm mixing for 18h. In all fermentation samples, 10.00% (v/v) of inoculum was used.
The fermentation experiments were performed in a 5.00-L bioreactor (Bio-T Mini Zeta) with a working volume of 1.50L. The temperature, agitation and pH were 37°C, 200rpm and 6.20, respectively. The pH was adjusted using 10N NaOH.
In the pulse feeding fed-batch fermentation, 2 pulses were made: one applied at 24h of fermentation and the other at 48h. Each pulse had the flow of 30.00mL/min and lasted for 2min and 18s.
For the constant rate fed-batch fermentation, the flow was 0.35mL/min. The feed was initiated at 18h of fermentation and lasted 16h.
Three different pH-stat fed-batch fermentation experiments were performed, each using a different base to adjust the pH. In the first, the feeding medium was composed of 500.00g/L of lactose, 7.50% of CSL and 10N NaOH. In the second, the feeding medium was composed of 500.00g/L of lactose, 7.50% of CSL and 5N NaOH. In the last, the feeding medium was composed of 500.00g/L of lactose and 7.50% of CSL. As the pH decreased, more feeding medium was added to the bioreactor.
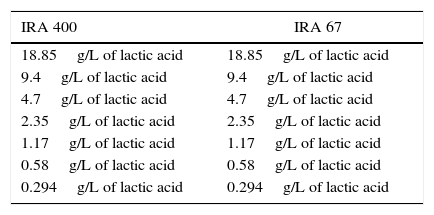
Resin selection: adsorption and recovery testTwo resins were used: IRA 400 (strongly basic anion-exchange resin) and IRA 67 (weakly basic anion-exchange resin). Both were treated to obtain the Cl− OH− or free-base form, according to its own nature (Amberlite IRA 67 could not be obtained in OH− form, the resin remained in the free-base form at pH>9.0). One gram of each resin IRA 400 and IRA 67, with 60% moisture, and 5.00mL of lactic acid solution of varying concentrations were mixed in Erlenmeyer flasks at 25°C and 150rpm (Table 1).
Concentration of lactic acid used in the adsorption study for each resin.
| IRA 400 | IRA 67 |
|---|---|
| 18.85g/L of lactic acid | 18.85g/L of lactic acid |
| 9.4g/L of lactic acid | 9.4g/L of lactic acid |
| 4.7g/L of lactic acid | 4.7g/L of lactic acid |
| 2.35g/L of lactic acid | 2.35g/L of lactic acid |
| 1.17g/L of lactic acid | 1.17g/L of lactic acid |
| 0.58g/L of lactic acid | 0.58g/L of lactic acid |
| 0.294g/L of lactic acid | 0.294g/L of lactic acid |
The initial pH of all samples was 5.00. After 12h, samples of supernatant were withdrawn and analyzed. When the equilibrium was achieved, samples were withdrawn, and the resin capacity was calculated according to Eq. (1).
where LA0 and LAf are the initial and final lactic acid (g/L) and w is weight of the resin (g).The resin with the highest adsorption capacity was selected to perform the recovery experiments: 1.00g of charged resin and different eluents (1N H2SO4, 1N HCl, 1N NaCl and 1N NaOH) were used in this test. The resin was mixed with 5.00mL of the each eluent in Erlenmeyer flasks for 2h in a shaker at 25°C and 150rpm. Samples of supernatant were withdrawn and analyzed.
Process of purificationThe broth from the sample with the best fermentation results was centrifuged to separate the cells from the broth. The broth was mixed with powdered activated carbon (18.72%) for 1.5h at ambient temperature. Then, the mixture was centrifuged, the pellet was washed twice and the supernatant was preserved.
The supernatant with 142.20g/L of lactic acid was pumped at flow of 1.50mL/min in a column (length 48cm and diameter 2cm) charged with 51.00g of resin and filled with water. Fractions of the eluent were collected and analyzed for their lactic acid, sugar and protein concentrations. When the concentration of lactic acid in the eluent was the same in the feed, the resin was considered saturated.
The interstitial solution was removed by pumping distilled water until the lactic acid concentration of the eluent was below 0.10g/L. The lactic acid was recovered with HCl solution (1N). Samples of eluent were collected until the concentration of lactic acid of the output was below 0.10g/L.
A wash step was performed to remove the HCl contained in the interstitial space, and then the resin was ready for a new cycle.
Analytical methodsThe optical purity of the lactic acid and the d-(−)- and l-(+)-lactic acid samples was determined using a high-performance liquid chromatography system (HPLC) equipped with a UV detector at 210nm using a Chirex 3126 Phenomenex (150.00mm×4.60mm) column with 1mM of CuSO4 as the mobile phase and a flow rate of 1.00mL/min (30°C).
Reducing sugars were measured using the 3,5-dinitro salicylic acid method17: the sample and the reagent (0.63% 3,5-dinitro salicylic acid, 18.2% Rochelle salts, 0.5%, phenol, 0.5% sodium bisulfite, and 2.14% sodium hydroxide) was mixed in a 1:1 ratio and heated for 5min in a boiling water bath. The color intensity was measured in UV–vis spectrophotometer at 540nm.
Cell growth was determined using a spectrophotometer at 650nm (OD650) after centrifuging and washing the cells. The dry mass was determined using a standard curve of optical density versus dry mass.
The total protein concentration was determined using the Lowry method18: in 1mL of the sample, 1.0mL of Reagent A (mix equal parts of: solution of about 20% sodium carbonate is added slowly while stirring to a solution of copper sulfate tartrate to give final concentrations of 0.1% copper sulfate (pentahydrate), 0.2% potassium tartrate, 10% sodium carbonate, NaOH (0.80N), SDS (10%), and H2O), is added and allow to stand for 10min at room temperature (20°C). Then, 0.5mL of Reagent B (1 volume of Folin–Ciocalteu phenol reagent is mixed with 5 volumes of distilled water) is added and mixed immediately. After 30min, the absorbance is read using a UV–vis spectrophotometer at 750nm.
The color determination was performed by calculating the luminescence19: this method is based in the transmittance measurement of the samples at 10 different wavenumbers: T489, T512, T529, T541, T551, T561, T572, T584, T600, and T627. The luminescence is the final value of the sum and multiplication by the factor of 0.1–10 of the obtained values of transmittance. The measurement was made in a UV–vis spectrophotometer. To calculate the color removal, the following equations were used:
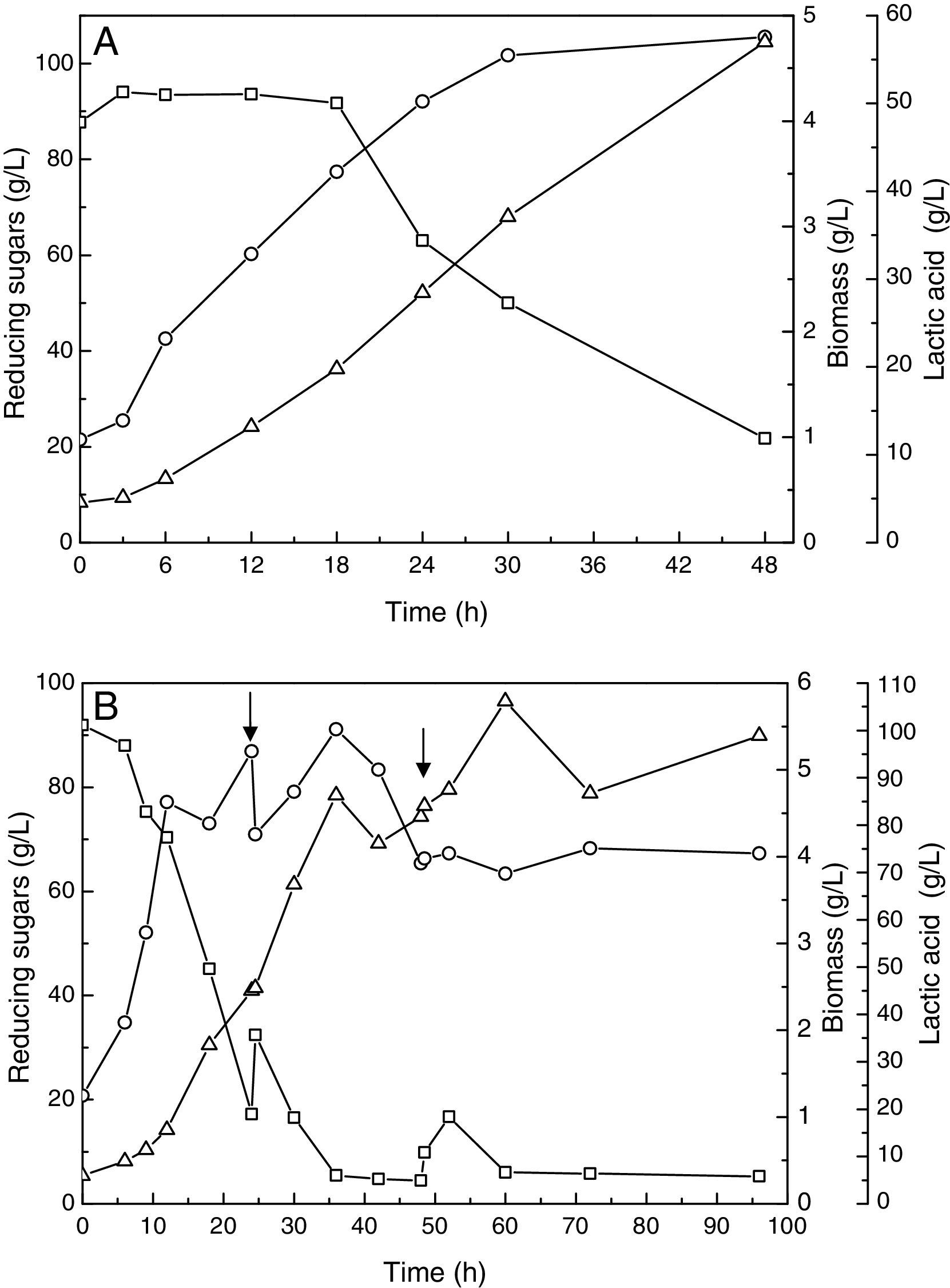
where Ai is the initial absorbance and Af is the final absorbancewhere A is the absorbance and L is the luminescence. The sum of transmittance percentages multiplied by 0.1 results in the luminescence, which is expressed as a percentage.Results and discussionBatch fermentationBatch fermentation was performed for 48h. The production of lactic acid, substrate consumption and growth are shown in Fig. 1A.
(A) Batch fermentation at 37°C, 200rpm and pH 6.20 controlled with NaOH (10N). (B) Pulse-fed batch at 37°C, 200rpm and pH 6.20 controlled with NaOH (10N). Pulse with flow of 30.00mL/min. The arrows indicate when the pulse occurred. Reducing sugars (square), biomass (circle) and lactic acid (triangle).
The highest production of l-(+)-lactic acid was 57.00g/L by the end of the fermentation, the final reducing sugar concentration was 21.75g/L and the maximum biomass obtained was 4.79g/L. At the end of the fermentation, the productivity of lactic acid was 1.18g/Lh. These results were similar to those obtained by De Lima et al.20 who achieved a production of lactic acid of 52.37g/L from 59.64g/L of lactose with productivity of lactic acid of 1.09g/Lh.
Fed-batch fermentationIn the pulse-fed batch fermentation, the highest production of lactic acid (106.20g/L) was achieved at 60h of fermentation. In addition, the residual reducing sugar was 5.30g/L, which was less than that obtained in the batch process (21.75g/L). These fermentation conditions led to an extension of the log phase (Fig. 1B). Compared with the conventional batch fermentation, the pulse-fed batch fermentation had an increased production of lactic acid of 41.90g/L at the end of the fermentation.
Li et al.21 using Lactobacillus rhamnosus and glucose (125.00g/L) as the carbon source, also increased lactic acid production from 90.00 to 130.00g/L by modifying the type of fermentation from batch fermentation to pulse-fed batch fermentation.
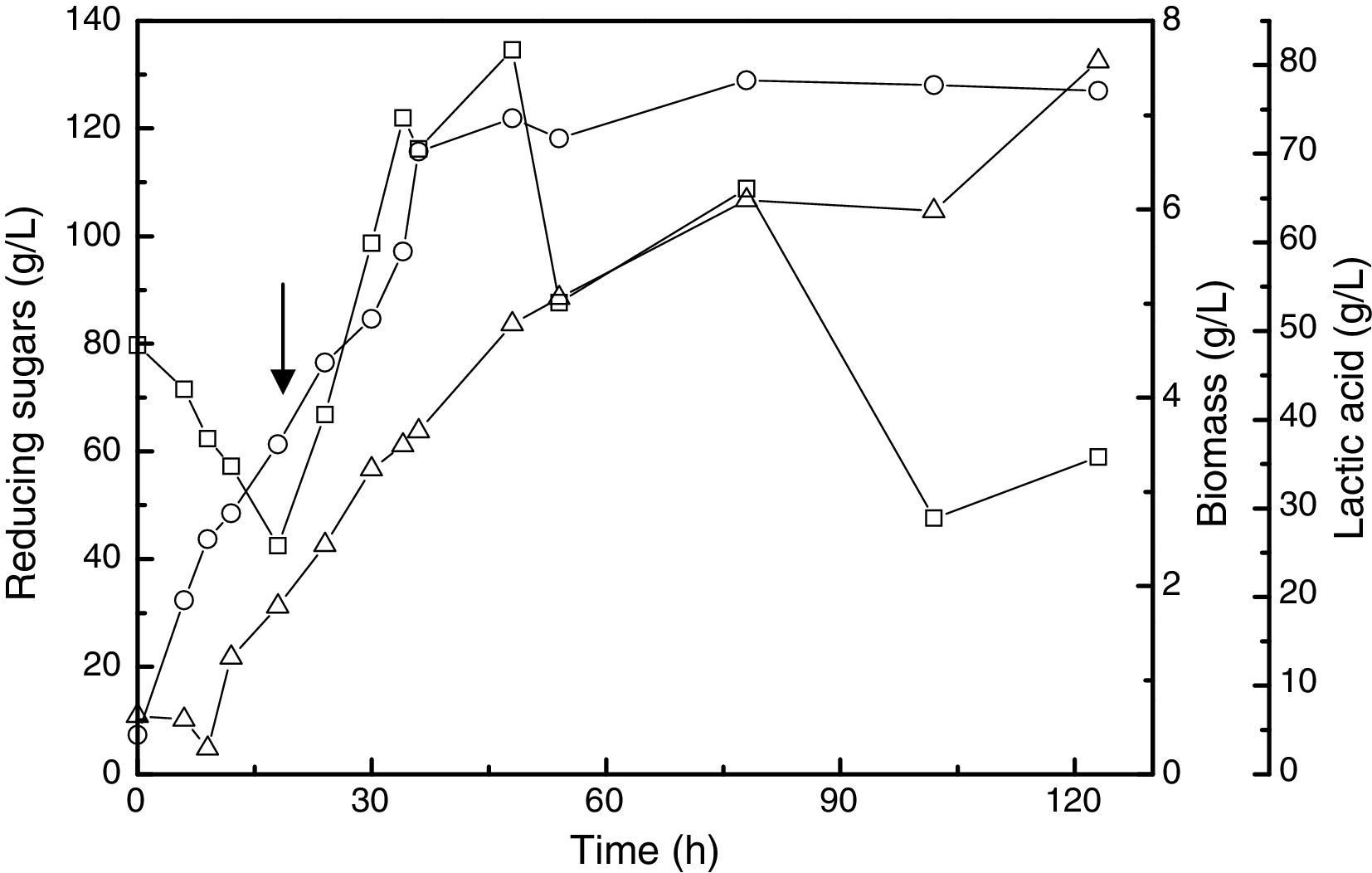
The use of a constant feed rate in the fed-batch fermentation was also investigated. In this case, at the end of the fermentation, there was high concentration of reducing sugars (58.94g/L) and the maximum lactic acid production and biomass were 80.45g/L and 7.36g/L, respectively (Fig. 2).
Bai et al.22 found that using a constant feed rate in fed-batch fermentation significantly increased the production of lactic acid by Lactobacillus lactis, to provide a final concentration of 210.00g/L of lactic acid and less than 0.50g/L of glucose.
The pulse-feed fed-batch fermentation was a better strategy than the constant feed rate batch fermentation because the production of lactic acid was higher, the increasing of lactic acid was 18.75g/L. Furthermore, the productivity for the pulse-feed fed-batch fermentation (1.17g/Lh) in 48h was better than that in the constant feed rate fermentation (1.05g/Lh).
In contrast with the results of this study, Ding and Tan7 found no difference in lactic acid production when these two strategies were used. Using constant feed rate fermentation, they achieved 135.00g/L for lactic acid production, and in the pulse-feed fed-batch fermentation, the production was 130.00g/L. These authors used glucose at 90.00g/L as carbon source and a feeding medium 850.00g/L.
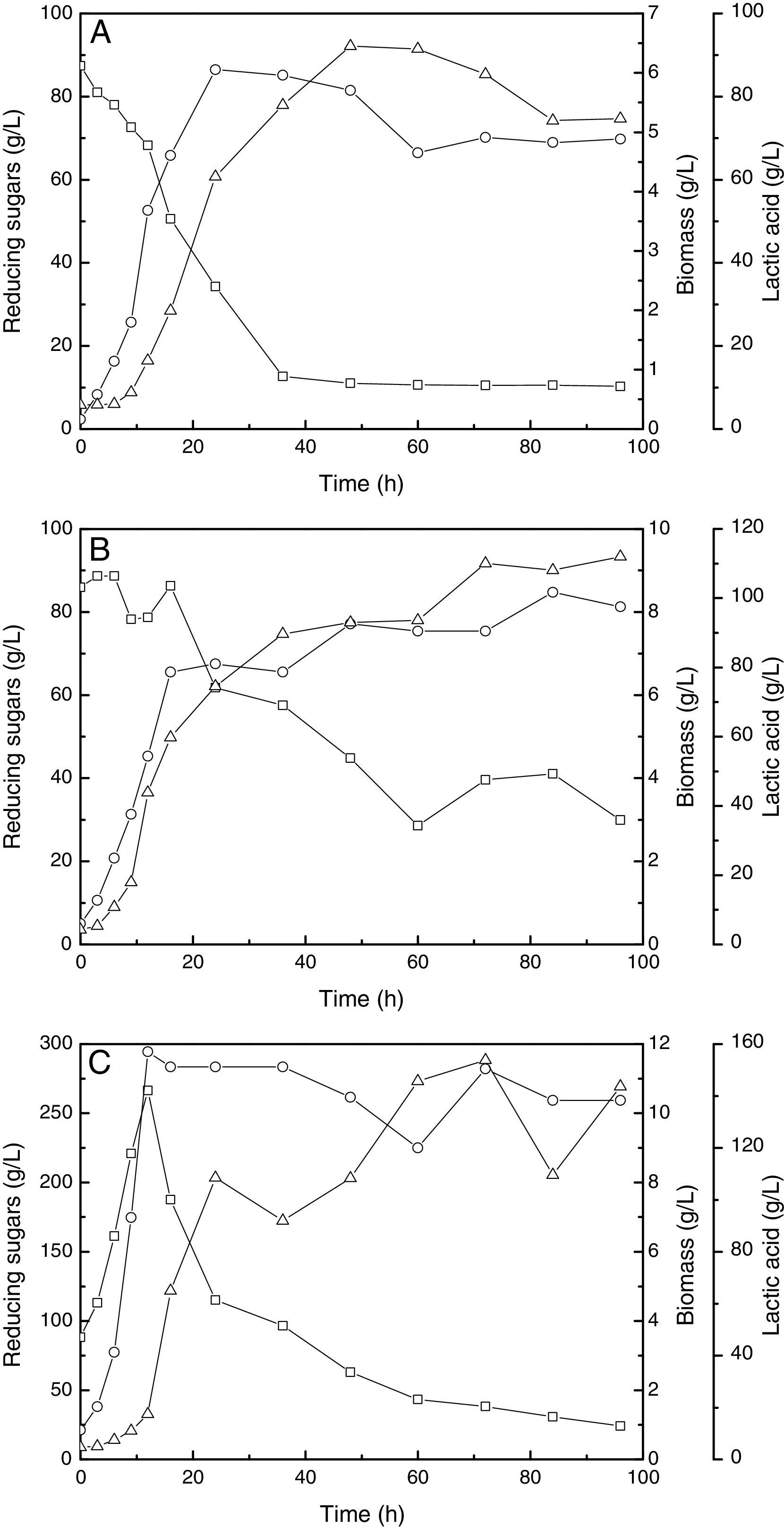
In the pH-stat fed-batch fermentation, the main goal is to add the substrate into the bioreactor and observe a decrease in pH. The decrease in the pH indicates that there was production of lactic acid and consumption of substrate. Thus, adding more substrate, a substantial improvement in the production of lactic acid was expected. In the pH-stat fed-batch fermentation with whey, CSL and NaOH (10N), the highest production of lactic acid was 92.15g/L. The final concentration of reducing sugars and biomass concentration were 0.25g/L and 4.88g/L, respectively (Fig. 3A).
(A) pH-stat fermentation at 37°C, 200rpm and pH 6.20 controlled with whey, CSL and NaOH (10N). (B) pH-stat fermentation at 37°C, 200rpm and pH 6.20 controlled with whey, CSL and NaOH (5N). (C) pH-stat fermentation (only whey and CSL) at 37°C, 200rpm and pH 6.20. Reducing sugars (square), biomass (circle) and lactic acid (triangle).
When whey, CSL and NaOH (5N) were used in the pH-stat fermentation, [0] the concentration of lactic acid increased to 112.00g/L, the concentration of the final reducing sugars increased (29.90g/L) and the biomass concentration improved to 8.10g/L (Fig. 3B).
The best fed-batch fermentation conditions used only whey and CSL to control the pH. Only 24.10g/L of reducing sugars remained following the fermentation. The final lactic acid concentration and biomass were 143.70g/L and 10.37g/L, respectively (Fig. 3C). Table 2 shows the kinetics factors from all the fermentation experiments.
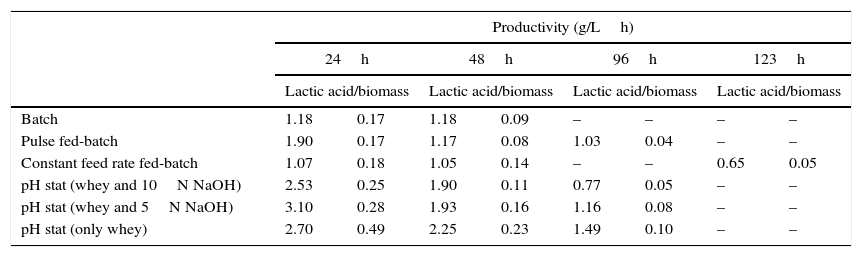
Productivity (g/Lh) of lactic acid and biomass across all fermentation conditions.
| Productivity (g/Lh) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 24h | 48h | 96h | 123h | |||||
| Lactic acid/biomass | Lactic acid/biomass | Lactic acid/biomass | Lactic acid/biomass | |||||
| Batch | 1.18 | 0.17 | 1.18 | 0.09 | – | – | – | – |
| Pulse fed-batch | 1.90 | 0.17 | 1.17 | 0.08 | 1.03 | 0.04 | – | – |
| Constant feed rate fed-batch | 1.07 | 0.18 | 1.05 | 0.14 | – | – | 0.65 | 0.05 |
| pH stat (whey and 10N NaOH) | 2.53 | 0.25 | 1.90 | 0.11 | 0.77 | 0.05 | – | – |
| pH stat (whey and 5N NaOH) | 3.10 | 0.28 | 1.93 | 0.16 | 1.16 | 0.08 | – | – |
| pH stat (only whey) | 2.70 | 0.49 | 2.25 | 0.23 | 1.49 | 0.10 | – | – |
Zhang et al.23 also reported a method of feeding based on pH, which was controlled with the substrate concentration, ammonium hydroxide and glucose. The authors used an initial glucose concentration of 38.00g/L and obtained 96.30g/L of lactic acid and a final concentration of glucose of 4.90g/L. Our experiment using only whey to control the pH was better in the production of lactic acid and in the required amount of sugar compared to this method. In addition, our method has the advantage that does not require hydroxide for pH control.
In the batch and pH-stat fed-batch (only whey and CSL) fermentation experiments, an elongation of the exponential phase of the biomass production, especially between 12 and 24h, was observed. Increasing the cell concentration is one of the objectives of fed-batch fermentation.24
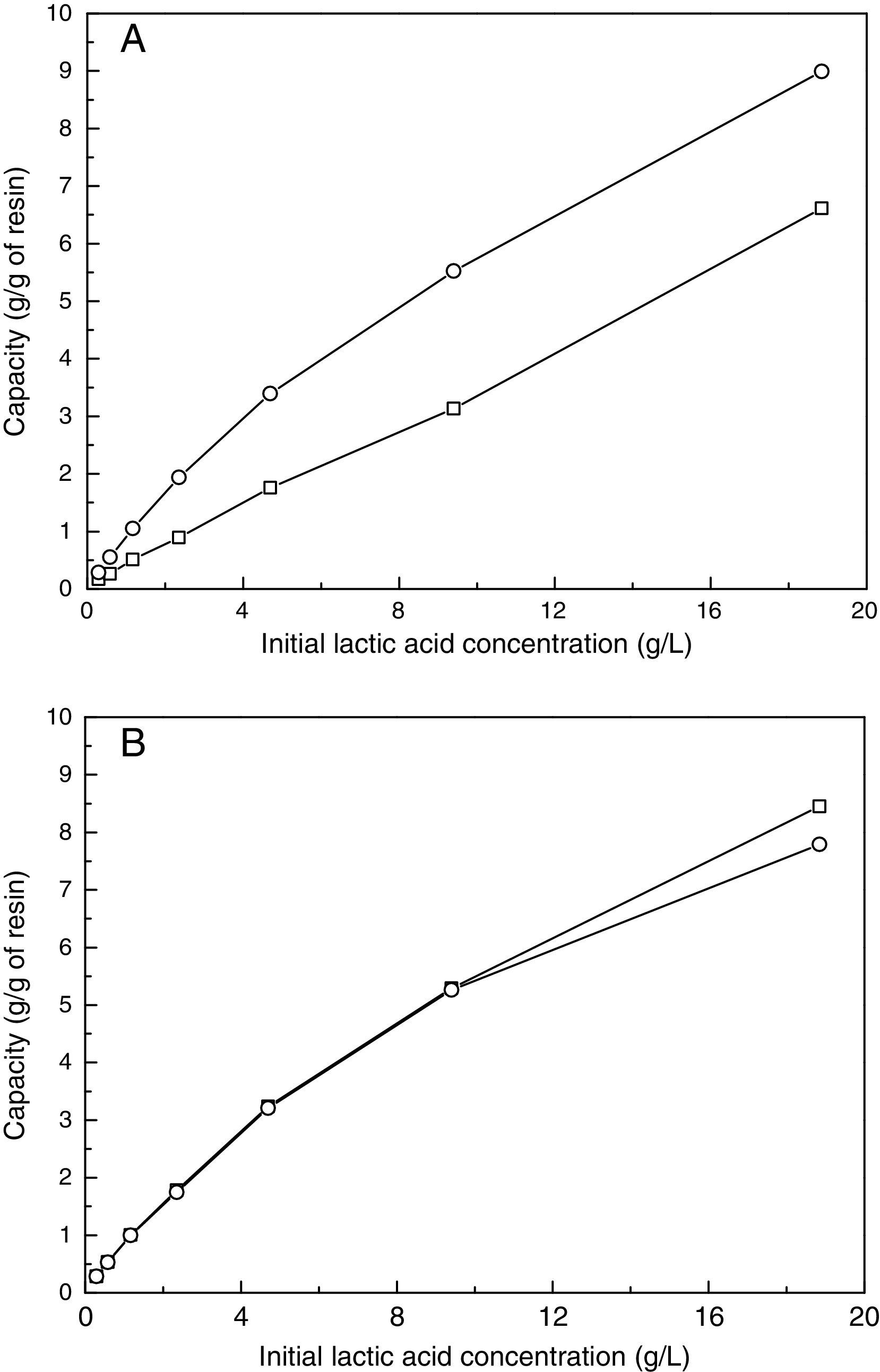
Resin selection: adsorption and recovery testsThe adsorption and recovery were analyzed, as described in the experimental section. When low lactic acid concentrations were tested, the two resins had high adsorption capacity. In contrast, when the lactic acid concentration was increased, the IRA 67 resin had the highest adsorption capacity in the Cl− form. Furthermore, the IRA 67 resin had better adsorption capacity than IRA 400 in both forms (Fig. 4A and B).
The results of the eluent study are shown in Table 3. NaCl had the most efficient recovery; however, considering the polymerization process, NaCl is not a good option because the salt will remain with the lactic acid solution. As a result, HCl was the best option because it provided the second-best recovery results and it can be easily removed by evaporation of the solution. This result is in accordance with work of John et al.25 that reported 1N hydrochloric acid the superior eluent for IRA 67 resin.
Purification processPurification procedures were performed, as described in the experimental section. The use of activated carbon in the first step of the purification led to color removal of 91.00%. In addition, the removal of lactose and proteins were 76.00% and 88.00%, respectively, with active carbon. Low concentrations of lactose and protein are important factors for the later procedures. The recovery of lactic acid was 77.00% using activated carbon.
The purification of lactic acid with an ion-exchange column in the second step provided an elution of lactic acid 63.70% with a recovery of 13.40%. The protein and color removal were both 100.00%, and the sugar removal was 99.10%.
The overall purification process produced had total lactic acid recovery of 10.30%, with purity of nearly 100%, resulting in only 0.28% of lactose (Table 4).
ConclusionsLactic acid production was enhanced by the feed strategy. The l-(+)-lactic acid concentration in the batch fermentation was 57.00g/L by the end of the fermentation. The final reducing sugar concentration was 21.75g/L, and the maximum biomass obtained was 4.79g/L. Using the pH-stat fermentation technique, the production of l-(+)-lactic acid was increased by 86.70g/L to provide a production of 143.70g/L with final reducing sugar concentration of 24.10g/L and final biomass of 10.37g/L. Agribusiness waste was used in this fermentation experiment. In addition, this method has the advantage of not requiring hydroxide for pH control.
A weak anion-exchange resin, amberlite IRA 67, was selected in the chloride form for purification. During the purification process, the use of powdered activated carbon provided a recovery of 77.00% of lactic acid, and the process using the IRA 67 resin led to a recovery 13.40% of l-(+)-lactic acid. A total recovery of lactic acid of 10.30% with 100% purity of proteins and only 0.28% of lactose was obtained in the end of the purification process.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to the Brazilian Fostering Agencies Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP- № 2011/15805-9) and CNPq for financial support. Also, the authors are grateful to the English review by BioMed Proofreading LLC.