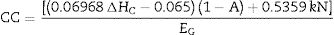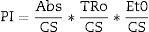Plants response to symbiosis with arbuscular mycorrhizal fungi (AMF) under water stress is important to agriculture. Under abiotic stress conditions native fungi are more effective than exotics in improving plant growth and water status. Mycorrhization efficiency is related to soil fungi development and energy cost-benefit ratio. In this study, we assessed the effect on growth, water status and energy metabolism of Cucurbita pepo var. pepo when inoculated with native AMF from the Sonoran desert Mexico (mixed isolate and field consortium), and compared with an exotic species from a temperate region, under drought, low and high salinity conditions. Dry weights, leaf water content, water and osmotic potentials, construction costs, photochemistry and mycorrhization features were quantified. Under drought and low salinity conditions, the mixed isolate increased plant growth and leaf water content. Leaf water potential was increased only by the field consortium under drought conditions (0.5–0.9MPa). Under high salinity, the field consortium increased aerial dry weight (more than 1g) and osmotic potential (0.54MPa), as compared to non-mycorrhized controls. Plants inoculated with native AMF, which supposedly diminish the effects of stress, exhibited low construction costs, increased photochemical capacity, and grew larger external mycelia in comparison to the exotic inoculum.
Aquifer over exploitation in semi-arid agricultural regions has increased soil salinity and caused water shortage.1 The direct effect of such increasing soil salinity on production has been documented in the coastal region of Hermosillo, Mexico.2 One of the proposals for mitigating these negative effects is the use of biofertilizers based on arbuscular mycorrhizal fungi (AMF), given that mycorrhizic symbiosis improves the establishment, vigor, productivity and survival of plants under water stress and salinity.3,4
The beneficial effects of AMF on plant development and soil features are considered relevant for sustainable agricultural management. However, conventional agriculture practices affect AMF diversity and density.5 Currently, isolated fungi species are used to colonize a wide variety of commercially important vegetables. Nonetheless, special interest has been given to characterization of native AMF species, since they are considered to be more efficient inoculates under environmental conditions similar to their origin, in contrast to exotic fungi from different environments.6
Since plant-AMF establish a compelled symbiosis, host responses to mycorrhization depend on the interaction efficiency between the symbionts, which in physiological terms is related to the amount of nutrients acquired by the host by unit of carbohydrates spent in supporting the guest. A study reporting the mycorrhization effect of Glomales on physiological and nutritional responses by several hosts found that water stress host tolerance varied depending on the associated AMF,3 and some fungi even diminished salinity and drought injuries.7,8
Ecophysiological studies found that mycorrhized plants response is better when accomplished by native strains. Apparently, this phenomenon is related to the fungi's environmental sensibility and to external mycelia development, which modifies water retention soil capacity and host water relations.9,10
Given the chemical composition of a plant, it is possible to estimate construction costs, defined as the total amount of photo-assimilates used for the construction of a gram of plant tissue, which is considered a useful measurement for evaluating plant development.11 Symbiosis may indirectly stimulate carbon fixation, eliminating symbiosis related costs in the plant energy budget providing a real benefit for both parts.12 Although the daily carbon flux between plant and fungus does not change, the final costs vary in relation to characteristics unique to each association. The resources efficient assimilation and the optimal biomass partition are determinant to the competitive ability and, in many cases, tolerance to stress conditions by hosts.13
One way of assessing the physiological response of mycorrhized plants subjected to stress is by measuring photosynthesis and chlorophyll fluorescence. Pinior et al.14 found that mycorrhization with Rhizophagus intraradices (N.C. Schenck and G.S. Sm.) C. Walker and A. Schüßler increased the tolerance of Rosa hybrid L. to water stress by increasing its photosynthetic activity, specifically in electron transport in photosystems I and II. Chlorophyll-a fluorescence analysis allowed the evaluation of AMF influence in reintroduced plants during their acclimation by quantifying the conservation of photosynthetic energy.15
Since it is possible to establish mycorrhization effects by evaluating host through assessing the photosynthetic and metabolic energies, in this work we evaluated some of the physiological responses to drought and salinity stresses in Cucurbita pepo var. pepo mycorrhized with native fungi from the Sonoran desert, with the objective of relating such changes in energy metabolism with a possible reduction of injuries caused by those stresses. Differences in plant growth and water status depend on the inoculum geographic origin. Mycorrhization with native organisms adapted to the stressing conditions improves cost-benefit ratio of association.
Materials and methodsIsolation of inoculatesTwo native AMF treatments were used in this study, a mixed isolate (MI) with two types of spores: Glomus sp. 1 (42sporesg−1) and Glomus sp. 4 (24sporesg−1), as well as a field consortium (FC) with seven types of spores: Glomus sp. 1 (103sporesg−1), Claroideoglomus claroideum (N.C. Schenck and G.S. Sm.) C. Walker and A. Schüßler (90sporesg−1), R. intraradices (34sporesg−1), Pacispora sp. 1 (27sporesg−1), Glomus sp. 3 (11sporesg−1), Glomus sp. 4 (10sporesg−1), Pacispora sp. 2 (7sporesg−1). AMF were selected from 9 abandoned fields in La Costa de Hermosillo (LCH), Sonora (28°35′–28°50′ N, 111°15′–111°35′ W). Field soils had salinity of 0.9 up to 5.2dSm−1, pH 8, sodium adsorption ratio of 3, exchangeable sodium percentage of 3.5 and 70% of sand; dominated by Atriplex canescens (Pursh) Nutt., Baccharis sarothroides A. Gray, and Encelia farinosa A. Gray ex Torr. The seven most frequently isolated AMF were included in this study. In order to increase the number of MI spores, Sorghum bicolor L. was cultivated with Glomus sp. 1 and 4, and for FC spores by cultivating C. pepo var. pepo in soil samples from LCH, obtaining an inoculum comprised of seven morphospecies of AMF.
Culture conditions and inoculate treatmentsFive seeds of C. pepo var. pepo were planted in 5L pots with a sterile substrate (5:2 peat moss: soil). Plants were grown under natural lightning until flowering, at 26.5% relative humidity and 24°C. For each treatment, 6 replicates were made, keeping three plants per pot. In all experiments, inoculation was performed 10d after planting, and treatments were established by inoculating hosts as follow: Inoculation of each plant with 3g of mixed isolate of native AMF (treatment MI), and 1.5g of the field consortium (FC). A third treatment was an exotic species, 1g of C. claroideum, from a temperate region of Central Mexico from Hueyotlipan, Tlaxcala (treatment EI). This strain was not included in FC. Negative controls for each stress treatment consisted in plants without AMF which received 1g of sterile soil as inoculate (treatment C).
Stress by drought and salinityExperiments to evaluate mycorrhization effects on plant responses to drought and salinity exposure were executed by separate. The first experiment was run for 57d and evaluated the effects of drought and AMF inoculation on C. pepo var. pepo. While the second experiment dealt with responses to high and low salinity and lasted 47d. Assessment of drought effects took place in the summer, while salinity experiments were carried out in the fall. Plants subjected to drought stress were irrigated with 25–50mL per day (soil humidity of 25–40%). Soil moisture was determined gravimetrically. Low salinity stress was created by irrigating plants with a 40mM NaCl solution, maintaining a salinity of 3.8±1.2dSm−1. High salinity was achieved by irrigation with a 50mM NaCl solution to maintain a mean salinity of 4.5dSm−1 (3.5–6dSm−1). Salinity was measured based on samples electrical conductivity values according to Shirokova et al.16 Non-stressed (no drought, neither salinity) controls, were irrigated with 100mL of water per day (soil moisture content of 50–60% and 1.4±0.4dSm−1).
Response assessmentTotal dry weights (both aerial and radicular) were measured by drying soil debris-free plant material in a lab oven (Blue M Stabil-Therm, model D-5226-Q, Evansville, IN, USA) at 60°C for 72h. Leaf water content was quantified by the difference between leaf sections fresh and dry weights and expressed on a percentage basis. Water potential (Ψw) was quantified with a Scholander pump (PMS Instrument Co., Albany, OR, USA). Samples were frozen at −80°C to obtain the intracellular exudate of the foliar tissue and then measure their osmolarity (Ci, mmol/kg) with an osmometer (Wescor Vapor 5520, Logan, UT, USA). Results of osmolarity were converted to osmotic potential (Ψs) through the Van’T Hoff equation: Ψs=−CiRT, where Ci refers to osmolarity, R gases constant and T to temperature (°K).
To estimate construction costs, leaves were harvested from every plant on each treatment and dried at 60°C for 24h. Glucose was estimated from the amount of carbon and energy required for tissue construction (g glucose g−1dry weight) by measuring the combustion heat (ΔHC) with a Parr calorimeter pump (model 1341, Moline, IL, USA), the ashes (A) in a muffle furnace (Thermolyne type, model 30400, New York, NY, USA), and nitrogen content (N) (LECO PF-528, St. Joseph, MI, USA). Resulting values were used to estimate the construction costs (CC) according to Carey et al.17:
where k is the oxidation state of the nitrogenized substrate (ranging from +5 nitrates to +3 ammonium) and EG is the conversion efficiency (0.89).18Chlorophyll fluorescence was measured at 25–28°C with a Handy PEA fluorimeter (Hansatech Instruments Ltd., King's Lynn, NO, England) equipped with a three high-intensity light emitting diode array at an excitation wavelength of 650nm and an emission wavelength range of 680–750nm, over a foliar surface of 4mm in diam. Readings were taken at 0.05, 0.1, 0.3, 2, and 30mm on the third leaf from the meristem after keeping the measured leaf section in absence of light for 30min.
Evaluated variables included basal fluorescence intensity (F0), maximum fluorescence intensity (Fm), and time to reach maximum fluorescence (Tfmax). These values were used to estimate the absorbed-electron flux per surface unit index (ABS/CS), the excitons canalized to the reaction center per surface unit index (TRo/CS), and the amount of electrons transferred to the electron transport chain for estimation of chemical energy (Et0/CS). Based on these values, the performance index (PI) was calculated using the equation, through the Handy PEA 32 bit Windows® software, as in Strasserf et al.19:
Mycorrhization was evaluated by processing samples through the wet-sieving method. The total number of spores (TNS) was quantified, morphospecies for each sample were identified through the saccharose floating method, and by visualization in an optical microscope. Mycorrhizal colonization (MC) was evaluated by dying with trypan blue 10–150mg of dry root (45°C). To measure the external mycelia, 0.02–0.25g aliquot parts of sieved material were placed in a microscope slide with glycerin and the number of hyphae was quantified counting the amount found along four evenly spaced lines marked in the cover slip. This methodology was based on that described by Herrera-Peraza et al.20
To identifying morphospecies, spores were separated using stereo microscope and then mounted on polyvinyl-lacto-glycerol (PVLG) and PVLG/Melzer (1:1 v/v) to observe the different structures through an optical compound microscope (Axioskop 2 plus, Zeiss, Oberkochen, Germany). Morphospecies were numbered as they were found through the samples, separating those identified to a described genus from those that were not identified. Morphospecies were classified by size, color, spore-wall thickness, presence and width of germinating walls, ornamentation, attachment to a suspending hypha, and the Melzer reaction based on the descriptions available on the International Culture Collection of Vesicular and Arbuscular Mycorrhizal Fungi website (http://invam.wvu.edu), http://www.agro.av.szczecin.pljblazkowski/index.html website, and the original descriptions of morphospecies.
Statistical analysisData were analyzed with general linear models using NCSS v2007.21 All data were submitted to analysis of variance (ANOVA) for a completely randomized design in a factorial arrangement of 4×2 (drought stress) and 4×3 (salinity stress). Results for radicular and aerial dry weights, aerial:radicular weight ratios, leaf water content, water and osmotic potentials, and construction costs for treatments (MI, FC and EI) were compared to negative controls (C) for each condition: irrigated, drought stressed, normal salinity, low salinity and high salinity. To analyze mycorrhization features (external mycelia, mychorrizal colonization, and total number of spores), data obtained from treatments MI and FC were compared to those from treatment EI. The development of each one of the morphospecies was estimated by the amount of spores (total and per species) of the six replicates. Significant differences (p≤0.05) by a combination of factors were compared based on standard error. For mycorrhization characteristics with high salinity an ANOVA was applied and statistical differences of means were compared by Tukey-Kramer (p≤0.05) method.
ResultsTable 1 shows growth, water status, and construction costs for control plants (C), as well as the mean differences for mycorrhized plants. The differences in plant growth grown irrigated or drought stresses were not statistically significant. Values for aerial:radicular ratio (A:R), aerial dry weight (ADW) and radicular dry weight (RDW) were similar when comparing the results obtained for non-inoculated controls and the different AMF inoculates (MI, FC and EI).
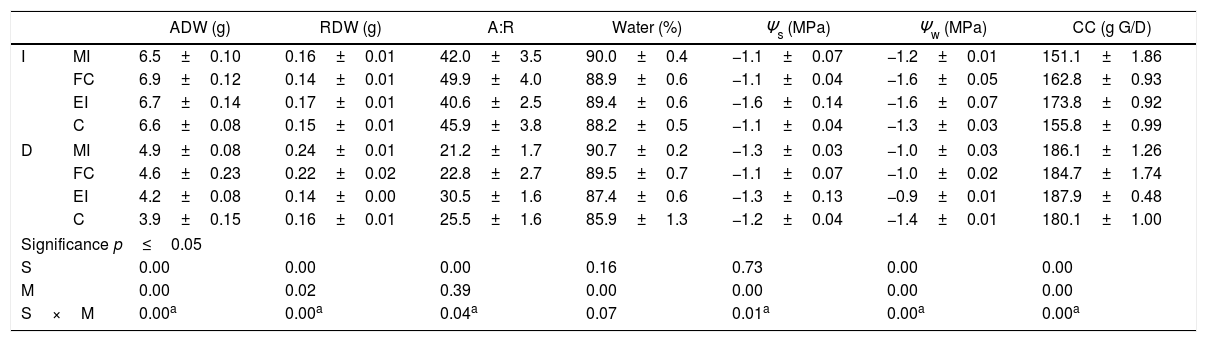
Effects of the mycorrhizal inoculation of Cucurbita pepo var. pepo irrigated (I) and stressed with drought (D). The assessed variables were: aerial dry weight (ADW), radicular dry weight (DRW), aerial: radicular dry weight ratio (A:R), foliar water content (water), osmotic potential (Ψs), water potential (Ψw), and construction costs (CC, g G/D: grams of glucose per dry weight).
| ADW (g) | RDW (g) | A:R | Water (%) | Ψs (MPa) | Ψw (MPa) | CC (g G/D) | ||
|---|---|---|---|---|---|---|---|---|
| I | MI | 6.5±0.10 | 0.16±0.01 | 42.0±3.5 | 90.0±0.4 | −1.1±0.07 | −1.2±0.01 | 151.1±1.86 |
| FC | 6.9±0.12 | 0.14±0.01 | 49.9±4.0 | 88.9±0.6 | −1.1±0.04 | −1.6±0.05 | 162.8±0.93 | |
| EI | 6.7±0.14 | 0.17±0.01 | 40.6±2.5 | 89.4±0.6 | −1.6±0.14 | −1.6±0.07 | 173.8±0.92 | |
| C | 6.6±0.08 | 0.15±0.01 | 45.9±3.8 | 88.2±0.5 | −1.1±0.04 | −1.3±0.03 | 155.8±0.99 | |
| D | MI | 4.9±0.08 | 0.24±0.01 | 21.2±1.7 | 90.7±0.2 | −1.3±0.03 | −1.0±0.03 | 186.1±1.26 |
| FC | 4.6±0.23 | 0.22±0.02 | 22.8±2.7 | 89.5±0.7 | −1.1±0.07 | −1.0±0.02 | 184.7±1.74 | |
| EI | 4.2±0.08 | 0.14±0.00 | 30.5±1.6 | 87.4±0.6 | −1.3±0.13 | −0.9±0.01 | 187.9±0.48 | |
| C | 3.9±0.15 | 0.16±0.01 | 25.5±1.6 | 85.9±1.3 | −1.2±0.04 | −1.4±0.01 | 180.1±1.00 | |
| Significance p≤0.05 | ||||||||
| S | 0.00 | 0.00 | 0.00 | 0.16 | 0.73 | 0.00 | 0.00 | |
| M | 0.00 | 0.02 | 0.39 | 0.00 | 0.00 | 0.00 | 0.00 | |
| S×M | 0.00a | 0.00a | 0.04a | 0.07 | 0.01a | 0.00a | 0.00a | |
These values correspond to the mean±standard error of the results for the mixed inoculums (MI), field consortium (FC), exotic inoculums (EI) and control (C). Factorial analysis asignificance p≤0.05 among the stress treatment (S) and mycorrhization (M).
After 57d of culture with irrigated treatment, changes in some variables related to the plant water status were observed when comparing C with the AMF inoculated treatments. A 0.7% decline in foliar water content was detected for MI, 0.33 vs. 0.6MPa in Ψs for EI and FC respectively. Also, the construction cost was less for FC (0.12g of glucoseg−1 of foliar weight) compared with C. In the 47d cultures with normal salinity, the water status of AMF inoculated plants was similar and the construction cost was equal or larger (0.02g of glucoseg−1 of dry weight for EI) with respect to C, except for MI, where Ψw increased in 0.9MPa and construction costs declined in 0.08g of glucoseg−1 of dry weight (Table 2).
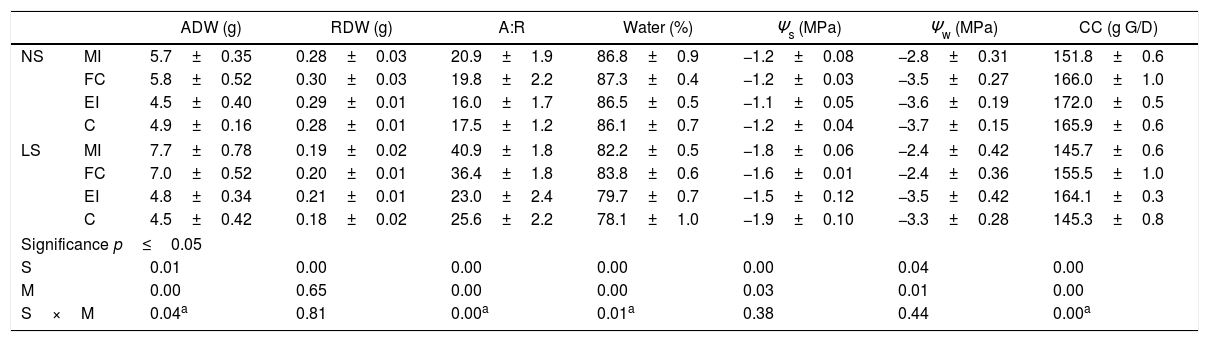
Effects of the mycorrhizal inoculation of Cucurbita pepo var. pepo normal salinity (NS) and stressed with low salinity (LS). The assessed variables were: aerial dry weight (ADW), radicular dry weight (DRW), aerial: radicular dry weight ratio (A:R), foliar water content (water), osmotic potential (Ψs), water potential (Ψw), and construction costs (CC, g G/D: grams of glucose per dry weight).
| ADW (g) | RDW (g) | A:R | Water (%) | Ψs (MPa) | Ψw (MPa) | CC (g G/D) | ||
|---|---|---|---|---|---|---|---|---|
| NS | MI | 5.7±0.35 | 0.28±0.03 | 20.9±1.9 | 86.8±0.9 | −1.2±0.08 | −2.8±0.31 | 151.8±0.6 |
| FC | 5.8±0.52 | 0.30±0.03 | 19.8±2.2 | 87.3±0.4 | −1.2±0.03 | −3.5±0.27 | 166.0±1.0 | |
| EI | 4.5±0.40 | 0.29±0.01 | 16.0±1.7 | 86.5±0.5 | −1.1±0.05 | −3.6±0.19 | 172.0±0.5 | |
| C | 4.9±0.16 | 0.28±0.01 | 17.5±1.2 | 86.1±0.7 | −1.2±0.04 | −3.7±0.15 | 165.9±0.6 | |
| LS | MI | 7.7±0.78 | 0.19±0.02 | 40.9±1.8 | 82.2±0.5 | −1.8±0.06 | −2.4±0.42 | 145.7±0.6 |
| FC | 7.0±0.52 | 0.20±0.01 | 36.4±1.8 | 83.8±0.6 | −1.6±0.01 | −2.4±0.36 | 155.5±1.0 | |
| EI | 4.8±0.34 | 0.21±0.01 | 23.0±2.4 | 79.7±0.7 | −1.5±0.12 | −3.5±0.42 | 164.1±0.3 | |
| C | 4.5±0.42 | 0.18±0.02 | 25.6±2.2 | 78.1±1.0 | −1.9±0.10 | −3.3±0.28 | 145.3±0.8 | |
| Significance p≤0.05 | ||||||||
| S | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | |
| M | 0.00 | 0.65 | 0.00 | 0.00 | 0.03 | 0.01 | 0.00 | |
| S×M | 0.04a | 0.81 | 0.00a | 0.01a | 0.38 | 0.44 | 0.00a | |
These values correspond to the mean±standard error of the results for the mixed inoculums (MI), field consortium (FC), exotic inoculums (EI) and control (C). Factorial analysis asignificance p≤0.05 among the stress treatment (S) and mycorrhization (M).
When stress by drought and inoculation with native AMF was applied (MI and FC), constructions costs were similar to C (Table 1). MI increased in ADW (1g), foliar water content (4.5%) and Ψw (0.45MPa) in relation to C. Minor increases were detected for FC, 0.6g in ADW, 3.6% in foliar water content and 0.38MPa in Ψw without decrease in Ψs. With the exotic species no differences were observed in most of the evaluated variables vs. C, except for the construction costs where an increase took place in EI (>0.03g of glucoseg−1 of dry weight compared to C).
When low-salinity stress was applied (Table 2), the inoculates composed by native AMF (treatments MI and FC) produced an increase in ADW, A:R and foliar water content of the plants. On the other side, construction cost did not change with MI, while with FC it was 0.04g of glucoseg−1 of dry weight more in relation to C. Under low-salinity stress, a larger benefit was observed in the increase of plant growth with the mixed native AMF inoculate in comparison to C (3g of ADW and 15 units of A:R with MI in comparison to 2.5g of ADW and 10 units of A:R quantified in FC). Foliar water content was improved with FC (5.7%), while with MI the difference was of 4.1%. In treatment EI, an increase of 0.9g of glucoseg−1 dry weight in construction cost was observed in respect to C; however, no statistically significant differences were observed in growth and water status.
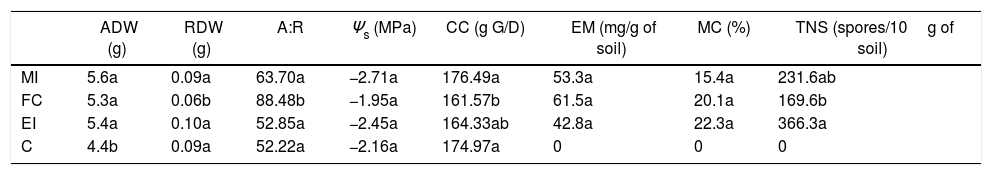
Under high-salinity, construction costs associated with FC was significantly higher than C (0.05g of glucoseg−1 of dry weight). Compared to C, plants inoculated with FC increased 0.9g of ADW, 36 units of A:R and radicular growth diminished (0.3g); while MI and C were similar in RDW, AR, Ψs, and CC (Table 3).
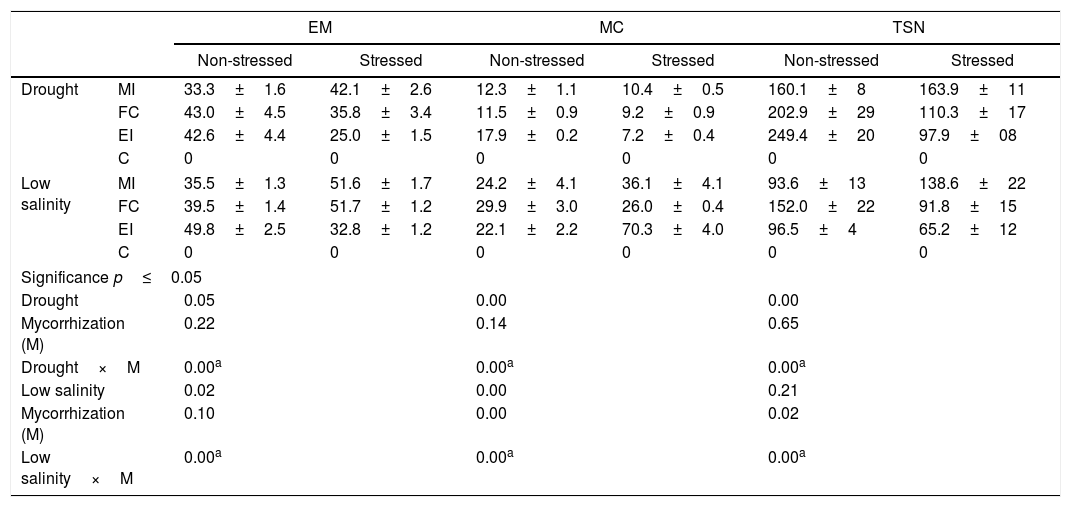
Mycorrhization characteristics and effects of the inoculation of Cucurbita pepo var. pepo with high salinity. The assessed variables were: aerial dry weight (ADW), radicular dry weight (DRW), aerial: radicular dry weight ratio (A:R), osmotic potential (Ψs), construction costs (CC, g G/D: grams of glucose per dry weight), external mycelium (EM), mycorrhizal colonization (MC), and total number of spores (TNS).
| ADW (g) | RDW (g) | A:R | Ψs (MPa) | CC (g G/D) | EM (mg/g of soil) | MC (%) | TNS (spores/10g of soil) | |
|---|---|---|---|---|---|---|---|---|
| MI | 5.6a | 0.09a | 63.70a | −2.71a | 176.49a | 53.3a | 15.4a | 231.6ab |
| FC | 5.3a | 0.06b | 88.48b | −1.95a | 161.57b | 61.5a | 20.1a | 169.6b |
| EI | 5.4a | 0.10a | 52.85a | −2.45a | 164.33ab | 42.8a | 22.3a | 366.3a |
| C | 4.4b | 0.09a | 52.22a | −2.16a | 174.97a | 0 | 0 | 0 |
These values correspond to the mean of the results for the mixed inoculums (MI), field consortium (FC), exotic inoculums (EI) and control (C). Means with different letters in a column are different (Tukey-Kramer p≤0.05).
The mycorrhization features for native inoculates were compared to those quantified with EI. It was observed that after 57d without stress applied, the external mycelia were similar, while the percentage of MC and TNS was significantly lower for MI and FC with respect to EI. On the other hand, after 47d in culture, the external mycelia for MI and FC was 10mgg−1 of soil less than in EI; MC was similar between all individuals and TNS only increased by 50 spores per 10g−1 of soil with FC vs. EI (Table 4).
Mycorrhization characteristics in Cucurbita pepo var. pepo under conditions of stress by drought, low salinity and non-stressed. The assessed variables were external mycelium (EM), mycorrhizal colonization (MC), and total number of spores (TNS).
| EM | MC | TSN | |||||
|---|---|---|---|---|---|---|---|
| Non-stressed | Stressed | Non-stressed | Stressed | Non-stressed | Stressed | ||
| Drought | MI | 33.3±1.6 | 42.1±2.6 | 12.3±1.1 | 10.4±0.5 | 160.1±8 | 163.9±11 |
| FC | 43.0±4.5 | 35.8±3.4 | 11.5±0.9 | 9.2±0.9 | 202.9±29 | 110.3±17 | |
| EI | 42.6±4.4 | 25.0±1.5 | 17.9±0.2 | 7.2±0.4 | 249.4±20 | 97.9±08 | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | |
| Low salinity | MI | 35.5±1.3 | 51.6±1.7 | 24.2±4.1 | 36.1±4.1 | 93.6±13 | 138.6±22 |
| FC | 39.5±1.4 | 51.7±1.2 | 29.9±3.0 | 26.0±0.4 | 152.0±22 | 91.8±15 | |
| EI | 49.8±2.5 | 32.8±1.2 | 22.1±2.2 | 70.3±4.0 | 96.5±4 | 65.2±12 | |
| C | 0 | 0 | 0 | 0 | 0 | 0 | |
| Significance p≤0.05 | |||||||
| Drought | 0.05 | 0.00 | 0.00 | ||||
| Mycorrhization (M) | 0.22 | 0.14 | 0.65 | ||||
| Drought×M | 0.00a | 0.00a | 0.00a | ||||
| Low salinity | 0.02 | 0.00 | 0.21 | ||||
| Mycorrhization (M) | 0.10 | 0.00 | 0.02 | ||||
| Low salinity×M | 0.00a | 0.00a | 0.00a | ||||
These values correspond to the mean±standard error of the results for the mixed inocula (MI), field consortium (FC), exotic inocula (EI) and control (C). Factorial analysis asignificance p≤0.05 among the stress treatment (drought or low salinity) and mycorrhization (M).
Under the three evaluated stress conditions, native AMF inoculates (MI and FC) presented a significantly higher growth of external mycelia (10–20mgg−1 of soil) than EI, at the high-salinity treatment a difference higher than 20mgg−1 of soil was found (Table 3). MC under drought conditions was similar between inoculates (Table 4), while salinity diminished the values for this variable with respect to EI, being 30% lower with native AMF (low-salinity treatment) and only 10% lower with MI (high-salinity treatment) (Table 4).
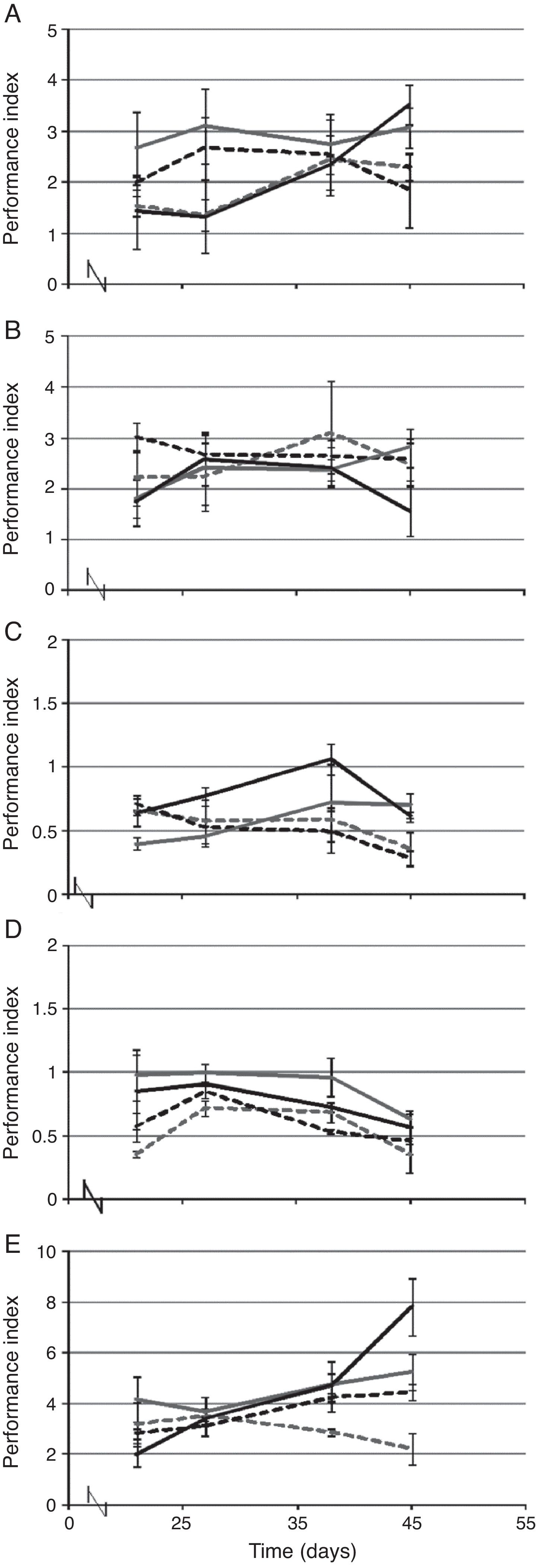
Fig. 1 shows differences in performance index (PI) between inoculation treatments were observed at day 45. The intervals for PI values were different between experiments for assessing drought (1–4 units), low salinity (0.3–1 units) and high-salinity (2–8 units). No significant differences were found among the different inoculation treatments under non-stressed conditions (Fig. 1B and D).
Performance index (PI) assessed at 21, 27, 38 and 45d in culture and calculated through readings of chlorophyll fluorescence in Cucurbita pepo var. pepo under conditions of drought (A), irrigated (B), low salinity (C), normal salinity (D), and high salinity (E). These values are the arithmetic mean for three replicates, vertical lines represent standard deviation. Treatment C (
), treatment EI (- - -), treatment FC (—), treatment MI (). Symbols for each inoculation treatment are the same as in Table 1.Performance index for C. pepo var. pepo applying drought is shown in Fig. 1A; when plants were treated with MI and FC, this index was higher than 3 units and significantly higher than EI (2.2 units) and C (1.8 units) after 45d. Under low-salinity conditions (Fig. 1C), plants inoculated with native fungi exhibited a PI higher than 0.6 units and higher than EI (0.35 units), while the lowest value was measured for C (0.28 units). For cultures under high-salinity stress, the highest PI was detected for treatment FC (7.8 units), while this value was similar among cultures treated with MI and C (4–5 units) and higher in relation to plants treated with EI (2.21 units).
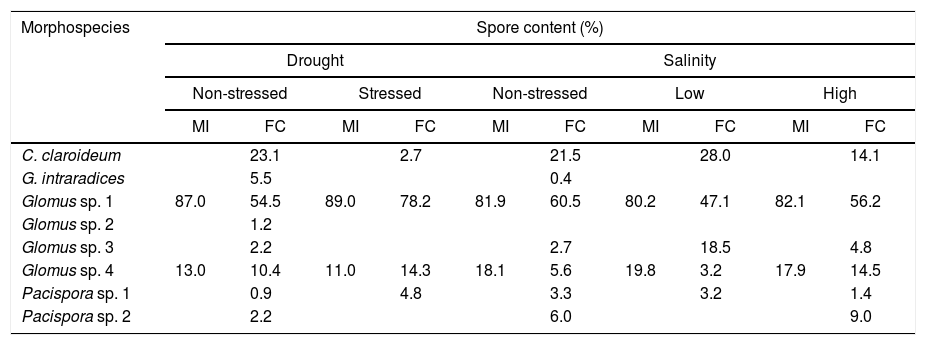
Table 5 shows the differences in spore content by morphospecies of native AMF inoculated on C. pepo var. pepo under conditions of stress by drought, low and high salinity, and non-stressed. When inoculating MI, regardless of stress condition, spore composition was Glomus sp. 1 and Glomus sp. 4 ranging from 80.2 to 89 and 11 to 19.8% respectively.
Native AMF morphospecies composition inoculated onto Cucurbita pepo var. pepo following exposure to different drought and salinity stresses.
| Morphospecies | Spore content (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drought | Salinity | |||||||||
| Non-stressed | Stressed | Non-stressed | Low | High | ||||||
| MI | FC | MI | FC | MI | FC | MI | FC | MI | FC | |
| C. claroideum | 23.1 | 2.7 | 21.5 | 28.0 | 14.1 | |||||
| G. intraradices | 5.5 | 0.4 | ||||||||
| Glomus sp. 1 | 87.0 | 54.5 | 89.0 | 78.2 | 81.9 | 60.5 | 80.2 | 47.1 | 82.1 | 56.2 |
| Glomus sp. 2 | 1.2 | |||||||||
| Glomus sp. 3 | 2.2 | 2.7 | 18.5 | 4.8 | ||||||
| Glomus sp. 4 | 13.0 | 10.4 | 11.0 | 14.3 | 18.1 | 5.6 | 19.8 | 3.2 | 17.9 | 14.5 |
| Pacispora sp. 1 | 0.9 | 4.8 | 3.3 | 3.2 | 1.4 | |||||
| Pacispora sp. 2 | 2.2 | 6.0 | 9.0 | |||||||
Spore content (%) was calculated based on the sum of spores (total and per species) of six replicates. Captions for each inoculation treatment are as in Table 1.
FC inoculated plants subjected to water stress yielded spores from only four morphospecies; according to their prevalence and in descending order, those were: Glomus sp. 1>Glomus sp. 4>Pacispora sp.>C. claroideum (Table 5). Under low salinity, a value of 47.1% was quantified for Glomus sp. 1, 28% for C. claroideum, 18.5% for Glomus sp. 3 and finally, 3.2% for Pacispora sp. and Glomus sp. 4. On the other hand, under high salinity conditions, 6 of the 7 inoculated morphospecies were found in the sporulate form. The lowest sporulation was found for Pacispora sp. (1.4%) and Glomus sp. 3. (4.8%); while the highest values corresponded to Glomus sp. 1 (56.2%). In all abiotic stress conditions, Glomus sp. 1 resulted in the highest spore count when plants were inoculated with FC.
DiscussionPrevious ecological and agronomical studies demonstrated how plant-AMF associations mitigate the damaging effects caused by water scarcity and soil salinity, improving plant growth and water status in several species, in contrast with non-mycorrhized plants under equal stressing conditions.9 However, plant response to stress may also vary depending on the fungi species.3,22,23 This study showed that symbiosis efficiency differed in relation to the inoculate composition and the origin of the AMF used. Under drought and salinity conditions, the dry weight and leaf water content of plants inoculated with an AMF species from a temperate region (EI) were similar to non-inoculated controls, while those inoculated with native AMF (MI and FC) did improved growth and water status (Tables 1 and 2).
Soil properties affect mycelium extension rate in several ways, since some AMF species are more susceptible to drought and saline conditions.3,10,24 The stress conditions assessed in this study reduced C. claroideum extracellular growth in comparison to the native species used as inoculum, since under drought and salinity, the amount of mycelia produced by MI and FC was over 50% highest (Tables 3 and 4).
Beneficial effects of mycorrhization with native AMF in growth and water status of C. pepo var. pepo can be related to the high external mycelia growth in MI and FC. A similar response was described in Phaseolus vulgaris and Latuca sativa grown under water stress, because a direct relationship was established between the greatest increases in biomass, soil water potential, and leaf lethal water potential with those AMF producing the largest extracellular mycelia.22,23
Salts and changes in water availability modify mycorrhizal colonization.25 Largest mycorrhizal colonization in Citrus tangerine resulted in improved dry weight and relative water content when plants were grown under drought.7 Our results are in agreement since the inoculants that mitigated drought negative effects (MI and FC) produced larger root mycorrhization than exotic inoculants (EI), which did not improve neither growth, nor plant water status.
In contrast to observations under drought, with high and low salinity the colonization percentage is smaller with the more effective treatments (Tables 3 and 4). However this does not agree with reports in Latuca sativa grown under saline stress, where the most beneficial AMF species showed the highest colonization.3 Plants inoculated with native AMF (MI and FC) and grown under drought or low salinity showed a significant increase in growth and water status, maintaining tissue construction costs similar or lower than those of non-inoculated plants (Tables 1 and 2). In a previous study with C. pepo grown under drought conditions, mycorrhization with R. intraradices improved stomata conductance and water potential gradient when water-loss rates varied by transpiration, while gas exchange increased to compensate for the additional carbon transferred to fungi.26
Performance index calculations, based in chlorophyll fluorescence readings, are measurements of plant relative vitality.19 Plant photochemistry assessment is a good indicator of its photoprotection under stress conditions,27 additionally this sort of assessment has been done to evaluate vitality in mycorrhized plants in terms of energy use efficiency during molecule biosynthesis.14,15 This study shows that the inoculation of native AMF (MI and FC) increased PI in comparison to non-stressed cultures and in relation to C and EI treatments, mostly under low salinity. Abo-Ghalia and Khalafallah25 found that, mycorrhization of Triticum aestivum under drought improved photosynthetic rates and increased carotenoid pigments and chlorophyll to counteract against photodestruction or photoinhibition.
Differences in intervals, including PI, in experiments 1 (drought assessment) and 2 (low salinity assessment) might have affected by changes in light intensity and temperature, given that experiments took place in different seasons. These differences have been previously documented in other plant species submitted to light and temperature stress.28
Mycorrhization implicates an increase in plant energy costs, however several studies prove that construction costs (CC) are lower when AMF improve efficiency in nutrient usage as well as the organism's adaptation to environmental conditions.29 In this case, the negative effects of drought and salinity over growth and water status are smaller when plants are mycorrhized by native fungi (Tables 1 and 2). The greatest benefit was obtained from MI and FC, where CC were similar or lower than C, while their PI's were highest.
The benefits given by AMF in terms of nutrition and water use efficiency compensate the energy expenditure implied in the transference of photoassimilates to the fungus.30 MI was most effective under stress by low salinity and drought, while FC was more effective under high salinity conditions. Additionally, there was an increased efficiency in the photochemistry capacity (measured through PI) in plants mycorrhized with native fungi, which suggests a compensation for the energy expend and carbon transference to the fungus. Thus tissue CC is similar or lower to those of plants associated with less effective AMF (EI).
Saline conditions either destroy PSII reaction centers or interrupt electron transference,31 such effects are mitigated when plants are associated with AMF by increasing chlorophyll content and photosynthetic capacity with respect to non-mycorrhized cultures.32,33 Changes in photosynthetic capacity are related to water status and gas exchange34 and also to electron transport capacity. In this study, under high salinity conditions, the greatest increase in PI was observed in those cultures grown in substrates inoculated with FC, which exhibited less damage by salts, allowing to the conservation of CC similar to non-mycorrhized plants and implying a similar efficiency to the non-stressed negative control (Table 3, Fig. 1E).
Even though the AMF-plant interaction is slightly specific, there are biochemical characteristics involved with the plant-fungus association function, which modify the success of the symbiosis.35 Even there are AMF ecotypes better adapted to certain environmental conditions.10,24 In the native inoculates (MI and FC), Glomus sp. 1 demonstrated to be most efficient in sporulation, despite all the assessed conditions. Thus, it is considered that this species associates better with C. pepo var. pepo and is adapted to the assessed stress conditions.
Also, some of the morphospecies initially present in FC showed no sporulation under drought or salinity stresses (Table 5). The variations in AMF diversity observed in these experiments were similar to those found in wheat inoculated with a mixed isolate of Glomus spp. under stress by drought, in which species diversity decreased as water availability abated.25
ConclusionAMF native from the Sonoran Desert mitigated the harmful effects of stress by drought and salinity on growth and water status of C. pepo var. pepo in comparison with C. claroideum native from a temperate region. The mixed inoculate MI, composed of two morphospecies of native AMF was the most beneficial under conditions of drought or low salinity. Under high salinity, the field consortium composed of 7 morphospecies of native AMF (FC) resulted better for plant adaptation to this stress condition. Benefits were related to the high amounts of external mycelia quantified in the soil, low CC, and to an increase of photochemical capacity measured as PI.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the financial support of project IAI-CRN II-14, US-NSF (GEO-04523250) and to CONACYT-Mexico for granting a PhD scholarship to CH. Thanks to J. Llano (UNISON), L. Contreras-Angulo and J. Antonio Orozco (CIAD) for their technical assistance. Thanks are also in order to MSc. L. Hernández-Cuevas (Universidad Autónoma de Tlaxcala) for providing the C. claroideum inoculate.