Vulvovaginal candidiasis (VVC) is an infection of the genital mucosa caused by different species of the genus Candida. Considering the lack of data on this topic in the south of Brazil, this study aimed to assess the prevalence of Candida spp. in the cervical-vaginal mucosa of patients treated at a university hospital in southern Rio Grande do Sul, as well as the etiology and the susceptibility of the isolates against fluconazole, itraconazole, miconazole and nystatin. Samples were collected at the gynecology clinic of the Federal Hospital of the University of Rio Grande, and the isolates were identified using phenotypic and biochemical tests. The susceptibility analysis was performed according to the CLSI M27-A2 protocol. Of the 263 patients included, Candida spp. was isolated in 27%, corresponding to a prevalence of approximately 15% for both VVC and colonization. More than 60% of the isolates were identified as Candida albicans; C. non-albicans was isolated at a rate of 8.6% in symptomatic patients and 14.3% in asymptomatic patients. The prevalence of resistance against fluconazole and itraconazole was 42% and 48%, respectively; the minimal inhibitory concentration of miconazole ranged from 0.031 to 8μg/mL, and that of nystatin ranged from 2 to >16μg/mL. The high rate of resistance to triazoles observed in our study suggests the necessity of the association of laboratory exams to clinical diagnosis to minimize the practice of empirical treatments that can contribute to the development of resistance in the isolates.
Vulvovaginal candidiasis (VVC) is characterized by an infection of the genital mucosa by Candida yeasts, which mainly undertakes the vulva and the vagina. The disease occurs endogenously due to predisposing factors that favor yeast multiplication. Signs and symptoms such as itching, burning, cracking, erythema and vulvar edema, leukorrhea and the presence of whitish plaques on the vaginal mucosa are common.1 VVC is considered the second most common cause of genital infection in women of reproductive age, and although it represents a problem of global importance in public health, its exact incidence is unknown.2,3
Risk factors for vulvovaginitis include pregnancy, use of oral contraceptives, genetic predisposition, and previous antibiotic therapy, among others. The incidence of infection, as well as the increase in colonization of the mucosa by the yeast, is also higher in women with diabetes due to their higher glycogen levels and in those with HIV due to immune suppression.4,5
Studies show that 70–75% of all women of reproductive age develop at least one case of VVC during their lifetime.6,7 The recurrence rate is 40–50%, and approximately 5–8% develop recurrent vulvovaginal candidiasis (RVVC), which is characterized by four or more episodes of disease over a period of twelve months.6–8
The main etiologic agent is Candida albicans, accounting for 70–90% of VVC cases.7,8 Among the non-albicans species C. glabrata is highlighted7–9 due to its prevalence and resistance to azoles.10,11
An increasing number of Candida spp. clinical isolates are resistant to antifungal agents routinely used for the treatment of VVC. In addition, studies in different regions of Brazil suggest that geographical factors interfere in the prevalence of Candida species as well as in the sensitivity of isolates to antifungals.1,11–13 Considering this and the lack of data on this topic in Rio Grande do Sul, this study aimed to identify the prevalence of Candida spp. isolated from the vaginal mucosa of patients with and without vulvovaginitis treated at a university hospital in extreme southern Brazil, as well as the susceptibility of these isolates to four antifungals used in gynecological routines.
Materials and methodsThe study included 263 patients attended on from April 2013 to October 2014 at the gynecology clinic of the University Hospital of the Federal University of Rio Grande (FURG) – Rio Grande do Sul – Brazil. The women agreed to participate by signing the Clarified Informed Consent form. The sample size, calculated using Epi Info 6.0 and considering a prevalence of 30%, a confidence level of 99%, maximum permissible error of 20% and losses of 10%, was estimated to be a sample of 249 patients.
During the gynecological exam, a sample of cervical-vaginal mucosa was collected using a sterile brush, which was stored in sterile tubes containing PBS and kept refrigerated until processing. The sample was sent to the Mycology Laboratory of the Medicine Faculty from FURG, where it was processed within 12h. The cultures were processed in Sabouraud agar with chloramphenicol 0.01% (SCl) and incubated at 37°C for seven days with daily assessment of growth. The yeasts were identified using phenotypic tests such as micromorphology, chromogenic medium (CHROM Agar Candida®), germ tube test, and microculture in agar cornmeal and were confirmed by biochemical automated method (VITEK® 2). Each isolate was maintained on potato dextrose agar (PDA) in the mycology collection at room temperature under freezing at −20°C in saline with 30% glycerol for subsequent susceptibility testing.
The variables evaluated in the study were obtained by self-administered pre-coded questionnaire and included age, skin color, educational level, marital status, family income, HIV infection status, pregnancy, contraception and vaginal pH at the time of sample collection. Signs and symptoms assessed during the gynecological exam were used to consider women as colonized or presenting VVC, the latter being those that had leukorrhea, pruritus, edema and/or vulvovaginal erythema associated with the presence of whitish plaques in the mucosa and the isolation of Candida spp. in mycological cultivation.
The in vitro susceptibility analysis of clinical isolates was performed by broth microdilution using the M27-A2 standard protocol CLSI (2002).14 For the standardization of fungal inoculum, isolates were subcultured on PDA for 24h at 37°C, young colonies were homogenized in sterile saline, and the turbidity was adjusted to 0.5 on the McFarland scale by spectrophotometry (530nm) to obtain a concentration of 1–5×106CFU/mL. Then, a dilution was performed at 1:100 and 1:20 in RPMI 1640, resulting in a concentration of 5×102 to 2.5×103CFU/mL. The inoculum concentration was confirmed using the Pour-plate technique. The susceptibility test was performed at concentrations of 64μg/mL to 0.125μg/mL for fluconazole and at concentrations ranging from 16μg/mL to 0.031μg/mL for itraconazole, miconazole and nystatin.
The 96-well microplates were filled with 100μL of the antifungal concentrations and 100μL of inoculum and were incubated at 37°C for 48h along with growth and sterility controls. Each isolate was tested in duplicate, and the results were visually evaluated by turbidity, comparing the fungal growth in the well with the growth control. The minimum inhibitory concentration (MIC) of fluconazole, itraconazole and miconazole was one that inhibited 80% of yeast growth, and the MIC of nystatin inhibited 100% of growth.
According to the MIC result for fluconazole and itraconazole, the isolates were classified as S for sensitive, SDD for sensitive dose-dependent and R for resistant, as established by the cutoff point protocol M27-A2. For miconazole and nystatin, the MIC50 and MIC90 were calculated corresponding to the antifungal concentration capable of inhibiting the growth of 50% and 90%, respectively, of the isolates tested. After reading the results of MIC, 10μL of the solutions from the wells related to concentrations higher than and equal to the MIC were plated on a Petri dish containing agar SCl and were incubated at 37°C for 48h to determine the fungicide minimum concentration (FMC).
The study was conducted according to ethical principles and was approved by the Research Ethics Committee of the Federal University of Rio Grande (65/2012). The results were compiled by conducting descriptive analysis of the data and chi-square tests for categorical variables using SPSS 19.0® software. p-values below 0.05 were considered statistically significant.
ResultsThe study population consisted mostly of women who had partners (64.5%), had white skin color (65.3%), were pregnant (60.3%) and were HIV-negative (72.9%). The mean age was 28.5 years and ranged from 12 and 68 years, with more than 65% of the women under 31 years of age (n=176) and 54.7% (n=135) using hormonal contraception (Table 1).
Descriptive analysis of the study population (n=263) and the influence of the variables studied (chi-square test) in the development of vulvovaginal candidiasis (VVC) (n=35).
| Variables | n (%) | VVC (n) | p value |
|---|---|---|---|
| HIV-positive | 71 (27.1) | 10 | 0.833 |
| HIV-negative | 191 (72.9) | 25 | |
| Pregnant | 158 (60.3) | 26 | 0.069 |
| Not pregnant | 104 (39.7) | 09 | |
| Skin color | 0.542 | ||
| White | 160 (65.3) | 20 | |
| Not white | 85 (34.7) | 13 | |
| Marital status | 0.891 | ||
| Without a partner | 89 (35.5) | 11 | |
| With partner | 162 (64.5) | 21 | |
| Educational level | 0.326 | ||
| 8 years or less | 131 (51) | 20 | |
| 9 years or more | 126 (49) | 14 | |
| Family income | 0.577 | ||
| Less than 1 wage | 56 (21.3) | 09 | |
| 1–2 wages | 86 (32.7) | 12 | |
| 2.1 or more | 79 (30) | 08 | |
| Contraception | 0.599 | ||
| Hormonal | 135 (54.7) | 20 | |
| Not hormonal | 112 (45.3) | 14 | |
Of the 263 patients, Candida spp. was isolated in the mycological culture from cervical-vaginal samples in 27% (n=71). Of these, vulvovaginal candidiasis was diagnosed in 49.3% (n=35), resulting in a prevalence in the population of 13.3%. The remaining 50.7% (n=36) that had no signs or symptoms of vulvovaginitis were classified as colonization cases.
The predominant species was Candida albicans in both groups, corresponding to 62.9% (n=22) of isolates originating from the group of women colonized and 74.3% (n=26) from the women with VVC. The other species identified colonizing the vaginal mucosa of healthy women were C. glabrata in 14.3% (n=5), followed by C. sphaerica in 8.6% (n=3) and C. parapsilosis complex in 2.9% (n=1). Among cases of VVC by C. non-albicans (n=5), 8.6% (n=3) were due to C. glabrata, 2.9% (n=1) were due to C. parapsilosis complex, and 2.9% (n=1) were due to C. tropicalis. In addition, 11.4% (n=4) of the isolates from colonization, and 11.4% (n=4) of the etiologic agents of VVC were identified only in general, being classified as Candida spp.
Regarding the variables analyzed, 82.4% (n=28) of patients diagnosed with VVC belonged to the group of women aged 30 years or younger (p=0.047). For the other variables such as skin color, marital status, educational level, family income, contraception, pregnancy and HIV infection, there was no significant difference between the group of women with and without VVC (Table 1). The same occurred with the vaginal pH at the time of collection, in which 133 patients had pH≤4.5, but only 14.3% (n=19) of them were diagnosed with VVC (p=0.948).
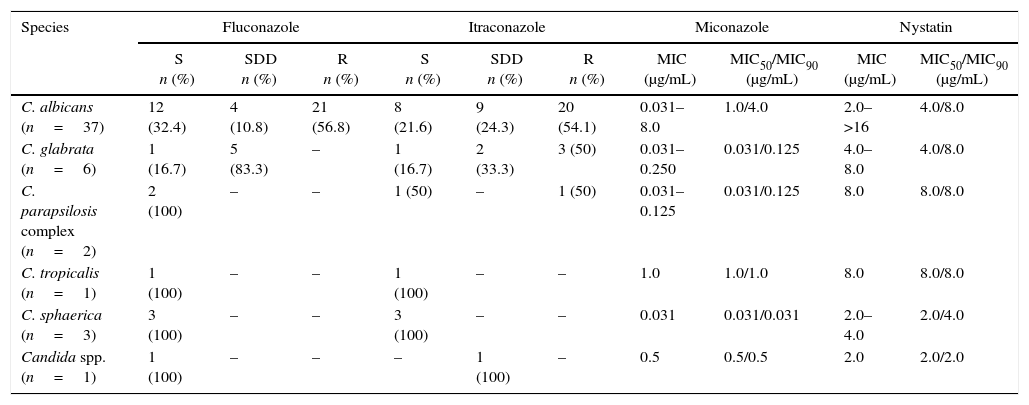
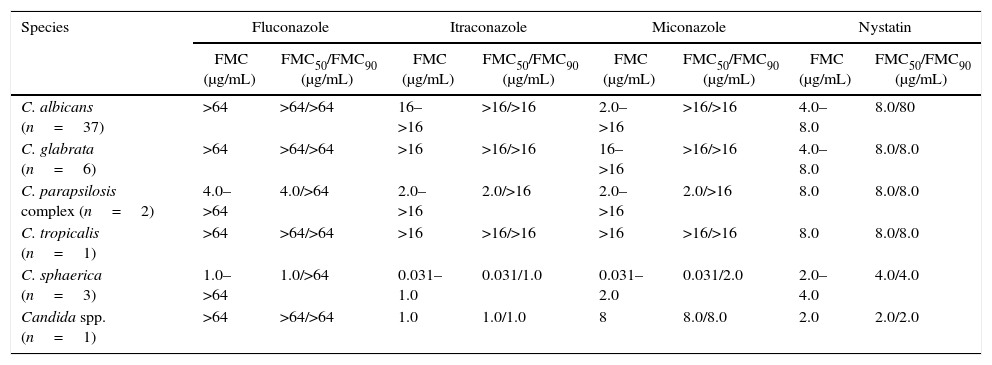
Of the 71 clinical isolates of Candida spp., 50 were subjected to in vitro susceptibility testing, 25 from healthy women and 25 from women with VVC. Considering all the isolates, regardless of species, the MIC to miconazole ranged from 0.031 to 8μg/mL, with MIC50=0.5μg/mL and MIC90=4μg/mL, and the MIC to nystatin ranged from 2 to >16μg/mL, with a MIC50=4μg/mL and MIC90=8μg/mL. Concerning fluconazole and itraconazole, resistance was observed in 42% and 48%, respectively, of the isolates (Table 2). No significant difference in MIC values was observed between clinical isolates of VVC and colonization. Concerning fluconazole, 100% of the isolates of C. albicans and C. glabrata showed an FMC>64μg/mL, and for itraconazole, 100% of C. glabrata and 97% of C. albicans showed an FMC>16μg/mL (Table 3).
Results of the in vitro susceptibility test of Candida spp. from colonized and/or VVC patients against four antifungal agents. S-sensitive, SDD-sensitive dose-dependent and R-resistant.
| Species | Fluconazole | Itraconazole | Miconazole | Nystatin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S n (%) | SDD n (%) | R n (%) | S n (%) | SDD n (%) | R n (%) | MIC (μg/mL) | MIC50/MIC90 (μg/mL) | MIC (μg/mL) | MIC50/MIC90 (μg/mL) | |
| C. albicans (n=37) | 12 (32.4) | 4 (10.8) | 21 (56.8) | 8 (21.6) | 9 (24.3) | 20 (54.1) | 0.031–8.0 | 1.0/4.0 | 2.0–>16 | 4.0/8.0 |
| C. glabrata (n=6) | 1 (16.7) | 5 (83.3) | – | 1 (16.7) | 2 (33.3) | 3 (50) | 0.031–0.250 | 0.031/0.125 | 4.0–8.0 | 4.0/8.0 |
| C. parapsilosis complex (n=2) | 2 (100) | – | – | 1 (50) | – | 1 (50) | 0.031–0.125 | 0.031/0.125 | 8.0 | 8.0/8.0 |
| C. tropicalis (n=1) | 1 (100) | – | – | 1 (100) | – | – | 1.0 | 1.0/1.0 | 8.0 | 8.0/8.0 |
| C. sphaerica (n=3) | 3 (100) | – | – | 3 (100) | – | – | 0.031 | 0.031/0.031 | 2.0–4.0 | 2.0/4.0 |
| Candida spp. (n=1) | 1 (100) | – | – | – | 1 (100) | – | 0.5 | 0.5/0.5 | 2.0 | 2.0/2.0 |
Fungicide Minimum Concentration (FMC) results of the four antifungals tested against Candida spp. isolated from colonized and/or VVC patients.
| Species | Fluconazole | Itraconazole | Miconazole | Nystatin | ||||
|---|---|---|---|---|---|---|---|---|
| FMC (μg/mL) | FMC50/FMC90 (μg/mL) | FMC (μg/mL) | FMC50/FMC90 (μg/mL) | FMC (μg/mL) | FMC50/FMC90 (μg/mL) | FMC (μg/mL) | FMC50/FMC90 (μg/mL) | |
| C. albicans (n=37) | >64 | >64/>64 | 16–>16 | >16/>16 | 2.0–>16 | >16/>16 | 4.0–8.0 | 8.0/80 |
| C. glabrata (n=6) | >64 | >64/>64 | >16 | >16/>16 | 16–>16 | >16/>16 | 4.0–8.0 | 8.0/8.0 |
| C. parapsilosis complex (n=2) | 4.0–>64 | 4.0/>64 | 2.0–>16 | 2.0/>16 | 2.0–>16 | 2.0/>16 | 8.0 | 8.0/8.0 |
| C. tropicalis (n=1) | >64 | >64/>64 | >16 | >16/>16 | >16 | >16/>16 | 8.0 | 8.0/8.0 |
| C. sphaerica (n=3) | 1.0–>64 | 1.0/>64 | 0.031–1.0 | 0.031/1.0 | 0.031–2.0 | 0.031/2.0 | 2.0–4.0 | 4.0/4.0 |
| Candida spp. (n=1) | >64 | >64/>64 | 1.0 | 1.0/1.0 | 8 | 8.0/8.0 | 2.0 | 2.0/2.0 |
The present study provides data about Candida spp. isolation from the vulvovaginal mucosa of women in southern Rio Grande do Sul, Brazil. We detected the yeast in 27% of the study population, corresponding to a prevalence of approximately 13% both for colonization and for vulvovaginal candidiasis. Similar and lower rates of fungal isolation in women with and without vulvovaginitis have been described in other studies of the southern region: 11 and 24.7% in patients in the state of Paraná,12–15 23.8% in patients in the state of Santa Catarina12 and 18.2% in the city of Santo Ângelo-RS,16 the latter being the only study held in Rio Grande do Sul. These differences in the isolation of Candida spp. from vaginal mucosa can be explained by cultural habits of different regions, especially in regard to hygiene practices, as this factor is directly related to self-contamination because the yeast belongs to the normal gastrointestinal tract microbiota.3,17,18 On the other hand, similar studies in northeastern Brazil show fungal isolation in women with and without vulvovaginitis of up to 46%.1,17,19,20 It is assumed that other factors besides those mentioned above may explain the high prevalence of Candida spp. in this region, such as weather conditions and social and environmental factors that are favorable to the reproduction of yeast.20
The rate of colonization of 13.7% observed in our study is consistent with the literature,3,9 which describes that Candida spp. is part of the genital microbiota of up to 30% of healthy women.1,18 Whereas the disease occurs endogenously, is important to differentiate between the colonization and infection of the vaginal mucosa.3,18
Ferrazza and co-workers12 found a VVC prevalence of 19.2% in Santa Catarina and of 9.3% in Paraná; these data are similar to the prevalence of VVC found in our study.12 However, it is below the 69% found by Holanda and co-workers17 in a study conducted in Natal, the 47.9% found by Andrioli and co-workers1 in Bahia, the 42.7% described by Sá and co-workers20 in Maranhão and the 39.6% found by Dias and co-workers7 in Mato Grosso. Considering that this disease is opportunistic, this discrepancy may be related to several factors related to the etiologic agent, the species distribution and/or virulence of the strains, the immune status of the host, or the environment, which influences the maintenance of temperature and humidity conducive to fungal proliferation.1,12,20,21
The differences in geographical location should be considered among the epidemiologic factors that also interfere in the prevalence of Candida spp. isolated from vaginal mucosa.12 In this study, C. albicans was predominant, accounting for over 60% of the isolates both in colonized patients and in those with VVC. In fact, this species is the most pathogenic of the gender, being related to most cases of VVC described, and generally represents more than half of the isolates identified in other studies.7,15,16,22 Ferraza and co-workers12 described the identification of this species in Santa Catarina in 100% and 72% of isolates from the asymptomatic and symptomatic patients, respectively, and in 66.7% of patients with VVC in state of Paraná. Similarly, Camargo and co-workers16 identified C. albicans in more than 80% of the isolates from patients with and without VVC in Santo Ângelo-RS.
Approximately 25% of cases of VVC were caused by non-albicans species in our study. Among these, C. glabrata, described as the second most important species in cases of VVC due to its frequency10,12,15,18,23 and greater resistance to antifungals10,22,24 was found in 8.6% of symptomatic patients and in 14.3% of colonized patients in our study, which highlights the importance of laboratory tests for correct identification of the etiologic agent, although the clinical diagnosis of the disease is easily performed and commonly applied.2,25
Regarding the variables studied, the largest number of patients with VVC was from the group of women younger than 31 years, being within the reproductive age group,3 which is considered a risk factor for VVC due to sexual activity.8 However, this difference in age between the group of women with VVC and the otherwise healthy group was not detected by other authors.1,17,19,23 Similarly, use of hormonal contraception or intrauterine devices, vaginal pH≤4.5, pregnancy and HIV infection, which are considered as risk factors for the development of VVC3,5,20,26 were not significantly associated with VVC infection in our study. This discrepancy may be due to the small number of women with VVC in our study (n=35), which does not allow for a robust statistical analysis for risk factors for the disease.
According to our study as well as that of Rodrigues and co-workers23 the other variables such as marital status, educational level and skin color do not exert significant influence on the development of VVC. Moreover, Álvares and co-workers4 state that the vaginal microbiota of black women has a lower incidence of bacterial species, and there would thus be a decrease in natural defenses against fungal growth, predisposing them to infection by Candida spp.4,20
In vitro studies have shown different resistance rates of Candida spp. from the vaginal mucosa to the azole drugs commonly used in the treatment and prophylaxis of VVC.7,8,10,11,13,22,24,27,28 Concerning fluconazole, the resistance rates range from 0.8% to 12.5%.7,10,11,24,28 These values are considerably lower than the results found in our study, in which 42% of the isolates were resistant to fluconazole. However, Dalazen and co-workers22 in a study conducted in Santa Catarina, found 100% of fluconazole resistance. With respect to itraconazole, resistance rates described in other studies range from 1.9 to 43%,10,13,27,28 also lower than the 48% found in our study. In addition to the high rate of resistance to fluconazole and itraconazole observed, the FMC of more than 90% of the isolates was greater than the maximum antifungal concentration tested. Therefore, there is a tendency to avoid the prophylactic use of low doses of antifungals in gynecological routines to prevent the emergence of drug-resistant isolates.2
Another group of azole drugs used for topical treatment of VVC is miconazole.22 In our study, the MIC for this drug ranged from 0.031 to 8μg/mL. These data were similar to those found in a study by Choukri and co-workers29 in which the MIC to miconazole varied between 0.015 and 8μg/mL and in a study by Richter and co-workers10 in which the variation was between 0.007 and 4μg/mL. Moreover, Dalazen and co-workers22 found a variation in the MIC of miconazole of between 0.097 and ≥100μg/mL. The different genotypes of the same species may be significantly different in their susceptibility to azoles.27
The polyenic agents are another group of drugs used for the treatment of VVC; this group includes nystatin, which is the most used in Brazil and is freely available in the Unified Health System (SUS) in Brazil.30 The MIC of nystatin found in our study ranged from 2 to >16μg/mL; however, in a study by Choukri and co-workers,29 the MIC varied between 1 and 4μg/mL, and in a study by Richter and co-workers,10 the concentration ranged from 1 to 16μg/mL. Knowledge of the patterns of susceptibility to typical drugs in isolates from different regions will allow for the rationalization of the empirical use of antifungal agents, thus contributing to the control of the isolated resistance to drugs.2
Our results will encourage the development of other studies related to the resistance mechanisms of these yeasts and also of clinical trials in the hospital to determine whether the high resistance to antifungals detected in vitro actually corresponds to therapeutic failure.
According to this data discrepancies and the lack of epidemiological data in the state of Rio Grande do Sul, the present study has been collaborating with local epidemiology, elucidating the etiological factors of VVC and warning of the high resistance rates found in vitro in Candida spp. isolates evaluated.
Conflicts of interestThe authors declare no conflicts of interest.